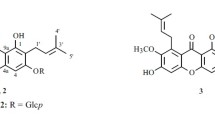
Abstract
Ginseng has been used for thousands of years in Asian countries as a traditional medicinal herb and has gained great popularity in the past decade. Ginsenosides are the major pharmacological components in ginseng. We here show that Cladosporium cladosporioide is able to convert the major ginsenoside Rb1 into four known metabolites (ginsenosides Rd, F2, CK and PPD) and two new metabolites [12β-hydroxydammar-3-one-20(S)-O-β-d-glucopyranoside (3-oxo-CK) and dammar-24-en-12β,20(S)-diol-3-one (3-oxo-PPD)]. CK, PPD and 3-oxo-PPD were shown to have a potent antiproliferative activity against A549 lung cancer cells. We found that Rb1 → Rd → F2 → CK → PPD or 3-oxo-CK → 3-oxo-PPD represents the ginsenoside metabolic pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As a traditional medicinal herb, ginseng has been used for thousands of years in the Far East. It has gained great popularity in the West during the past decade (Attele et al. 1999; Ang-Lee et al. 2001). Ginsenosides are the major pharmacological components in ginseng with Rb1, Rb2, Rc, Rd, Rg1 and Re as the major compounds (Yang et al. 2014; Cheng et al. 2008). For instance, Rb1 is the major component of the protopanaxadiol group saponins in roots, making up 23.8 % of the total ginsenosides (Son et al. 2008).
Orally ingested major ginsenosides are activated by intestinal bacterial deglycosylation (Hasegawa 2004). In recent decades, many studies have reported the successful transformation of major ginsenosides into more active ginsenosides such as Rg3, CK and PPD. For instance, CK has anti-cancer (Kim et al. 2009; Ming et al. 2011), anti-angiogenic (Jeong et al. 2010), anti-inflammation (Joh et al. 2011) and hepatoprotective effects (Lee et al. 2005), while PPD provides anti-lung cancer (Zhang et al. 2013) and anti-prostate cancer activity (Cao et al. 2014). The natural availability of these ginsenosides in ginseng is low. The conversion of major ginsenosides to the more active minor ginsenosides can be accomplished through a number of methods such as acid treatment (Bae et al. 2004), alkali treatment (Yang et al. 2003), heating (Sun et al. 2009) and microbial transformation (Wu et al. 2012). Chemical transformation produces nonspecific racemic mixtures of ginsenosides, which is why Microbial transformation is preferred (Quan et al. 2011). Microbial modification includes side-chain oxidation–reduction, hydroxylation and ketonization (Liu et al. 2011; Chen et al. 2013; Jin et al. 2014).
Lung cancer is the leading cause of cancer death in the world (Ferlay et al. 2010). Non-small-cell lung carcinoma (NSCLC) is the most common type of lung cancer accounting for 85–90 % of the cases (Gong et al. 2011). A large number of bioactive compounds are used to treat cancers, including vinblastine, paclitaxel and camptothecin. Still it is important to search for alternative therapeutic agents. Here we show that Cladosporium cladosporioide KACC 43926 converts Rb1 into several ginsenosides with potent antiproliferative activity against A549 lung cancer cells.
Materials and methods
Materials and organisms
Yeast mold (YM) broth was purchased from Difco (USA). The Silica gel 60 F254 plates and silica gel 60 (Merck, Germany) was used for TLC and column chromatography. All chemicals and solvents were analytical or HPLC grade. The strain C. cladosporioide KACC 43926 was purchased from Korean Agricultural Culture Collection (Suwon, Republic of Korea).
Preparation of ginsenoside Rb1
American ginseng extract with a ginsenoside content ≥ 80 % (w/w) was purchased from Jiuhui Co. Ltd. (Changsha, Hunan, China). The crude extract was subjected to a silica gel column and eluted with CHCl3–MeOH–H2O (5:1:3–65:35:5, v/v/v). The eluate was then purified by semi-preparative HPLC to yield pure Rb1.
Biotransformation of ginsenoside Rb1
C. cladosporioide KACC 43926 was incubated in 150 mL YM broth containing 0.4 mg/mL Rb1 as the carbon source in flasks at 28 °C and 150 rpm. After 10 days of incubation, the reaction mixture was extracted three times with water-saturated butanol. TLC analysis was performed using Silica Gel 60 plates and CHCl3–CH3OH–H2O (65:35:10 v/v/v). Compounds were detected by spraying 10 % (v/v) H2SO4 followed by heating at 110 °C for 10 min (Shibata et al. 1965). HPLC was performed using an Agilent 1260 system (Agilent). The separation was performed on a C18 column (50 × 4.6 mm, ID 2.6 μm) with H2O (solvent A) and acetonitrile (solvent B) at A/B ratios of 81:19, 81:19, 71:29, 71:29, 60:40, 44:56, 30:70, 10:90, 10:90, 81:19, and 81:19; with run times of 0–7, 7–11, 11–14, 14–25, 25–28, 28–30, 30–31.5, 31.5–34, 34–34.5, and 34.5–40 min, respectively. The flow rate was 0.6 mL min−1 and detection wavelength was 203 nm.
Isolation of metabolites
Fractions A–C were obtained by separating the reaction extract on silica gel column chromatography using CHCl3–CH3OH (13:1). Fraction A was separated with CH2Cl2–EtOH (50:1–1:1) yielding fractions D-E. Fraction were further purified by semi-preparative HPLC. Metabolites were dissolved in pyridine-d 5 and identified by 1H, 13C, and 2D NMR using an FT-NMR spectrometer (400 MHz; Varian Inova AS 400, Varian, Palo Alto, CA, USA). Chemical shifts are given in δ (ppm) based on tetramethylsilane (TMS) as internal standard.
Proton NMR data of metabolite 5 were: 1H-NMR (400 MHz, pyridine-d 5, δH) 5.25 (1H, dd, J = 6.8, 6.4 Hz, H-24), 5.16 (1H, d, J = 7.6 Hz, H-1′), 4.46–3.89 (sugar moieties), 1.60 (9H, s, H-28, 21, 29), 1.11 (3H, H-26), 1.03 (3H, s, H-27), 0.96 (3H, s, H-18), 0.91 (3H, s, H-19), 0.87 (3H, s, H-30). Proton NMR data of metabolite 6 were: 1H-NMR (400 MHz, pyridine-d 5, δH) 5.47 (1H, dd, J = 6.4, 5.6 Hz, H-24), 3.89 (1H, m, H-12), 1.69 (3H, s, H-28), 1.66 (3H, s, H-21), 1.40 (3H, s, H-29), 1.13 (3H, H-26), 1.04 (3H, s, H-27), 0.99 (3H, s, H-18), 0.92 (3H, s, H-19), 0.87 (3H, s, H-30).
Cytotoxicity assay
Cell viability was measured by using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) assay (Mosmann 1983). A549 Lung Cancer cells were cultured for 24 h with different 0-300 μM ginsenoside in 96-well plates at a density of 1 × 104 cells/well. After incubation at 37 °C in a humidified incubator containing 5 % CO2, 10 μL of MTT (5 mg/mL) was added to each well and incubated for an additional 4 h. Precipitated formazan was dissolved in 100 μL of DMSO for 30 min. The absorbance was recorded with a plate reader (Bio-Tek Instrument, USA) at a test wavelength of 570 nm and a reference wavelength of 630 nm.
Results
Biotranformation of ginsenoside Rb1
C. cladosporioide KACC 43926 converted ginsenoside Rb1 into several products as shown by TLC and HPLC analysis. Reaction products were separated on silica gel column chromatography. As a result, 4 known metabolites (ginsenoside Rd (metabolite 1), F2 (metabolite 2), CK (metabolite 3) and PPD (metabolite 4)) and 2 new metabolites (metabolites 5, 6) were obtained (Figs. 1, 2). Metabolite 1, 2 are intermediate metabolites, while metabolites 3, 4, 5, 6 represent final products.
Structure of metabolites
Metabolite 5 was obtained as a white powder. The 1H and 13C NMR and DEPT (distortionless enhancement by polarization transfer) spectra of metabolite 5 were very similar to CK except for the appearance of a ketone (δC 216.2, C-3) and the disappearance of an oxygenated methine signal. The molecular weight of metabolite 5 was 2 daltons less than that of CK, indicating that the ketonization was introduced at C-3. The proton and carbon signals attributed to the sugar moiety suggested the presence of a β-glucopyranosyl group. The correlation between δH 5.16 (H-1′) and δC 83.3 (C-20) in the HMBC (heteronuclear multiple bond correlation) spectrum and the chemical shift of the anomeric carbon signal (δC 98.2) supported the presence of a glucopyranosyl group at C-20. Therefore, the structure of metabolite 5 was determined as 12β-hydroxydammar-3-1-20(S)-O-β-d-glucopyranoside (3-oxo-CK).
Metabolite 6 was obtained as a white powder. The 13C-NMR spectrum of metabolite 6 displayed 30 carbon signals. All signals could be assigned based on a DEPT experiment, the HSQC (heteronuclear single quantum coherence) spectrum, and a comparison with the 13C-NMR data of PPD (Asakawa et al. 1977) (Table 1). The data were similar to PPD with the exception of the proton and carbon resonances indicating the presence of a carbonyl functional group instead of an oxygenated methane at the C-3 position. The 1H and 13C NMR spectra (Table 1) showed signals of an olefine methine group [δH 5.47 (J = 6.4, 5.6 Hz, H-24), δc 126.0 (C-24)], eight tertiary methyl groups (Table 1) and an oxygenated methine group [δH 3.89 (H-12), δc 70.5 (C-12)]. A ketone signal at δc 215.9 (C-3) was observed in the low magnetic field. In comparison to a previously isolated compound, metabolite 6 lacked a sugar moiety at the C-20 position. Consequently, the structure of metabolite 6 was determined to be dammar-24-en-12β,20(S)-diol-3-one (3-oxo-PPD) (Anufriev et al. 1997).
Biotransformation pathway
TLC analysis was performed to identify the metabolic pathway of the ginsenosides. To this end, samples of the reaction mixture were taken in time (Fig. 3). Ginsenoside Rb1 was converted into ginsenoside Rd by hydrolysis of a glucose unit at C-20 position. Then, ginsenoside F2 was produced from ginsenoside Rd by additional hydrolysis of a single glucose moiety at C-3 position. Ginsenoside F2 was converted into CK by hydrolysis of a glucose unit at C-3 position. Ginsenoside CK was transformed into PPD by hydrolysis of a glucose unit at C-20 position or 3-oxo-CK by ketonization at C-3 position. Finally, ginsenoside PPD and 3-oxo-CK were transformed into 3-oxo-PPD by ketonization at C-3 position and hydrolysis of a glucose unit at C-20 position, respectively. These results suggest that C. cladosporioide KACC 43926 has potent β-glucosidase and ginsenoside dehydrogenase activity.
In vitro cytotoxicity assay in A549 lung cancer cells
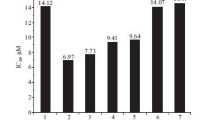
Cell viability effects of four derivatives (CK, 3-oxo-CK, PPD, 3-oxo-PPD) on A549 cells was evaluated by MTT assay. The 3-oxo-CK did not affect A549 cell growth in 24 h (data not shown). However, proliferation of A549 cells was suppressed in a dose-dependent way by CK, PPD and 3-oxo-PPD (Fig. 4). CK, PPD and 3-oxo-PPD significantly inhibited the growth of A549 cells at 50, 100 and 300 μM, while 3-oxo-PPD only showed activity at 10 μM. Thus, CK and PPD are more cytotoxic than 3-oxo-PPD.
Discussion
The natural major ginsenosides are excreted after uptake by the human body but when modified by the intestinal microbiota these bioactive compounds are retained. For instance, the ginsenosides Rb1, Rb2, Rc and Rd are metabolized into CK by intestinal microbiota (Hasegawa et al. 1996; Akao et al. 1998). Oral administration of ginsenoside Rb1 resulted in eight metabolites in rats, including gypenoside XVII, ginsenosides Rd, F2, CK and 3-oxo-CK (Chen et al. 2008). In addition, Jin et al. (2013) reported that PPD was metabolized into 3-oxo-PPD in rats after oral administration. It should be noted that individuals have their own characteristic indigenous gut microbiota (Simon and Gorbach 1986; Rumney and Rowland 1992; Wakabayashi et al. 1997) and their ginsenoside modifying activity can thus be different. Moreover, microbial intestinal composition can be affected disease, unbalanced diet, stress and lifestyles. Hence, the pharmacological effects of ginseng will depend on how effectively a person can metabolize and absorb ginsenosides. To circumvent this, ginsenosides can be modified in vitro.
This study shows for the first time that C. cladosporioide KACC 43926 can convert the major ginsenoside Rb1 into bioactive compounds that can also be found after oral administration. Evidence was presented that the metabolic pathway of Rb1 follows Rb1 → Rd → F2 → CK → PPD or 3-oxo-CK → 3-oxo-PPD. C. cladosporioide KACC 43926 has potent β-glucosidase for hydrolysis of a glucose unit at C-3 or C-20 position and ginsenoside dehydrogenase activity for ketonization at C-3 position.
The non-polar ginsenosides, such as Rg3, Rh2, Rk1, Rg5, CK and PPD are taken up in human breast cancer cells. The most non-polar ginsenoside PPD has the highest uptake rate, followed by CK (Ha et al. 2010). The Rb1 metabolites of C. cladosporioide should thus be easily absorbed in the body even in the absence of an intestinal microbiota that can metabolize ginsenosides. Based on the cytotoxicity results of four compounds, the ketonized compounds may reduce the cytotoxicity in cell lines. CK, PPD and 3-oxo-PPD significantly inhibited growth of A549 lung cancer cells at 50–300 μM. Therefore, these compounds have both high uptake and potent antiproliferative activity against these cells.
References
Akao T, Kida H, Kanaoka M, Hattori M, Kobashi K (1998) Intestinal bacterial hydrolysis is required for the appearance of compound K in rat plasma after oral administration of ginsenoside Rb1 from Panax ginseng. J Pharm Pharmacol 50:1155–1160
Ang-Lee MK, Moss J, Yuan CS (2001) Herbal medicines and perioperative care. JAMA 286:208–216
Anufriev VP, Malinovskaya GV, Denisenko VA, Uvaròva NI, Elyakov GB, Kim SI, Baek NI (1997) Synthesis of ginsenoside Rg3, a minor constituent of Ginseng Radix. Carbohydr Res 304:179–182
Asakawa J, Kasai R, Yamasaki K, Tanaka O (1977) Carbon-13 NMR study of Ginseng sapogenins and their related dammarane type triterpenes. Tetrahedron 33:1935–1939
Attele AS, Wu JA, Yuan CS (1999) Ginseng pharmacology: multiple constituents and multiple actions. Bioche Pharmacol 58:1685–1693
Bae EA, Han MJ, Kim EJ, Kim DH (2004) Transformation of ginseng saponins to ginsenoside Rh2 by acids and human intestinal bacteria and biological activities of their transformants. Arch Pharm Res 27:61–67
Cao B, Qi Y, Yang Y, Liu X, Xu D, Guo W, Zhan Y, Xiong Z, Zhang A, Wang AR, Fu X, Zhang H, Zhao L, Gu J, Dong Y (2014) 20(S)-protopanaxadiol inhibition of progression and growth of castration-resistant prostate cancer. Plos One 9:e111201
Chen G, Yang M, Song Y, Lu Z, Zhang J, Huang H, Guan S, Wu L, Guo DA (2008) Comparative analysis on microbial and rat metabolism of ginsenoside Rb1 by high-performance liquid chromatography coupled with tandem mass spectrometry. Biomed Chromatogr 22:779–785
Chen G, Yang M, Nong S, Yang X, Ling Y, Wang D, Wang X, Zhang W (2013) Microbial transformation of 20(S)-protopanaxadiol by Absidia corymbifera. Cytotoxic activity of the metabolites against human prostate cancer cells. Fitoterapia 84:6–10
Cheng LQ, Na JR, Bang MH, Kim MK, Yang DC (2008) Conversion of major ginsenoside Rb1 to 20(S)-ginsenoside Rg3 by Microbacterium sp. GS514. Phytochemistry 69:218–224
Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM (2010) Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 127:2893–2917
Gong HC, Wang S, Mayer G, Chen G, Leesman G, Singh S, Beer DG (2011) Signatures of drug sensitivity in nonsmall cell lung cancer. Int J Proteomics 2011:215496
Ha YW, Ahn KS, Lee JC, Kim SH, Chung BC, Choi MH (2010) Validated quantification for selective cellular uptake of ginsenosides on MCF-7 human breast cancer cells by liquid chromatography-mass spectrometry. Anal Bioanal Chem 396:3017–3025
Hasegawa H (2004) Proof of the mysterious efficacy of ginseng: basic and clinical trials: metabolic activation of ginsenoside: deglycosylation by intestinal bacteria and esterification with fatty acid. J Pharmacol Sci 95:153–157
Hasegawa H, Sung JH, Matsumiya S, Uchiyama M (1996) Main ginseng metabolites formed by intestinal bacteria. Planta Med 62:453–455
Jeong A, Lee HJ, Jeong SJ, Lee HJ, Lee EO, Bae H, Kim SH (2010) Compound K inhibits basic fibroblast growth factor-induced angiogenesis via regulation of p38 mitogen activated protein kinase and AKT in human umbilical vein endothelial cells. Bio Pharm Bull 33:945–950
Jin X, Li SL, Zhang ZH, Zhu FX, Sun E, Wei YJ, Jia XB (2013) Characterization of metabolites of 20(S)-protopanaxadiol in rats using ultra-performance liquid chromatography/quadrupole-time-of-flight mass spectrometry. J Chromatogr B 933:59–66
Jin Y, Jung SY, Kim YJ, Lee DY, Min JW, Wang C, Yang DC (2014) Microbial ketonization of ginsenosides F1 and C-K by Lactobacillus brevis. Antonie Van Leeuwenhoek 106:1215–1221
Joh EH, Lee IA, Jung IH, Kim DH (2011) Ginsenoside Rb1 and its metabolite compound K inhibit IRAK-1 activation–the key step of inflammation. Biochem Pharmacol 82:278–286
Kim DY, Park MW, Yuan HD, Lee HJ, Kim SH, Chung SH (2009) Compound K induces apoptosis via CAMK-IV/AMPK pathways in HT-29 colon cancer cells. J Agric Food Chem 57:10573–10578
Lee HU, Bae EA, Han MJ, Kim NJ, Kim DH (2005) Hepatoprotective effect of ginsenoside Rb1 and compound K on tert-butyl hydroperoxide-induced liver injury. Liver Int 25:1069–1073
Liu X, Qiao LR, Xie D, Dai JG (2011) Microbial deglycosylation and ketonization of ginsenosides Rg1 and Rb1 by Fusarium oxysporum. J Asian Nat Prod Res 13:652–658
Ming Y, Chen Z, Chen L, Lin D, Tong Q, Zheng Z, Song G (2011) Ginsenoside compound K attenuates metastatic growth of hepatocellular carcinoma, which is associated with the translocation of nuclear factor-κB p65 and reduction of matrix metalloproteinase-2/9. Planta Med 77:428–433
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Quan LH, Piao JY, Min JW, Yang DU, Lee HN, Yang DC (2011) Bioconversion of ginsenoside Rb1 into compound k by Leuconostoc citreum LH1 isolated from kimchi. Braz J Microbiol 42:1227–1237
Rumney CJ, Rowland IR (1992) In vivo and in vitro models of the human colonic flora. Crit Rev Food Sci Nutr 31:299–331
Shibata S, Tanaka O, Soma K, Ando T, Iida Y, Nakamura H (1965) Studies on saponins and sapogenins of ginseng: the structure of panaxatriol. Tetrahedron Lett 42:207–213
Simon GL, Gorbach SL (1986) The human intestinal microflora. Dig Dis Sci 31:147–162
Son JW, Kim HJ, Oh DK (2008) Ginsenoside Rd production from the major ginsenoside Rb1 by beta-glucosidase from Thermus caldophilus. Biotechnol Lett 30:713–716
Sun BS, Gu LJ, Fang ZM, Wang CY, Wang Z, Lee MR, Li Z, Li JJ, Sung CK (2009) Simultaneous quantification of 19 ginsenosides in black ginseng developed from Panax ginseng by HPLC-ELSD. J Pharm Biomed Anal 50:15–22
Wakabayashi C, Hasegawa H, Murata J, Saiki I (1997) In vivo antimetastatic action of ginseng protopanaxadiol saponins is based on their intestinal bacterial metabolites after oral administration. Oncol Res 9:411–417
Wu LP, Jin Y, Yin CR, Bai LL (2012) Co-transformation of Panax major ginsenosides Rb1 and Rg1 to minor ginsenosides C-K and F1 by Cladosporium cladosporioides. J Ind Microbiol Biotechnol 39:521–527
Yang L, He KJ, Y. Yang (2003) A preparation method of less polar individual ginsenosides and saponins by base hydrolysis. China Patent CN03134090.3
Yang WZ, Hu Y, Wu WY, Ye M, Guo DA (2014) Saponins in the genus Panax L. (Araliaceae): a systematic review of their chemical diversity. Phytochemistry 106:7–24
Zhang YL, Zhang R, Xu HL, Yu XF, Qu SC, Sui DY (2013) 20(S)-protopanaxadiol triggers mitochondrial-mediated apoptosis in human lung adenocarcinoma A549 cells via inhibiting the PI3 K/Akt signaling pathway. Am J Chin Med 41:1137–1152
Acknowledgments
This research was supported by Korea Institute of Planning & Evaluation for Technology in Food, Agriculture, Forestry & Fisheries (KIPET NO: 313038-03-1-SB010) and Next-Generation BioGreen 21 Program (SSAC, Grant#: PJ009529032014), Republic of Korea.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Jin, Y., Jung, S.Y., Kim, YJ. et al. Microbial deglycosylation and ketonization of ginsenoside by Cladosporium cladosporioide and their anticancer activity. Antonie van Leeuwenhoek 109, 179–185 (2016). https://doi.org/10.1007/s10482-015-0619-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10482-015-0619-8