Abstract
The fermentative and aromatic capabilities of Kloeckera africana/Hanseniaspora vineae K1, K. apiculata/H. uvarum K2, and Saccharomyces cerevisiae S1 and S2 were studied in pure and mixed culture fermentations using Agave tequila juice as the culture medium. In pure and mixed cultures, Kloeckera/Hanseniaspora strains showed limited growth and sugar consumption, as well as low ethanol yield and productivity, compared to S. cerevisiae, which yielded more biomass, ethanol and viable cell concentrations. In pure and mixed cultures, S. cerevisiae presented a similar behaviour reaching high biomass production, completely consuming the sugar, leading to high ethanol production. Furthermore, the presence of S. cerevisiae strains in the mixed cultures promoted the production of higher alcohols, acetaldehyde and ethyl esters, whereas Kloeckera/Hanseniaspora strains stimulated the production of ethyl acetate and 2-phenyl ethyl acetate compounds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tequila is a Mexican alcoholic beverage produced by distilling the fermented juice of the Agave tequilana Weber blue variety (Agave azul) plant (NOM-006-SCFI-2012 2012). Tequila production involves several steps: harvesting and cooking the A. azul plant, milling the cooked A. azul, fermenting the sugars, and distilling the must (Cedeño 1995). During tequila fermentation, yeasts convert sugars into ethanol and volatile compounds, which form the bouquet of the alcoholic beverage (Swiegers et al. 2005; Díaz-Montaño et al. 2008). As in wine fermentation, in traditional tequila distilleries spontaneous fermentation is carried out by a succession of different yeast strains. The first stages of fermentation are characterized by the growth of certain non-Saccharomyces species, mainly Torulaspora delbrueckii, Kluyveromyces marxianus, and Hanseniaspora spp. (Lachance 1995). Their growth is quickly inhibited by the increase in ethanol concentration (Kunkee 1984) or by a nutritional limitation in the culture medium (Díaz-Montaño et al. 2010), progressively ceding the way to more alcohol-tolerant strains, especially Saccharomyces cerevisiae (Ciani and Picciotti 1995; Romano et al. 1997; Pérez-Nevado et al. 2006). Despite their limited growth during fermentation, non-Saccharomyces species produce a large number of important metabolites such as esters, acetic acid, and acetoin, which can have a significant influence on the sensory properties of the final product (Romano et al. 1997, 2003; Ciani and Picciotti 1995). In addition, non-Saccharomyces strains are associated with the synthesis of several extracellular enzymes such as β-glucosidases, which release monoterpenes derived from their glycosylated forms. These compounds give higher fruit-like aromatic characteristics to wine and other alcoholic beverages (Swangkeaw et al. 2010; Díaz-Montaño and Córdova 2009).
Volatile compounds provide taste and odor to alcoholic beverages. Most of these compounds are produced by yeast during fermentation. Many studies have examined tequila’s aromatic profile. Benn and Peppard (1996) distinguished more than 175 compounds in three types of tequila (white, rested and aged). Díaz-Montaño et al. (2008) analyzed the volatile composition of agave juice fermented by Saccharomyces and Kloeckera strains, and determined that Kloeckera yeasts lead to higher concentrations of ethyl acetate, acetic acid, and 2-phenyl ethyl acetate than Saccharomyces strains, which predominantly produce higher alcohols, acetaldehyde, glycerol, isoamyl acetate, and ethyl hexanoate. Modern distilleries often select specific yeast strains to achieve a specific aroma profile (Ugliano et al. 2010). However, tequila is typically fermented with an inoculum of S. cerevisiae; due to its higher fermentative capacity (Hernández-Cortés et al. 2010). Nevertheless, several reports on wine fermentation have revealed the importance of using mixed cultures of non-Saccharomyces yeasts in combination with S. cerevisiae since they increase the aromatic fraction of the fermented media, therefore improving the sensory quality of the final product (Rojas et al. 2003; Romano et al. 2003; Ciani et al. 2006; Moreira et al. 2005). For example, mixed culture fermentations of non-Saccharomyces and S. cerevisiae have shown the production of higher concentrations of esters, terpenes, and acetoin than the must fermented by S. cerevisiae in monoculture (Mendes-Ferreira et al. 2001; Romano et al. 1997, 2003; Rojas et al. 2003).
To our knowledge, studies using mixed culture fermentations of non-Saccharomyces and S. cerevisiae native strains have not been reported previously in tequila and their potential use as an alternative to diversify or modify the overall flavour quality of the final product. Therefore, the main purpose of this work was to investigate the fermentative capability, growth, and synthesis of volatile compounds during the fermentation of agave juice, using pure and mixed cultures of Kloeckera/Hanseniaspora and Saccharomyces native strain
Materials and methods
Yeast strains
Four strains (S1, S2, K1 and K2) were isolated from A. tequilana Weber blue variety juice (A. azul juice) obtained from a tequila distillery in Jalisco, Mexico, and deposited in the culture collection at the Centro de Investigación y Asistencia en Tecnología y Diseño del Estado de Jalisco (CIATEJ). They were identified by biochemical and molecular tests by Díaz-Montaño et al. (2008). The four strains, S. cerevisiae (S1 and S2), Kloeckera africana/Hanseniaspora vineae (K1), and K. apiculata/H. uvarum (K2) were stored in 1 ml vials at −70 °C in a 1:1 mixture of the propagation medium and a 50 % glycerol solution.
Inoculum and fermentation media
The A. tequilana Weber blue variety juice used was kindly supplied by La Quemada tequila distillery. The agave juice at 20°Bx was filtered and stored at −20 °C. Pre-inoculum and inoculum media were prepared in diluted agave juice adjusted to 3°Bx (31 ± 1.3 g/l of reducing sugars) with distilled water and supplemented with ammonium sulfate (1 g/l). A pre-inoculum was prepared by transferring 1 ml of conserved cells at –70 °C to 50 ml of medium contained in a 200 ml Erlenmeyer flask and incubated at 30 °C and 250 rpm for 24 h. Then, an inoculum was prepared by adding 20 ml of pre-inoculum in 200 ml of medium contained in a 1 l Erlenmeyer flask and incubated at 30 °C and 250 rpm for 12 h. Fermentation media were prepared with agave juice adjusted to a reducing sugars concentration of 100 ± 13 g/l supplemented with ammonium sulfate (1 g/l) as it is common done in the tequila industry (Cedeño 1995). Fermentation and growth media were sterilized at 121 °C for 15 min.
Fermentation conditions
Pure and mixed culture fermentations were performed under anaerobic growth conditions in 500 ml-Erlenmeyer flasks containing 300 ml of agave juice at 30 °C and 250 rpm. These cultures were protected from exposure to oxygen using stoppers. Pure culture fermentations were inoculated with 3.5 × 106 cells/ml of the selected yeast strain. Meanwhile, mixed culture fermentations were inoculated with 1.75 × 106 cells/ml of each specific yeast. Prior to inoculation, the yeast population was estimated with a Neubauer chamber. All fermentations were stopped after 72 h and performed in duplicate for the concentration of biomass, ethanol, reducing sugars and volatile compounds.
Analytical methods
The yeast growth during fermentation was obtained by viable cell quantification using the classical plate count method. Samples were taken aseptically throughout the fermentations and diluted appropriately with distilled water. The enumeration of the yeasts in pure culture was accomplished in YEPD-agar medium (containing 20 g/l glucose, 20 g/l peptone, 20 g/l agar, and 10 g/l yeast extract at pH 4.7). In mixed culture fermentations, the enumeration of Kloeckera/Hanseniaspora yeasts (K1 and K2) cells was performed using YEPD-agar medium supplemented with 0.5 μg/ml of cycloheximide (Fluka analytical) (YEPD + CYH) (Pérez et al. 2000). The number of viable cells of S. cerevisiae strains (S1 and S2) in mixed cultures was given as the difference between the total number of colonies on YEPD-agar plates and the total number of colonies on YEPD + CYH plates. YEPD-agar and YEPD + CYH plates were incubated at 30 °C for 2–6 days. The number of cells for each yeast strain was calculated when no increase in the number of colony forming units (CFU) was observed.
Biomass concentration was determined by measuring the dry weight of 5 mL fermented must centrifuge as reported by Díaz-Montaño et al. (2010). Must supernatants were used to assay sugars, ethanol and volatile compounds. Reducing sugars and ethanol concentrations were measured using the modified DNS method (Díaz-Montaño, 2004) and enzymatic analyzer (YSI model 2700 select, Yellow Springs Instruments) respectively.
Volatile compounds concentrations were determined by duplicated by gas chromatography (GC) following Valle-Rodríguez et al. (2012) conditions. A Hewlett Packard Head-space HP 7694E model connected to a Hewlett-Packard 6890 Series gas chromatograph equipped with a flame ionization detector (FID) and a 60 m × 320 µm × 0.25 µm thickness film HP-Innowax capillary column were used. The oven temperature was held at 35 °C for 10 min, then increased at a rate of 3.5 °C min−1 until 175 °C for 2 min, and finally increased at 7 °C min−1 until 250 °C were reached. Helium was the carrier gas at 1.5 mL min−1. The detector and injector temperatures were 260 °C and 240 °C, respectively. The Headspace system program temperatures were 80, 110, and 115 °C for vial, loop and R-line respectively. The times for the gas chromatograph cycle, vial equilibrium, pressurization, filling loop, equilibrium of loop, injection and agitation were: 41, 10, 0.2, 0.2, 0.5, 1 and 5 min, respectively. Quantification was based on the external standard method using different diluted solutions containing 0.05, 0.2, 0.4, 0.6, 0.8, 1 and 2 mg/l of isoamyl acetate, ethyl hexanoate, ethyl octanoate, ethyl decanoate and 2-phenyl ethyl acetate; 1, 4, 8, 12, 16, 20 and 40 mg/l of n-butanol, acetaldehyde and ethyl acetate; 2, 8, 16, 24, 32, 40 and 80 mg/l of n-propanol, isobutanol and 2-phenyl ethanol, 20, 60, 120, 180, 240, 300 and 600 mg/l of isoamyl alcohol, and 10, 40, 80, 120, 160, 200 and 400 mg/l of methanol. Calibration curves gave a correlation coefficient (R 2 ) greater than or equal to 0.999 for each compound as determined using the HP Chemstation software Rev. A.05.04.
Data treatment and statistical analysis
The response variables data (biomass, ethanol and reducing sugar) of the two fermentations for each yeast strain in pure and mixed culture were compared using the Student’s t test for means comparison of paired samples at a 95 % probability. When significant differences were found in response variable data between replicates, the experiment was performed again two times in order to obtain more reliable data. Experimental data were adjusted by using the Curve Expert 1.3 program to determine the kinetic parameters (EBT Comm, Columbus, USA). The statistical method used for comparing yeast strain performance was the one-way variance analysis (ANOVA). The response variables measured were as follows: final concentration of different volatile compounds, biomass, ethanol, and consumed substrate, as well as maximal value of the specific growth rate, ethanol production rate, and sugar consumption rate. The differences in the amounts of volatile compounds were analyzed by principal component analysis (PCA). The ANOVA analysis employed Statgraphics plus software (Manugistics Inc; Rockville, USA). PCAwas performed using Simca software P7.01.
Results and discussion
Kinetic analysis of Kloeckera/Hanseniaspora and Saccharomyces strains in pure and mixed cultures.
Pure cultures
Fermentations by pure cultures of S. cerevisiae (S1 and S2), K. africana/H. vineae (K1) and K. apiculata/H. uvarum (K2) were performed under anaerobic growth at 30 °C and 250 rpm. Viable cell (CFU), biomass, reducing sugar consumption, and ethanol production profiles for each yeast strain in pure cultures are plotted in Fig. 1, and the kinetic parameters are shown in Table 1. The behaviour of Saccharomyces and Kloeckera/Hanseniaspora strains in pure culture was different. Both strains of S. cerevisiae grew faster than Kloeckera/Hanseniaspora yeasts reaching 4.22 and 3.68 g/l of biomass (S1 and S2) in 24 and 16 h of fermentation respectively. On the one hand, Kloeckera/Hanseniaspora (K1 and K2) strains grew slowly; reaching maximal concentrations of 1.83 and 1.93 g/l at the end of fermentation (Fig. 1). On the other hand, the viable cells (CFU) of S. cerevisiae (S1 and S2) strains remained constant for a much longer time, reaching 98.5 and 147 × 106 CFU/ml at 16 h of fermentation. Kloeckera/Hanseniaspora strains (K1 and K2) showed a maximum level at 16 and 20 h of fermentation, reaching 74 and 62.5 × 106 CFU/ml respectively (Fig. 1). However, the viable cell number for Kloeckera/Hanseniaspora yeasts declined gradually to 30 × 106 CFU/ml at the end of fermentation (Fig. 1).
Kinetic profiles of fermentations by pure culture of S. cerevisiae S1 ( ), S. cerevisiae S2 (
), S. cerevisiae S2 ( ), K. africana/H. vineae K1 (filled square) and K. apiculata/H. uvarum K2 (filled inverted triangle) strains in Agave tequilana juice media at 12°Brix supplemented with ammonium sulphate (1 g/l). CFU: viable cell (a, c, e, g); biomass: biomass concentration profile (filled circle); reducing sugar: reducing sugar concentration profile (filled triangle) and ethanol: ethanol concentration profile (
), K. africana/H. vineae K1 (filled square) and K. apiculata/H. uvarum K2 (filled inverted triangle) strains in Agave tequilana juice media at 12°Brix supplemented with ammonium sulphate (1 g/l). CFU: viable cell (a, c, e, g); biomass: biomass concentration profile (filled circle); reducing sugar: reducing sugar concentration profile (filled triangle) and ethanol: ethanol concentration profile ( ). Each value represents the average ± SD of two fermentations. Vertical bars represent SD
). Each value represents the average ± SD of two fermentations. Vertical bars represent SD
Significant differences were observed for sugar consumption and ethanol production among the yeast strains in pure culture (95 % LSD). Both Saccharomyces strains (S1 and S2) completely consumed all the fermentable sugars from agave juice after 24 and 36 h of fermentation and produced high ethanol concentration >36 g/l (Fig. 1). By contrast, Kloeckera/Hanseniaspora species showed low fermentative capacity compared to Saccharomyces, leaving around 40 g/l of residual sugar in the culture medium at 72 h of fermentation and producing lower ethanol concentrations (≥10 g/l) (Fig. 1).
Growth yields were also different: Saccharomyces strains converted sugars into ethanol more efficiently than Kloeckera/Hanseniaspora strains, with ethanol/sugar yields reaching 0.33 and 0.34 g/g. These values are close to the theoretical ethanol/sugars yield (0.51). In contrast, the Kloeckera/Hanseniaspora strains presented a lower ethanol/sugar yield of 0.10 and 0.18 g/g (Table 1). No significant differences were observed in biomass/sugar yields (Yx/s) among strains. Moreover, statistical analysis (95 % LSD) showed significant differences among yeast strains in all kinetic parameters. As expected, the higher values of specific growth rate (µmax), sugar consumption rate (qpmax), and ethanol production rate (qpmax) were reached by Saccharomyces strains (Table 1). Previous tequila studies have reported similar results. Díaz-Montaño et al. (2008) observed that Kloeckera/Hanseniaspora strains presented a lower fermentative capability than Saccharomyces, probably due to a nutritional limitation and/or to the presence of toxic compounds in agave juice. However, K. africana/H. vineae in pure culture in the presence of specific growth factors such as amino acids and vitamins presents a better growth and fermentative efficiency (Díaz-Montaño et al. 2010; Valle-Rodríguez et al. 2012). This can be explained since the presence of amino acids in the medium may increase the ability for rapid synthesis of degraded proteins as glucose transporters, which allow the yeast to achieve complete fermentations in the particular case of non-Saccharomyces strains (Díaz-Montaño et al. 2008, 2009). With regard to the selectivity of sugar transport in S. cerevisiae, it has been considered that this yeast is mostly glucosophilic and its high fermentative capacity is mainly attributed to its ability to metabolize both inorganic and organic N-sources from the culture medium (Díaz-Montaño et al. 2010). Nevertheless, other mechanisms could be implicated during the fermentation of agave using mixed cultures.
Mixed cultures
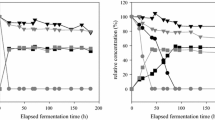
Development of biomass, viable cell (CFU), sugar consumption, and ethanol production versus time were plotted in Fig. 2 and Table 2 shows the kinetic parameters obtained from mixed culture fermentations of Kloeckera/Hanseniaspora and S. cerevisiae. Three different yeast groups were tested during the fermentation of agave juice; Group 1, a mixed culture of K. africana/H. vineae and S. cerevisiae (K1 + S1 − G1), Group 2, a mixed culture of K. apiculata/H.uvarum and S. cerevisiae (K2 + S1 − G2) and Group 3, a mixed culture of K. apiculata/H. uvarum and S. cerevisiae (K2 + S2 − G3). During the first eight hours of culture, K. africana/H. vineae (K1) and K. apiculata/H. uvarum (K2) presented similar growth rates than S. cerevisiae (S1 and S2). However, the proportion of S. cerevisiae strains in the mixed cultures increased during fermentation, while the proportion of Kloeckera/Hanseniaspora decreased (Fig. 2a, c, e). S. cerevisiae strains reached maximum growth of 16.8 (K1 + S1 − G1), 10.9 (K2 + S1 − G2), and 17.1 × 106 CFU/ml (K2 + S2 − G3) at 24, 36 and 16 h of fermentation (Fig. 2). The maximum growth of Kloeckera/Hanseniaspora strains in mixed cultures with S. cerevisiae reached 9.95 (K1 + S1 − G1), 7.5 (K2 + S1 − G2), and 10.7 × 106 CFU/ml (K2 + S2 − G3) at 24, 20 and 16 h of fermentation respectively. In all cases, the population of Kloeckera/Hanseniaspora dropped below 6.5 × 106 CFU/ml (Fig. 2). Although Saccharomyces strains remained active for a longer period than Kloeckera/Hanseniaspora strains, their growth in Group 1 and Group 2 declined at 36 h and 24 h to cell concentrations of 4.15 and 4.95 × 106 CFU/ml at the end of fermentation (Fig. 2a, b). However, the viable cells (CFU) of S. cerevisiae S2 (group 3) showed higher values until the end of fermentation (Fig. 2e).
Kinetic profiles of fermentations by mixed culture of S. cerevisiae S1 ( ), S. cerevisiae S2 (
), S. cerevisiae S2 ( ), K. africana/H. vineae K1 (filled square) and K. apiculata/H. uvarum K2 (filled inverted triangle) strains in Agave tequilana juice media at 12°Brix supplemented with ammonium sulphate (1 g/l). Group 1: K1 + S1; Group 2: K2 + S1 and Group 3: K2 + S2. CFU: viable cell (a, c, e) biomass: biomass concentration profile (filled circle); reducing sugar: reducing sugar concentration profile (filled triangle) and ethanol: ethanol concentration profile (
), K. africana/H. vineae K1 (filled square) and K. apiculata/H. uvarum K2 (filled inverted triangle) strains in Agave tequilana juice media at 12°Brix supplemented with ammonium sulphate (1 g/l). Group 1: K1 + S1; Group 2: K2 + S1 and Group 3: K2 + S2. CFU: viable cell (a, c, e) biomass: biomass concentration profile (filled circle); reducing sugar: reducing sugar concentration profile (filled triangle) and ethanol: ethanol concentration profile ( ). Each value represents the average ± SD of two fermentations. Vertical bars represent SD
). Each value represents the average ± SD of two fermentations. Vertical bars represent SD
No significant differences were observed (95 % LSD) among the mixed cultures regarding biomass concentration (values ≥5 g/l at 36 h of fermentation), ethanol production (>42 g/l in 24 h of fermentation) and sugar consumption (Fig. 2b, c, f). Although fermentable sugars were completely consumed in all mixed cultures, there were significative differences in maximum specific rate of sugar consumtion (qsmax) and ethanol production. Mixed culture group 2 presented a higher rate of maximum specific sugar consumption (16.6 g/g h−1) than the other two mixed cultures (5.24 and 8.65 g/g h−1 for G1 and G3 respectively), depleting the sugar in 24 h of fermentation, while the other two groups consumed it in approximately 50 h. Furthermore, mixed culture group 2 presented a higher rate of maximum specific ethanol production (2.12 g/g h−1) than the G1 and G3 mixed cultures (0.91 and 0.78 g/g h−1 respectively). Otherwise, no significant differences were observed (95 % LSD) in the maximum specific growth rate (μ max) among the three mixed cultures (G1, G2, G3), which reached 0.22, 0.27 and 0.31 h−1 respectively. Moreover, no significant differences were observed in either ethanol/sugar (Yp/s) or biomass/sugar (Yx/s) yields among three groups (95 % LSD).
Mixed cultures of Kloeckera/Hanseniaspora and S. cerevisiae presented similar fermentative capacity with respect sugar consumption and ethanol production than S. cerevisiae in pure culture, which in mixed cultures was the dominant yeast. However, significant differences were observed regarding CFU profiles. Pure cultures of Saccharomyces and Kloeckera/Hanseniaspora presented higher culture stability and cell concentration than mixed cultures, which exhibited a different performance in each culture (Figs. 1, 2). Our results are in agreement with previous studies conducted in wine fermentation. Mendoza et al. (2007) reported that K. apiculata and S. cerevisiae in pure cultures reached higher cell concentrations than mixed cultures. The maximum cell density in both cultures decrease; possible due to mechanisms related to ethanol concentration, killer toxins, and competition for assimilable nitrogen compounds. However, the persistence of non-Saccharomyces yeasts during fermentation may depend upon many other factors, such as the presence of toxic compounds other than ethanol, availability of nutrients, fermentation temperature, oxygen availability, and the strength of the Saccharomyces inoculum (Díaz-Montaño et al. 2010; Mancilla-Margalli and López 2002; Erten 2002; Holm Hansen et al. 2001; Nissen et al. 2003). The main cause reported for the early death of non-Saccharomyces yeasts during mixed culture fermentation with S. cerevisiae is usually the lower ethanol tolerance of non-Saccharomyces in comparison to S. cerevisiae. In spite of that, it has been reported in tequila fermentation that K. africana K1 requires N-organic sources and certain growth factors such as asparagine to stimulate and improve its growth and fermentative efficiency (Díaz-Montaño et al. 2010; Valle-Rodríguez et al. 2012).
Since K. africana K1 is one of the strains used in this study, the low availability of N-organic sources and vitamins in the agave juice could be the main cause of the limited growth of Kloeckera yeasts in mixed culture fermentation with Saccharomyces. Although the present study provides a better understanding regarding the fermentative capabilities of non-Saccharomyces strains during agave juice fermentation in mixed culture with S. cerevisiae under similar conditions to those used in the tequila industry, the effect of supplementing the agave juice with specific amounts of growth factors and nitrogen sources have been performed and an increase in growth and fermentative capability of K. africana/H. vineae in co-culture with S. cerevisiae has been observed (González-Robles 2012).
Volatile compounds produced by Kloeckera/Hanseniaspora and Saccharomyces yeasts in pure and mixed cultures
This study determined secondary metabolites such as higher alcohols, esters and aldehydes, obtained from pure and mixed cultures of Saccharomyces and Kloeckera/Hanseniaspora strains (Table 3). Saccharomyces strains (S1 and S2) in pure and mixed cultures produced higher concentrations of acetaldehyde, isobutanol, isoamyl alcohol and 2-phenyl ethanol than pure cultures of Kloeckera/Hanseniaspora (K1 and K2), which generated higher amounts of ethyl acetate, isoamyl acetate and 2-phenyl ethyl acetate (Table 3). K. africana/H. vineae K1 in pure culture produced the highest concentration of n-butanol (Table 3). No significant differences were observed in n-propanol or methanol concentrations among pure and mixed cultures (95 % LSD) (Table 3). Methanol concentration was similar in all fermentations since it is produced mainly during the agave-cooking step (Cedeño 1995).
Other authors reported similar results. Zironi et al. (1993) reported that S. cerevisiae in pure and mixed cultures produced higher concentrations of higher alcohols than K. apiculata and H. guillermondii in pure cultures, which only produced greater amounts of 2,3-butanediol and acetoin. Díaz-Montaño et al. (2008) reported that Saccharomyces strains produced higher concentrations of higher alcohols and acetaldehyde than Kloeckera, which produced high concentrations of ethyl acetate, 2-phenetyl acetate, and acetic acid. Higher alcohols confer a strong pungent taste and odor to alcoholic beverages. In concentrations below 300 mg/l, they contribute to desired complexity but in concentrations greater than 400 mg/l, there is a negative effect on the aroma (Swiegers et al. 2005; Díaz-Montaño and Córdova 2009). The concentration of higher alcohols depends on several factors, including the type of yeast strain, fermentation temperature, pH, and amino acid composition of the culture medium (Swiegers et al. 2005; Pinal et al. 1997). Acetaldehyde at low concentrations (<100 mg/l) contributes to the sensory properties of the beverage by conferring apple-like, citrus-like and nutty descriptors. However, high concentrations result in an irritating, spicy scent (Schreier and Jennings 1979). Moreover, a high concentration of ethyl acetate (>200 mg/l) does not improve the aroma of the fermented beverage it generates an off-flavour instead. Yet, at low concentrations (50–80 mg/l), it contributes to the quality of beverage by conferring fruity notes (Zohre and Erten 2002; Moreira et al. 2005). Our results are in accordance with those reports since the Kloeckera/Hanseniaspora strains (K1 and K2) in pure and mixed cultures produced greater amounts of ethyl acetate, in the order of 33.13–74.22 mg/l (Table 3). Besides ethyl acetate, other acetate esters that have a positive effect on the overall flavour of the beverage are isoamyl acetate and 2-phenyl ethyl acetate. These were produced in greater amounts by Kloeckera/Hanseniaspora yeasts in pure cultures, providing sweet, rose-like, fruity and banana-like descriptors (Díaz-Montaño and Córdova 2009; Swiegers et al. 2005). Nevertheless ethyl hexanoate, ethyl octanoate, and ethyl decanoate were mainly produced by Saccharomyces yeast strains in pure and mixed cultures (Table 3; Fig. 3).
PCA of a the volatile compounds and b the yeast strains in pure and mixed cultures. acet acetaldehyde, met methanol, ethyl-acet ethyl acetate, iso-acet isoamyl acetate, phenethyl-acet 2-phenyl ethyl acetate, ethyl-hex ethyl hexanoate, ethyl-oct ethyl octanoate, ethyl-dec ethyl decanoate, prop n-propanol, but n-butanol, isob isobutanol, isoamyl isoamyl alcohol, phen-eth 2-phenyl ethanol. K1: K. africana/H. vineae; K2: K. apiculata/H. uvarum; S1: S. cerevisiae; S2: S. cerevisiae; G1: mixed culture of K1 and S1; G2: mixed culture of K2 and S1; G3: mixed culture of K2 and S2
PCA grouped volatile compounds produced by Kloeckera/Hanseniaspora and Saccharomyces yeasts in pure and mixed cultures into two principal components with a 73.8 % explained variance (Fig. 3; Table 4). Volatile compounds with a greater weight in the first component (PC-1) were isoamyl acetate, 2-phenyl ethyl acetate, and n-butanol with negative weights, and acetaldehyde, 2-phenyl ethanol, isoamyl alcohol, and isobutanol with positive weights. Meanwhile, the volatile compounds for PC-2 were methanol and 2-phenyl ethyl acetate with negative weights; and n-propanol and ethyl decanoate with positive weights (Fig. 3; Table 4).
Conclusions
Kloeckera africana/H. vineae, K. apiculata/H. uvarum, S. cerevisiae S1 and S2 were examined in pure and mixed culture fermentations of agave juice. Results showed that Kloeckera/Hanseniaspora strains grew poorly in the presence of S. cerevisiae. Possible hypotheses to explain the growth inhibition include a nutritional limitation in agave juice or the presence of toxic compounds. On the other hand, Saccharomyces yeast strains showed high fermentative efficiency, remaining active for much longer time during fermentation. They also exhibited higher ethanol and biomass concentrations than fermentations with Kloeckera/Hanseniaspora strains. In addition, the presence of Kloeckera/Hanseniaspora yeast strains in mixed cultures had a significant influence on the sensorial profile; since high ester concentration like ethyl acetate, isoamyl acetate and 2-phenyl ethyl acetate were present at the end of fermentation. Indeed, lower concentrations of the volatile compounds regulated under the Official Mexican Standard (NOM-006-SCFI-2012 2012) were observed from the mixed culture than from the pure culture of Saccharomyces.
These results suggest the potential and beneficial use of using mixed cultures during tequila fermentation because could produce beverages with distinctive sensory properties and low concentrations of regulated volatile compounds. However, further studies are needed to assure all the implications of their application during tequila production.
References
Benn SM, Peppard TL (1996) Characterization of tequila flavour by instrumental and sensory analysis. J Agric Food Chem 44:557–566
Cedeño M (1995) Tequila production. Crit Rev Biotechnol 15:1–11
Ciani M, Picciotti G (1995) The growth kinetics and fermentation behavior of some non-Saccharomyces yeast associated with wine-making. Biotechnol Lett 17:1247–1250
Ciani M, Beco L, Comitini F (2006) Fermentation behaviour and metabolic interactions of multistarter wine yeast fermentations. Int J Food Microbiol 108:239–245
Díaz-Montaño DM (2004) Estudio fisiológico y cinético de dos cepas de levaduras involucradas en la etapa fermentativa de la elaboración de tequila. Dissertation, Universidad de Guadalajara-Institut National Polytechnique de Toulouse, France
Díaz-Montaño DM, Córdova J (2009) The fermentative and aromatic ability of Kloeckera and Hanseniaspora yeasts. In: Satyanarayana T, Kunze G (eds) Yeast biotechnology: diversity and applications. Springer, Dordrecht, pp 281–301
Díaz-Montaño DM, Délia ML, Estarrón-Espinosa M, Strehaiano P (2008) Fermentative capability and aroma compound production by yeast strains isolated from Agave tequilana Weber juice. Enzym Microb Technol 42:608–616
Díaz-Montaño DM, Favela-Torres E, Córdova J (2010) Improvement of growth, fermentative efficiency and ethanol tolerance of Kloeckera africana during the fermentation of Agave tequilana juice by addition of yeast extract. J Sci Food Agric 90:321–328
Erten H (2002) Relations between elevated temperatures and fermentation behavior of Kloeckera apiculata and Saccharomyces cerevisiae associated with winemaking in mixed cultures. J Microbiol Biotechnol 18:377–382
González-Robles IW (2012) Estudio de la fermentación del jugo de agave en cultivos mixtos de levaduras tequileras del género Kloeckera y Saccharomyces: “Efecto del sistema fermentativo y las condiciones nutrimentales”. Master dissertation, Centro de Investigación en Asistencia en Tecnología y Diseño del Estado de Jalisco A.C
Hernández-Cortés G, Cordova-López J, Herrera-López EJ, Morán-Marroquín GA, Valle-Rodríguez JO, Díaz-Montaño DM (2010) Effect of pH, aeration and feeding non-sterilized agave juice in a continuous agave juice fermentation. J Sci Food Agric 90:1423–1428
Holm Hansen E, Nissen P, Sommer P, Nielsen JC, Arneborg N (2001) The effect of oxygen on the survival of non-Saccharomyces yeasts during mixed culture fermentations of grape juice with Saccharomyces cerevisiae. J Appl Microbiol 91(3):541–547
Kunkee RE (1984) Selection and modification of yeasts and lactic acid bacteria for wine fermentation. Food Microbiol 1:315–332
Lachance MA (1995) Yeast communities in a natural tequila fermentation. Antonie Van Leeuwenhoek 68:151–160
Mancilla-Margalli NA, López MG (2002) Generation of Maillard compounds from inulin during the thermal processing of Agave tequilana Weber Var. azul. J Agric Food Chem 50:806–812
Mendes-Ferreira A, Clímaco MC, Mendes-Faia A (2001) The role of non-Saccharomyces species in releasing glycosidic bound fraction of grape aroma components—a preliminary study. J Appl Microbiol 91:67–71
Mendoza LM, Manca de Nadra MC, Farías ME (2007) Kinetics and metabolic behavior of a composite culture of Kloeckera apiculata and Saccharomyces cerevisiae wine related strains. Biotechnol Lett 29:1057–1063
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Moreira N, Mendes F, Hogg T, Vasconcelos I (2005) Alcohols, esters and heavy sulphur compounds production by pure and mixed cultures of apiculate wine yeasts. Int J Food Microbiol 103:285–294
Nissen P, Nielsen D, Arneborg N (2003) Viable Saccharomyces cerevisiae cells at high concentrations cause early growth arrest of non-Saccharomyces yeasts in mixed cultures by a cell-cell contact-mediated mechanism. Yeast 20:331–341
NOM-006-SCFI-2012 (2012) Bebidas alcohólicas-Tequila-Especificaciones. México: Diario Oficial de la Federación, p 20
Pérez F, Regodón JA, Valdés ME, De Miguel C, Ramírez M (2000) Cycloheximide resistance as a marker for monitoring yeast in wine fermentations. Food Microbiol 17:119–128
Pérez-Nevado F, Albergaria H, Hogg T, Girio F (2006) Cellular death of two non-Saccharomyces wine-related yeasts during mixed fermentations with Saccharomyces cerevisiae. Int J Food Microbiol 108:336–345
Pinal L, Cedeño M, Gutiérrez H, Alvarez-Jacobs J (1997) Fermentation parameters influencing higher alcohol production in the tequila process. Biotechnol Lett 19:45–47
Rojas V, Gil JV, Piñaga F, Manzanares P (2003) Acetate ester formation in wine by mixed cultures in laboratory fermentations. Int J Food Microbiol 86:181–188
Romano P, Suzzi G, Comi G, Zironi R, Maifreni M (1997) Glycerol and other fermentation products of apiculate wine yeasts. J Appl Microbiol 82:615–618
Romano P, Fiore C, Paraggio M, Caruso M, Capece A (2003) Function of yeast species and strains in wine flavour. Int J Food Microbiol 86:169–180
Schreier P, Jennings WG (1979) Flavour composition of wines: a review. Crit Rev Food Sci Nutr 12:59–111
Swangkeaw J, Vichitphan S, Butzke CE, Vichitphan K (2010) Characterization of β-glucosidases from Hanseniaspora spp. and Pichia anomala with potentially aroma-enhancing capabilities in juice and wine. World J Microb Biotechnol 27:423–430
Swiegers JH, Bartowsky EJ, Henschke PA, Pretorius IS (2005) Yeast and bacterial modulation of wine aroma and flavor. Aust J Grape Wine Res 11:139–173
Ugliano M, Travis B, Francis IL, Henschke PA (2010) Volatile composition and sensory properties of Shiraz wines as affected by nitrogen supplementation and yeast species: rationalizing nitrogen modulation of wine Aroma. J Agric Food Chem 16:12417–12425
Valle-Rodríguez JO, Hernández-Cortés G, Cordova-López J, Estarrón-Espinosa M, Díaz-Montaño DM (2012) Fermentation of Agave tequilana juice by Kloeckera africana: influence of amino-acid supplementations. Antonie Van Leeuwenhoek 101:195–204
Zironi R, Romano P, Suzzi G, Battistutta F, Comi G (1993) Volatile metabolites produced in wine by mixed and sequential cultures of Hanseniaspora guillermondii or Kloeckera apiculata and Saccharomyces cerevisiae. Biotechnol Lett 15:235–238
Zohre DE, Erten H (2002) The influence of Kloeckera apiculata and Candida pulcherrima yeasts on wine fermentation. Process Biochem 38(3):319–324
Acknowledgments
This research was funded by CONACYT (Mexican National Council of Science and Technology) under the project SEP-CONACYT 24547. Ivonne Wendolyne Gonzalez Robles thanks CONACYT for the scholarships granted. The authors would also like to thank John Dye of Peace Corps Mexico for revising the language of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dulce María Díaz-Montaño—Research leader retired.
Rights and permissions
About this article
Cite this article
González-Robles, I.W., Estarrón-Espinosa, M. & Díaz-Montaño, D.M. Fermentative capabilities and volatile compounds produced by Kloeckera/Hanseniaspora and Saccharomyces yeast strains in pure and mixed cultures during Agave tequilana juice fermentation. Antonie van Leeuwenhoek 108, 525–536 (2015). https://doi.org/10.1007/s10482-015-0506-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10482-015-0506-3