Abstract
This study aimed to improve the fermentation efficiency of Kloeckera africana K1, in tequila fermentations. We investigated organic and inorganic nitrogen source requirements in continuous K. africana fermentations fed with Agave tequilana juice. The addition of a mixture of 20 amino-acids greatly improved the fermentation efficiency of this yeast, increasing the consumption of reducing sugars and production of ethanol, compared with fermentations supplemented with ammonium sulfate. The preference of K. africana for each of the 20 amino-acids was further determined in batch fermentations and we found that asparagine supplementation increased K. africana biomass production, reducing sugar consumption and ethanol production (by 30, 36.7 and 45%, respectively) over fermentations supplemented with ammonium sulfate. Therefore, asparagine appears to overcome K. africana nutritional limitation in Agave juice. Surprisingly, K. africana produced a high concentration of ethanol. This contrasts to poor ethanol productivities reported for other non-Saccharomyces yeasts indicating a relatively high ethanol tolerance for the K. africana K1 strain. Kloeckera spp. strains are known to synthesize a wide variety of volatile compounds and we have shown that amino-acid supplements influenced the synthesis by K. africana of important metabolites involved in the bouquet of tequila. The findings of this study have revealed important nutritional limitations of non-Saccharomyces yeasts fermenting Agave tequilana juice, and have highlighted the potential of K. africana in tequila production processes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tequila is a distilled alcoholic beverage produced from juice extracted from Agave tequilana Weber blue variety (NOM-006-SCFI-2005, 2006). The steps for tequila production included: (i) harvesting the agave plant, (ii) steaming the head (core) to hydrolyze the fructans, (iii) milling the cooked agave heads to extract the juice, (iv) fermenting the sugars from the extracted juice, (v) double distillation of the must to produce “blanco” tequila and eventually, (vi) aging either 3 or 12 months in white oak barrels to get “reposado” (rested) or “añejo” (aged) tequila, respectively (Cedeño 1995).
Fermentation has an important effect on the tequila bouquet, because yeasts produce both ethanol and many volatile compounds that form the bouquet (Soufleros and Bertrand 1979). The optimal characteristics of yeast used for fermentation include: high ethanol production, high fermentation rate, efficient substrate-ethanol conversion, tolerance to ethanol and osmotic pressure, balanced production of desirable volatile compounds and low biomass production (Pretorius 2000). Kloeckera spp. strains are particularly interesting yeast because they synthesize a great variety of volatile compounds contributing decisively to the beverage’s bouquet (Pretorius 2000; Díaz-Montaño et al. 2008; Gil et al. 1996). In fact, in musts fermented by mixed cultures of non-Saccharomyces yeasts and Saccharomyces cerevisiae, higher concentrations of esters, terpenes and acetoin have been found than in musts fermented by S. cerevisiae monocultures (Mendes-Ferreira et al. 2001; Romano et al. 2003).
Amino-acids supplementation has been used to improve fermentation performance; moreover, it was evidenced that yeasts prefer consume some specific amino-acids (Albers et al. 1996; Manginot et al. 1998).
In wine fermentations S. cerevisiae prefers glutamic acid, glutamine, aspartic acid, asparagine, threonine, histidine, alanine, tyrosine and arginine have been reported (Cooper 1982; Gorinstein et al. 1984; Henschke and Jiranek 1993; Jiranek et al. 1991; Pierce 1982). Additionally, in yeast cultures supplemented with amino-acids, the synthesis of proteins and higher alcohols is increased (Arias-Gil et al. 2007; Bely et al. 1994). Serine, valine, isoleucine, leucine, phenylalanine, tyrosine, tryptophan and methionine, are the precursors used in the Ehrlich pathway, for the synthesis of glycol, 2-methyl-1-propanol, 2-methyl-1-butanol, 3-methyl-1-butanol, phenethyl alcohol, tyrosol, tryptophol and methionol, respectively (Henschke and Jiranek 1993).
The aim of this work was to determine the amino-acids required by K. africana cultured in agave juice for achieving complete fermentation with high ethanol production. In addition, the correlation of the amino-acids to the synthesis of several secondary metabolites was studied using statistical techniques. This study also contributes to the understanding of the early death of non-Saccharomyces yeasts in spontaneous alcoholic fermentations.
Materials and methods
Yeast strain
Kloeckera africana K1 was isolated from a spontaneous fermentation of agave juice collected from a tequila distillery in the state of Jalisco, Mexico and it was maintain at −70°C, adding glycerol (25% v/v) in 1.5 ml micro-tubes.
Inoculum and fermentation media
The Agave tequilana Weber blue variety juice (agave juice) used in this work was supplied by “La Quemada” (a tequila distillery). The agave juice (at 20° Bx) was filtered and conserved at −20°C. Pre-inoculum and inoculum media consisted of agave juice adjusted to 3°Brix (27 ± 2 g/l of reducing sugars) and supplemented with either 1 g/l of yeast extract for pre-inoculum or 1 g/l of ammonium sulfate for inoculum. A pre-inoculum was prepared by transferring 1 ml of conserved cells (at −70°C) to 50 ml of medium contained in a 200 ml Erlenmeyer flask and incubating at 30°C and 250 rpm for 24 h. Then, an inoculum was prepared by adding 20 ml of pre-inoculum in 200 ml of medium contained in a 1 l Erlenmeyer flask and incubated at 30°C and 250 rpm for 12 h. Fermentation media were prepared with agave juice adjusted to a reducing sugars concentration of 100 ± 13 g/l, supplemented as described below. Fermentation and growth media were sterilized at 121°C for 15 min.
Fermentation conditions
The fermentations were carried out at 30°C and 250 rpm, inoculating 3.5 × 106 cells/ml of medium. The effect of nitrogen (N) sources on the fermentative performance of K. africana, was studied on continuous cultures using a 3 l bioreactor (Applikon, The Netherlands), with a working volume of 1.5 l. The fed media were supplemented with ammonium sulfate (220 mg N/l) or the mixture of the 20 amino-acids, each one added at 0.5 mM, to obtain a total concentration of 220 mg N/l. To reach the steady state of the culture, at least three residence times were set after modifying a fermentation condition. Samples were taken every 12 h till attaining the first three residence times and then every 6 h for each experimental condition.
The amino-acids preference of K. africana was determined on batch cultures using 500 ml Erlenmeyer flasks containing 100 ml of media supplemented with 440 mg N/l of the following sources: each of the 20 basic amino-acids added separately, a mixture of all these amino-acids, yeast extract and ammonium sulfate. Amino-acids were sterilized by filtration through a cellulose acetate membrane (0.22 μm). A culture control (with no N-source added) was simultaneously run. All the fermentations were stopped after 80 h and assayed in duplicate for the concentrations of biomass, ethanol, reducing sugars and amino-acids. Each experimental unit was performed in duplicate.
Analytical methods
Amino-acids concentration in the agave juice was quantified using HPLC as reported by Vázquez-Ortiz et al. (1995). Biomass concentration was determined by measuring the dry weight as reported by Díaz-Montaño et al. (2010). Fermented musts supernatants were used to assay reducing sugars, ethanol, amino-acids, glycerol, acetoin, acetic acid, succinic acid and volatile compounds. Reducing sugars and ethanol concentrations were determined using the DNS method (Miller 1959) and enzymatic analyzer (YSI model 2700 select, Yellow Springs Instruments), respectively. Amino-acids concentrations (for the K. africana preference experiment) were determined by the reaction with ninhydrin, employing a spectrophotometer (Bio-Rad Microplate Reader Model 680 XR). Some modifications were made to the original method (Moore and Stein 1948): absorbance was read at 450 nm for the proline reaction and at 540 nm for all the other amino-acids reactions, a 67 mM ninhydrin solution was prepared, and isoleucine (30 mM) was used as standard.
Glycerol, acetoin, acetic acid and succinic acid concentrations were determined in duplicate by HPLC (Varian, Inc.) as reported by Díaz-Montaño et al. (2008). Five-point calibration curves for each standard (0.4, 0.8, 1.2, 1.6 and 2.0 g/l) were used. Volatile compounds were assayed in duplicate by gas chromatography (GC). A Hewlett-Packard Headspace HP 7694E model was connected to a Hewlett-Packard 6890 Series gas chromatograph equipped with a flame ionization detector (FID) and a 60 m × 320 × 0.25 μm thickness film HP-Innowax capillary column. The column temperature was held at 35°C for 10 min, and then increased to 210°C at a rate of 3.5°C min−1. Helium was the carrier gas used at 1.5 ml min−1. The FID detector was operated at 260°C, and the hydrogen and nitrogen (auxiliary gas) were at 30 ml min−1 and air at 300 ml min−1 flow rates. Quantification was based on the external standard method by using five-point calibration curves for each standard: 0.2, 0.4, 0.6, 0.8 and 1 mg/l of isoamyl acetate, ethyl hexanoate, ethyl octanoate, α-terpineol or 2-phenyl ethyl acetate; 4, 8, 12, 16 and 20 mg/l of n-butanol, acetaldehyde or ethyl acetate; 8, 16, 24, 32 and 40 mg/l of n-propanol, isobutanol or 2-phenyl ethanol; 60, 120, 180, 240 and 300 mg/l of methanol and isoamyl alcohol. Calibration curves showed a determination coefficient (R 2 ) equal or greater than 0.99 for each compound as determined using the HP Chem Station software Rev. A. 05.04. Assays were performed twice.
Data treatment and statistical analysis
One way variance analysis (ANOVA) was used to compare the effect of N-sources on the fermentative performance of K. africana with Statgraphics plus 4 software (Manugistics Inc.). The response variables were: concentrations of biomass, ethanol, consumed substrate, major volatile compounds; yields of biomass and ethanol production; instantaneous rates of biomass and ethanol production, and sugars consumption.
Results and discussion
Effect of the nitrogen source on the fermentative capability
The amino-acids composition in agave juice was firstly analyzed, showing a total content of 2.38 mg/l, with serine at the highest concentration (0.37 mg/l) (Table 1). However, it is well known that serine is not a good nutrient for yeasts (Gorinstein et al. 1984; Henschke and Jiranek 1993; Pierce 1982; Cooper 1982; Jiranek et al. 1995). Amino-acids are the most important nitrogen source in agave juice; however, their natural concentrations (0.02 mg N/l) are not enough to support balanced yeast growth and the complete fermentation of sugars. In wines, 120 mg N/l was reported as the minimum S. cerevisiae requirement for achieving maximal growth and/or sugar catabolic rates (Jiranek et al. 1995). This report suggests agave juice supplementation with a appropriate N-source. It is important to note that ammonium sulfate is the most common N-source supplemented in the tequila industry at 1 g/l (Cedeño 1995).
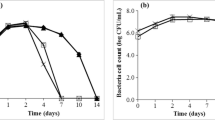
A continuous culture at a dilution rate (D) of 0.04 h−1 was realized to study the effect of the addition of N-sources (ammonium sulfate or mixture of amino-acids), on K. africana fermentative capability. In the fermentation feeding agave juice supplemented with ammonium sulfate, the biomass and ethanol production and the reducing sugars consumption were 0.62, 13.3 and 33.6 g/l, respectively. Whereas in fermentation supplemented with the mixture of the 20 amino-acids, biomass and ethanol productions, and reducing sugars consumption increased 4.17, 2.45 and 1.96 times, respectively (Fig. 1). Also, other kinetic parameters increased in the culture supplemented with amino-acids compared to that supplemented with ammonium sulfate: instantaneous rates of biomass and ethanol productions, and of sugar consumption increased 4, 2.5 and 2 times, respectively; yields of biomass and ethanol increased 27.6 and 22.6%, respectively (Table 2).
Effect of ammonium sulfate and amino-acids supplementation (both at 220 mg N/l) to the agave juice fed in continuous culture (D = 0.04 h−1) using K. africana. Biomass (x, triangle), ethanol (p, diamond) and reducing sugars (s, square). Data represent the mean ± standard deviation of four samples taken at the steady state. No statistical differences (P < 0.05) among these parameters were found on the steady state of each tested experimental condition. τ is the residence time = 25 h
It should be noted that the ethanol/reducing sugars yield, reached in the fermentation supplemented with amino-acids, was particularly high (0.488), since the maximum (theoretical) yield value of ethanol from glucose or fructose is 0.51. It is important to mention also that agave juice contains a low concentration (4 ± 1 g/l) of non-reducing sugars (partially hydrolyzed fructose polymer) that potentially could slightly increase product concentration (e.g. biomass and ethanol) and overestimate the yields values. These results showed that the agave juice supplementation with amino-acids improved K. africana fermentative capacity. Nevertheless, the even high concentration of residual sugars remaining in the must revealed a limitation in N-source. Because of this, for the following experiments, the N-source concentration was increased from 220 to 440 mg N/l.
Study of the amino-acids preference of K. africana
A unifactorial experiment was conducted to determine the influence of each amino-acid on the growth and fermentative capability of K. africana cultured in agave juice. Two treatments were also incorporated in this study: the supplementation with either yeast extract or with ammonium sulfate. Figure 2a shows that K. africana was more efficient in consuming sugars when the agave juice was supplemented with yeast extract (98.81%), glutamine (96.90%), asparagine (95.89%) and glutamic acid (92.87%). On the other hand, low sugar consumptions were observed for the supplementation with cysteine (11.15%), ammonium sulfate (25.85%) and the control (32.34%, no N-source added). K. africana showed the highest biomass production (6.18 g/l) when supplemented with asparagine. It is important to mention that this biomass concentration represents the highest value ever reported for this yeast genus in alcoholic fermentation (Díaz-Montaño et al. 2008, 2010). Good biomass production was also obtained with yeast extract (3.38 g/l), glutamine (3.35 g/l) and arginine (3.09 g/l) (Fig. 2c). The amino-acids significantly improving the alcohol production of K. africana were (in descending order, in g/l): asparagine (33.8), glutamine (27.3), glutamic acid (25.1), histidine (22.4) and tyrosine (20.1) (Fig. 2b).
Kloeckera africana fermentation of agave juice supplemented with different nutrients. a Percentage of sugars consumption, b Ethanol production, c Biomass production and d Percentage of amino-acid consumption. Cultures were all stopped at 80 h and then the assays were performed. Nutrients were added at the same total nitrogen content (440 mg N/l). YE yeast extract, 20AAs mixture of the 20 amino-acids, AS ammonium sulfate, Control without addition of N-source. Data represent the mean and standard deviation of four assays
It was clear that not all amino-acids had the same effect in promoting the growth of yeast and ethanol production. In tequila production, yeast extract has been reported to stimulate the growth, fermentative capacity and alcohol tolerance of K. africana. In fact, when K africana fermentation was supplemented with yeast extract, maximum specific growth rate (μmax), ethanol production and sugar consumption reached similar values to those calculated for S. cerevisiae cultured in agave juice supplemented with ammonium sulfate (Díaz-Montaño et al. 2010). When the fermentation medium was supplemented with asparagine, K. africana showed a fermentative capacity similar to that obtained using yeast extract. In comparison to the other amino-acids, the supplementation with asparagine showed the highest ethanol production (33.8 g/l), outstanding biomass formation (6.18 g/l) and high sugar consumption (95.9%).
On the other hand, several amino-acids were preferentially consumed by K. africana (Fig. 2d). In the fermentations supplemented with glutamine, valine and the mixture of the amino-acids, high amino-acids consumption was found (68.25, 67.99 and 66.70%, respectively). In contrast, low consumption was found by supplementing tryptophan, arginine, cysteine and threonine (26, 24.5, 13.23 and 12.52%, respectively). The amino-acids preference of K. africana was different to that manifested by S. cerevisiae in wine fermentations, suggesting a nutrimental requirement diversity within genres. Jiranek et al. (1995) studied the preferences for 18 amino-acids by nine S. cerevisiae wine strains in a chemically defined medium resembling the composition of grape juice. All of the S. cerevisiae strains, used mainly arginine, serine, glutamate, threonine, aspartate and lysine, which contributed between 70.6 and 79% of the total nitrogen consumed.
This study showed that most of the consumed amino-acids did not necessarily enhance the fermentative capability of K. africana. For example, although valine was greatly consumed (67.99%), it did not improve the alcoholic efficiency (27%), even though it stimulated high consumption of sugars (82.93%) and production of biomass and glycerol (2.10 and 0.46 g/l, respectively). Some of these amino-acids, such as asparagine, glutamine and glutamic acid, could be considered as important nutrients for improving the fermentative capability of K. africana. Most of them are also important for yeasts involved in wine fermentation (Cooper 1982; Gorinstein et al. 1984; Henschke and Jiranek 1993; Jiranek et al. 1991; Pierce 1982). Interestingly, threonine, considered to be an important nutrient for S. cerevisiae in wine fermentation, was not significant for K. africana.
Others metabolites produced at high concentrations during alcoholic fermentations are glycerol, acetoin, acetic acid and succinic acid. It was observed that the productions of these compounds were dependent on the N-sources added to the agave juice (Table 3). Thus, glycerol production increased by supplementing the agave juice with asparagine and ammonium sulfate (3.19 and 2.39 g/l, respectively). These glycerol concentrations were comparable to those concentrations found in K. apiculata tequila fermentation (2.5 g/l) (Díaz-Montaño et al. 2008) and in K. apiculata and Hanseniaspora uvarum wine fermentations (2.7 and 3.2 g/l, respectively) (Granchi et al. 2002; Ciani and Picciotti 1995). In this work, it was also observed that some amino-acids did not enhance the glycerol production when added to the agave juice. These included tryptophan, valine and tyrosine (0.37, 0.46 and 0.50 g/l, respectively) (Table 3).
On the other hand, the production of acetic acid was high when agave juice was supplemented by histidine, lysine and threonine (0.78, 0.67 and 0.66 g/l, respectively) (Table 3). This acetic acid production was similar to the production found in wine fermentation with K. corticis, Hanseniaspora osmophila and H. uvarum (0.84, 0.91 and 1.0 g/l, respectively) (Granchi et al. 2002; Ciani and Picciotti 1995). The fermentation of synthetic grape juice with glutamic acid as the sole nitrogen source presented higher acetic acid concentration than those with other kinds of amino-acids (Albers et al. 1996). In contrast, the acetic acid production in this study was low for K. africana fermentation supplemented with glutamic acid or with cysteine (0.14 and 0.15 g/l−, respectively) (Table 3). Similar low acetic acid concentration was found in K. apiculata wine fermentation (0.25 g/l) (Ciani and Picciotti 1995). These results suggested that the metabolic regulation of Kloeckera is quite different compared to that of S. cerevisie.
A high acetoin production was reached when the agave juice sugars were depleted and an elevated yield of ethanol was reached, as occurred with the fermentations supplemented with yeast extract and asparagine (330 and 260 mg/l of acetoin) (Table 3). These acetoin concentrations were higher than those found in wine fermentation using K. apiculata, H. uvarum and K. corticis (29.3, 34.4 and 160 mg/l, respectively) (Granchi et al. 2002; Ciani and Picciotti 1995). On the other hand, K. africana did not produce acetoin when the agave juice was supplemented by the mixture of amino-acids, ammonium sulfate or arginine, or when no nutrient was added (control) (Table 3).
On the other hand, a high succinic acid production was obtained when agave juice was supplemented by isoleucine, valine and tyrosine (0.66, 0.63 and 0.57 g/l, respectively) (Table 3). These productions were higher than those found in K. apiculata and H. uvarum wine fermentations (0.25 and 0.54 g/l, respectively) (Ciani and Picciotti 1995). Conversely, succinic acid production was low for K. africana fermentation supplemented with threonine, glutamine and ammonium sulfate (0.04, 0.13 and 0.13 g/l, respectively). A similar succinic acid concentration was reported in tequila K. apiculata fermentation (0.13 g/l) (Díaz-Montaño et al. 2008).
Kinetics of growth, sugar consumption and ethanol production in batch fermentation supplemented with asparagine or ammonium sulfate
Due to the advantages previously mentioned, asparagine was selected for the supplementation of agave juice (at 440 mg N/l) in a fermentation performed in a batch bioreactor, in order to characterize the kinetics of growth, substrate consumption and ethanol production (Fig. 3). These results were also compared to a batch fermentation supplemented with ammonium sulfate (at 440 mg N/l). In the fermentation supplemented with asparagine, K. africana depleted reducing sugars from agave juice after 72 h of fermentation, and increased biomass and ethanol production by 30 and 45% respectively, in comparison to that fermentation supplemented with ammonium sulfate. Maximal instantaneous rates of growth and sugars consumption of K. africana also increased in 64 and 16%, respectively. It is worth noting that with the addition of asparagine, K. africana converted reducing sugars efficiently into ethanol, with a yield (0.48) close to the theoretical (0.51) and ethanol attained a high concentration (47.8 g/l). These results evidenced a nutritional limitation in K. africana K1 which was eliminated by supplementing with asparagina. Conversely, other workers have suggested that the growth of non-Saccharomyces yeasts stop early due to ethanol intolerance (Fleet 1990; Kunkee 1984). Our results support the exclusion of this supposition. Both maximal biomass values were attained using different culture conditions. 6.18 g/l was obtained using Erlenmeyer flask, whereas 2.4 g/l was obtained using the bioreactor. It is worth noting that K. africana biomass concentration using agave juice supplemented with asparagine was higher when cultured in Erlenmeyer flask when compared to bioreactor. This can be attributed to inherent differences between both types of cultures, mainly aeration.
Kloeckera africana kinetics of growth, substrate consumption and ethanol production in the fermentation of agave juice supplemented with asparagine (closed symbols) or ammonium sulfate (open symbols). Biomass (filled triangle, open triangle), ethanol (filled diamond, open diamond) and reducing sugars (filled square, open square). Data represent the mean of four assays. Each experimental unit was carried out in duplicate
The fermented must obtained from agave juice supplemented with asparagine showed a different profile of volatile compounds compared to that supplemented with ammonium sulfate. Statistical differences were found for the concentrations of every one of volatile compounds studied (P < 0.05), with the exception of methanol (Table 4). Compared to the ammonium sulfate supplement, for the fermentation supplemented with asparagine, higher concentrations of acetaldehyde, higher alcohols (n-propanol, n-butanol, isobutanol, isoamyl alcohol 2-phenyl ethanol), α-terpineol and ethyl acetate were obtained. In contrast, the concentrations of the esters (except for the ethyl acetate) were lower (Table 4). Remarkably, the major volatile compounds concentrations in fermentation supplemented with asparagine was in compliance with the Mexican Official Standard for tequila (NOM-006-SCFI-2005), suggesting the possibility of using K. africana in tequila fermentation process.
Conclusions
The results showed that K. africana K1 requires amino-acids supplementation for fermenting agave juice efficiently. The amino-acid type influenced K. Africana fermentative capacity and the synthesis of important metabolites involved in the tequila bouquet. The high consumption of a particular amino-acid did not necessarily enhance the K. africana fermentative capability. Asparagine was found as the main nutrient. When supplementing agave juice, it improved K. africana fermentative capability, depleting sugars and reaching ethanol production close to that predicted by the theoretical yield. In fact, ethanol concentrations and yields obtained in this study were higher than those reported in the scientific literature and in the tequila industry. This can be considered as a great result with clear economical implications. This finding also suggests that the low Kloeckera spp. fermentative capability observed in wine fermentations can be due to a nutritional limitation instead of a low ethanol tolerance. It is worth noting that one of most intricate problems to be solved in the tequila industry is the high residual sugars concentration at the end of the fermentation. That problem has both ecological and economical repercussions. In this work, we are proposing a way to reduce organic discharges and increase tequila yields. On the other hand, the tequila bouquet from the agave fermentations supplemented with different nitrogen sources has not even been characterized and a sensorial test will be performed shortly. However, preliminary results have revealed that the tequila sensory quality is certainly influenced by the N source added to the agave juice fermentation.
Although K. africana realized an efficient fermentation of sugars in the fermentation supplemented with asparagine, the growth, sugars consumption and ethanol production rates were slow compared to those reached by S. cerevisiae fermenting agave juice (Díaz-Montaño et al. 2008). Ongoing work is exploring this slowness.
The utilization of K. africana in the tequila industry is promising, giving its variety of synthesized volatile compounds, which would enrich the attributes of this beverage.
References
Albers E, Larsson C, Lidén G, Niklasson C, Gustafsson L (1996) Influence of the nitrogen source on Saccharomyces cerevisiae anaerobic growth and product formation. Appl Environ Microbiol 62:3187–3195
Arias-Gil M, Garde-Cerdán T, Ancín-Azpilicueta C (2007) Influence of addition of ammonium and different amino acid concentrations on nitrogen metabolism in spontaneous must fermentation. Food Chem 103:1312–1318
Bely M, Salmon JM, Barre P (1994) Assimilable nitrogen addition and hexose transport system activity during enological fermentation. J Inst Brew 100:279–282
Cedeño MC (1995) Tequila Production. Crit Rev Biotechnol 50:1–11
Ciani M, Picciotti G (1995) The growth kinetics and fermentation behaviour of some non-Saccharomyces yeasts associated with wine-making. Biotechnol Lett 17:1247–1250
Cooper TC (1982) Nitrogen metabolism in Saccharomyces cerevisiae. In: Strathern JN, Jones EW, Broach JR (ed) The molecular biology of the yeast Saccharomyces: metabolism and gene expression, vol. 2. Cold Spring Harbor Laboratory, Cold Springs Harbor, pp 39–100
Díaz-Montaño DM, Délia ML, Estarrón-Espinosa M, Strehaiano P (2008) Fermentative capability and aroma compound production by yeast strains isolated from Agave tequilana Weber juice. Enzyme Microb Technol 42:608–616
Díaz-Montaño DM, Favela-Torres E, Córdova J (2010) Improvement of growth, fermentative efficiency and ethanol tolerance of Kloeckera africana during the fermentation of agave tequilana juice by addition of yeast extract. J Sci Food Agric 90:321–328
Fleet GH (1990) Growth of yeasts during wine fermentations. J Wine Res 1:211–223
Gil JV, Mateo JJ, Jiménez M, Pastor A, Huerta T (1996) Aroma compounds in wine as influenced by apiculate yeasts. J Food Sci 61:1247–1250
Gorinstein S, Goldblum A, Kitov S, Deutsch J (1984) Fermentation and post-fermentation changes in Israeli wines. J Food Sci 49:251–256
Granchi L, Ganucci D, Messini A, Vincenzini M (2002) Oenological properties of Hanseniaspora osmophila and Kloeckera corticis from wines produced by spontaneous fermentations of normal and dried grapes. FEMS Yeast Res 2:403–407
Henschke PA, Jiranek V (1993) Yeast-Metabolism of nitrogen compounds. In: Fleet GH (ed) Wine, microbiology and biotechnology. Harwood Academic Publishers, Chur, pp 77–165
Jiranek V, Langridge P, Henschke PA (1991) Yeast nitrogen demand: selection criterium for wine yeasts for fermenting low nitrogen musts. In: Rantz J Proceedings of the international symposium on nitrogen in grapes and wines. American Society for Enology and Viticulture, Seattle, pp 266–269
Jiranek V, Langridge P, Henschke PA (1995) Amino acid and ammonium utilization by Saccharomyces cerevisiae wine yeasts. Am J Enol Vitic 46:75–83
Kunkee RE (1984) Selection and modification of yeasts and lactic acid bacteria for wine fermentation. Food Microbiol 1:315–332
Manginot C, Roustan JL, Sablayrolles JM (1998) Nitrogen demand of different yeast strains during alcoholic fermentation. Importance of the stationary phase. Enzyme Microb Technol 23:511–517
Mendes-Ferreira A, Clímaco MC, Mendes-Faia A (2001) The role of non-Saccharomyces species in releasing glycosidic bound fraction of grape aroma components––a preliminary study. J Appl Microbiol 91:67–71
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Moore S, Stein WH (1948) Photometric ninhydrin method for use in the chromatography of amino acids. J Biol Chem 176:367–388
Pierce JS (1982) Amino acids in malting and brewing. J Inst Brew 88:228–233
Pretorius IS (2000) Tailoring wine yeast for the new millennium: novel approaches to the ancient art of winemaking. Yeast 16:675–729
Romano P, Fiore C, Paraggio M, Caruso M, Capece A (2003) Function of yeast species and strains in wine flavour. Int J Food Microbiol 86:169–180
Soufleros E, Bertrand A (1979) Role of “yeast strain” in the production of volatile compounds during grape juice fermentation. Connaissance de la Vigne et du Vin 13:181–198
Vázquez-Ortiz FA, Caire G, Higuera-Ciapara I, Hernández G (1995) High performance liquid chromatographic determination of free amino acids in shrimp. J Liquid Chromatogr Relat Technol 18:2059–2068
Acknowledgments
This research was funded by CONACYT Consejo Nacional de Ciencia y Tecnología (Mexican National Council of Science and Technology) supporting SEP-CONACYT 24547 project. Juan O. Valle-Rodríguez and Guillermo Hernández-Cortés thank CONACYT for the scholarships which were granted.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Valle-Rodríguez, J.O., Hernández-Cortés, G., Córdova, J. et al. Fermentation of Agave tequilana juice by Kloeckera africana: influence of amino-acid supplementations. Antonie van Leeuwenhoek 101, 195–204 (2012). https://doi.org/10.1007/s10482-011-9622-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10482-011-9622-x