Abstract
Serious cyanobacterial blooms lead to the release of harmful microcystin-LR and ammonia to eutrophic waters, thereby posing serious challenges to zooplankton. The physiological and ecological responses of Brachionus calyciflorus Pallas to microcystin-LR and ammonia stresses were evaluated. Neonates (< 2 h old) were exposed to microcystin-LR (0, 10, 50, 100, and 200 µg L−1) and ammonia (0, 740, and 1580 µg L−1) single solutions and mixtures. High doses of 200 µg L−1 microcystin-LR and 1580 µg L−1 ammonia and mixtures of these two toxicants exhibited negative effects on the rotifer net reproduction rate (R0), intrinsic growth rate (rm), and enzymatic activities of glutathione (GSH), glutathione peroxidase (GPx), and glutathione S-transferase (GST). Microcystin-LR combined with ammonia decreased the generation time (T), Na+/K+–adenosine triphosphatase (Na+/K+–ATPase) activity, and glutathione reductase (GR) activity, but promoted the reactive oxygen species (ROS) production in rotifers (p < 0.05). The ROS levels were negatively correlated with GSH-related enzymatic activities (p < 0.01). Microcystin-LR and ammonia exerted synergistic effects on R0, rm, and ROS levels and GSH, GR, and GST activities (p < 0.05), but had antagonistic effects on T, Na+/K+–ATPase activity, and GPx activity (p > 0.05). Microcystis metabolites negatively affected growth and reproductive performance of B. calyciflorus. In addition, the life-cycle parameters were related to the effects of microcystin-LR and ammonia on the GSH-related antioxidant defense system of rotifers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water blooms of cyanobacteria pose a worldwide environmental threat (Mowe et al. 2015). Cyanotoxins, the secondary metabolites of toxic cyanobacteria, are released into eutrophic waters during severe cyanobacterial bloom breakouts and pose hazards to animals and humans (Bláha et al. 2009; Pantelić et al. 2013). Microcystins are hepatotoxins that are widely distributed worldwide. Microcystin-LR has the broadest distribution and the most toxic among microcystins (Kim et al. 2015). The microcystin-LR concentration in eutrophic water bodies usually reaches up to 200 µg L−1 and is even high in areas of serious Microcystis blooms (Thirumavalavan et al. 2013). Microcystin-LR inhibits protein phosphatases, induces oxidative stress, and causes intracellular problems (Zong et al. 2018). Elevated ammonia and microcystin-LR are released into water bodies during toxic cyanobacterial blooms (Xia et al. 2018). The concentrations of ammonia reach 200–3400 µg L−1 in some areas of a eutrophic lake, such as Lake Taihu, China (Zhang et al. 2010). High concentrations of microcystin-LR and ammonia are deleterious to animals and cause issues in aquatic communities (Chen et al. 2015; Xia et al. 2018). Stressors derived from Microcystis blooms, such as microcystin-LR and ammonia, often threaten aquatic organisms by association (Zhu et al. 2015; Liang et al. 2018).
Microcystin-LR and ammonia induce changes on the intracellular enzymatic activity and antioxidant physiological response in organisms (Chen et al. 2015). Reactive oxygen species (ROS) at high levels cause oxidative stress and disrupt the physiological function in animals (Park et al. 2017). The increase in ROS production is harmful to life parameters and antioxidant systems of zooplankton (Lee et al. 2017). Na+/K+–adenosine triphosphatase (Na+/K+–ATPase) is a membrane protein whose main function is to provide energy for ion regulation and maintain the osmoregulation of organisms (Li et al. 2014). Na+/K+–ATPase activity changes by toxicants exhibit negative effects on the physiological enzyme activity and cellular homeostasis of the exposed organisms (Kopecka-Pilarczyk 2010). Maternal exposure to microcystin-LR and ammonia mixtures resulted in adverse effects on the growth of Daphnia offspring. This phenomenon leads to the argument among the authors that most energy was allocated to resist toxicant stress, whereas growth and reproductive performance consumed less energy (Dao et al. 2010; Zhu et al. 2015).
The antioxidant defense system, which eliminates excessive ROS, is important for protecting the organism from oxidation damage (Wang et al. 2015). Glutathione (GSH)-related enzymes are considered oxidative stress indicators in aquatic animals (Couto et al. 2016). GSH is important in intracellular protective mechanism against oxidative stress and participates in the reduction of peroxides with concomitant formation of oxidized glutathione disulfide (GSSG) (Yilmaz et al. 2009; Park et al. 2017). Glutathione peroxidase (GPx) breaks down hydrogen dioxide into water and oxygen (Rocha et al. 2015). Glutathione reductase (GR) reduces GSSG to GSH at the cost of reducing nicotinamide adenine dinucleotide phosphate under normal physiological conditions. This phenomenon is responsible for maintaining the proper GSH activity (Pannala et al. 2013). Moreover, GSH forms S-conjugates with toxic metabolites, and the S-conjugation reaction is accelerated by glutathione S-transferase (GST) (Guo and Xie 2011). Microcystin-LR and ammonia induce the ROS production, which affects Na+/K+–ATPase activity and antioxidant enzymatic activities (López-López et al. 2011; Chen et al. 2015; Liang et al. 2017). Variation in the activities of GPx, GR, and GST causes disturbances in GSH activity. These disturbances adversely affect the growth and reproduction of animals (Lee et al. 2017).
Rotifers are key grazers in aquatic ecosystems. They exhibit sensitivity to toxic substances. Thus, they are commonly used in ecotoxicology tests (Snell and Joaquim-Justo 2007). Brachionus calyciflorus is a widely used test animal for toxicity evaluation worldwide and serves as a sensitive species that is used to quantify the toxic effects of toxicants (Snell and Janssen 1995). Algae and cyanobacteria are usually consumed by rotifers in waters. Thus, metabolic products of toxic Microcystis deleteriously affect the reproductive performance of B. calyciflorus (Liang et al. 2018). Following a breakout of cyanobacterial blooms, rotifers change their growth and reproduction performance to improve their fitness in harsh water environments (Zweerus et al. 2017). The correlation between the enzyme activities and Brachionus reproduction is sufficient for evaluating stressors (Wang et al. 2015). Acute toxicity of microcystin-LR and ammonia to zooplankton and the reproductive and physiological responses to these toxicants were determined (Leung et al. 2011; Zhu et al. 2015). The zooplankton growth in response to cyanobacterial metabolites has been previously studied (Huang et al. 2012; Cao et al. 2014). Several studies reported that the toxicological effects of pollutants (microcystins and nitrogen-containing wastes) are derived from cyanobacterial blooms on different species of aquatic organisms (Ge et al. 2012; Sun et al. 2011, 2013), and a number of researchers have focused on freshwater cladoceran species (Yang et al. 2011; Lyu et al. 2013, 2016). However, research evaluating the influence of combined exposure to microcystin-LR and ammonia on the life-cycle parameters, Na+/K+–ATPase activity, and GSH, GPx, GR, and GST activities of B. calyciflorus is limited.
This current study aimed to examine the responses of life-cycle parameters and GSH-related enzyme activities to microcystin-LR and ammonia to reveal the mechanism of cyanobacterial secondary metabolites on B. calyciflorus reproduction. We hypothesized that (1) dissolved microcystin-LR and ammonia exhibit interactive toxic effects on the life-cycle parameters of rotifers, and (2) microcystin-LR combined with ammonia is detrimental to GSH-related antioxidant defense system in terms of changes in antioxidant enzymatic activities in B. calyciflorus. Toxicology studies on rotifers are scientifically significant for future ecotoxicological studies regarding the effects of other cyanobacterial metabolites on aquatic animals and provide a reference for using zooplankton as ecotoxicology test models.
Materials and methods
Test animal
Rotifers B. calyciflorus Pallas, 1766 were originally collected from Moon Lake, wherein toxic cyanobacteria bloom, in Nanjing, China (32°6′35.24″N, 118°54′32.71″E), and continuously cultured in the laboratory. Cysts were used to obtain test animals in the ecotoxicology experiments with B. calyciflorus (Snell and Janssen 1995). Initial neonates (< 2 h old) were collected directly from cultures in modified freshwater Environmental Protection Agency (EPA) medium using the formula from ASTM (2001): 96 mg NaHCO3, 60 mg CaSO4·H2O, 123 mg MgSO4 (originally 60 mg MgSO4), and 4 mg KCl in 1 L deionized water at 25 °C and pH 7.8. Chlorella pyrenoidosa Chick, 1903 (1 × 106 cells mL−1, Institute of Hydrobiology, Chinese Academy of Sciences, Wuhan, China) cultured in a 5-L bag containing Bold’s basal medium was used as food for the rotifers. Bold’s basal medium was prepared according to the protocol described in a previous study (Stanier et al. 1971). A linear relationship was observed between C. pyrenoidosa concentration and A680 nm absorbance. Chlorella density was obtained by measuring light absorption at 680 nm by using a spectrophotometer (Ultrospec 2100, Biochrom Ltd., Cambridge, UK) with double-distilled water based on linear regression. Brachionus and Chlorella were both cultured in an illuminated incubator. The conditions were maintained at 25 ± 1 °C under a 2500 lx constant fluorescent illumination and at 14 h:10 h light/dark photoperiod.
Experimental design
To evaluate the influence of pure microcystin-LR and ammonia on the life-cycle parameters and GSH-related antioxidant enzyme activities of rotifers, microcystin-LR and ammonia were diluted to a range of toxin doses. Microcystin-LR (Express, Beijing, China) was dissolved in modified EPA medium, and the microcystin-LR concentrations were set according to the levels when Microcystis blooms collapsed (Zhu et al. 2015). Ammonium chloride (NH4Cl, Sinopharm, Shanghai, China) dissolved in the EPA medium was used to obtain the ammonia test solutions. Ammonia concentrations are calculated according to Eq. 1, as follows (Emerson et al. 1975):
where the dissociation constant pKa = 0.09018 + 2729.92/T. T is the temperature in K, and the pH is stable at 7.5. The ammonia doses were set according to field observations based on toxicant concentrations in the field while severe Microcystis blooms break out (Zhang et al. 2010) and on the 24 h LC50 values of the two toxicants (microcystin-LR LC50: 56.2 µg L−1, ammonia LC50: 838.8 µg L−1). Microcystin-LR and ammonia doses were set as 0, 10, 50, 100, and 200 µg L−1 (M0, M10, M50, M100, and M200) and 0, 740, and 1580 µg L−1 (N0, N740, and N1580), respectively. Fifteen combinations of N × M were determined, and the N0M0 treatment was used as the control in all experiments. Microcystin-LR and ammonia solutions were replaced every 24 h to maintain constant toxicant doses.
Life-cycle experiments were conducted in 6-well microplates. Ten neonates (< 2 h old) were introduced into each well containing 10 mL of different toxicant solutions to evaluate the life-cycle parameters of the rotifers. Brachionus has a small body size (< 0.5 mm) and can be cultured in a small volume of solution (Snell and Janssen 1995; Sun et al. 2019). Thus, 10 mL test solution was used to assess the toxicity of microcystin-LR and ammonia in this study. Rotifer cysts, which were cultured in the illuminated incubator, were hatched in 6-well microplates containing the EPA medium and subjected to a 2500 lx constant fluorescent illumination at 25 °C. The rotifers that hatched from the resting eggs were used for the experiments, and each test solution treatment had 12 replicates (N = 12, N stands for biological replicates) in the life-cycle experiments. The animals received the same biomass of C. pyrenoidosa every 12 h. Test solutions were replaced at 24-h intervals. The experimental animals were monitored at 6-h interval under an inverted microscope (Motic SMZ168, Motic Instruments, China). The numbers of living original individuals and neonates produced were recorded. Meanwhile, the newborn rotifers were systematically removed from the toxicant solutions. The original females were transferred into freshly toxicant solutions containing 1 × 106 cells mL−1 of C. pyrenoidosa every 24 h until all the initial experimental females were dead. The toxicant solution (10 µL), which included the initial individuals, was transferred by a micropipette. The errors made, e.g., killing and/or damaging rotifers during daily handling, were excluded during the life-cycle experiments. The ecological responses of B. calyciflorus under the stress of microcystin-LR and ammonia were assessed by life-cycle parameters. The net reproduction rate (R0), generation time (T), and intrinsic growth rate (rm) are related to survival and reproductive performance and are the ecologically important parameters to evaluate the population growth potential of rotifers (Snell and Janssen 1995). The three variables are calculated using Eqs. 2–4 (Krebs 1994):
where x is the rotifer age, lx is the proportion of individuals surviving at age x compared with its original cohort, and mx is the mean number of female offspring produced in a unit of time by a female at age x.
In physiology-based experiments, the initial culture density of rotifers was 600–900 individuals per treatment. The exposure time of test animals to each N × M treatment was 24 h. Rotifers were fed with C. pyrenoidosa at 12 h. The ROS levels, Na+/K+–ATPase activity, and GSH-related enzyme activities in B. calyciflorus were determined after the exposure time of 24 h. Rotifers were collected into 1.5-mL centrifugal tubes by using precision test sieves with mesh size of 37.4 µm. The rotifer bodies gathered at the bottom of the tubes after centrifugation at 6000 rpm for 15 min at 4 °C. Stress and errors from the collection and centrifugation of the animals were ruled out. Four hundred rotifers chosen from the initial individuals were separated in a 1.5-mL centrifuge tube (400 individuals tube−1) and weighed (W) with the difference between the weight of the tube containing 400 individuals (W1) and an empty tube (W2) by an electronic balance (W = W1 − W2). We omitted the influence of Chlorella (consumed by rotifers within 12 h) and test solutions (removed from tubes by a sterile syringe). The chosen 400 rotifers were homogenized in nine volumes (v/w) with cold 0.1 mol L−1 pH 7.3 phosphate buffer. The supernatants with different treatments were stored at − 80 °C for biochemical measurement after the rotifer homogenates were centrifuged at 3000 rpm for 10 min at 4 °C. Three replicates were performed for each treatment to measure the ROS levels, Na+/K+–ATPase activity, and GSH-related enzyme activities (N = 3, N stands for biological replicates).
Biochemical assay
ROS levels, GSH, GPx, GR, and GST enzymatic activities were assayed by kits (Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s instructions. The ROS levels and GSH-related enzyme activities were assayed with a microplate reader in 96-cell plates. All enzyme activities were calculated with reference to the protein content. The rotifer protein content was evaluated according to the study of Bradford (1976).
Measurement of Na + /K + –ATPase
Total ATPase activity was estimated by determining the accumulation of inorganic phosphate in a medium with the following composition: 100 mM NaCl, 20 mM KCl, 5 mM MgCl2, 3 mM Na2–ATPase, and 50 mM Tris–HCl at pH 7.4 and 37 °C. Na+/K+–ATPase activity was the difference between the activities of total and Mg2+–ATPase recorded in the same medium but without KCl (Li et al. 2014).
Statistical analysis
Data feasibility analysis was tested with the analysis of variance (ANOVA) based on the Kolmogorov–Smirnov tests and Levene’s test for data distribution and homogeneity, respectively. Duncan’s and Tukey’s multiple range tests were conducted, revealing no differences in the data trends. The life-cycle parameters, ROS levels, Na+/K+–ATPase activity, and GSH-related antioxidant enzyme activities of rotifers B. calyciflorus under the stress of microcystin-LR and ammonia were assessed by two-way ANOVA, followed by Tukey’s multiple range test. Values are the means ± standard error (SE). The maximum mean value was marked “a” under a certain toxin dose. “a,” “b,” “c,” “d,” and “e” indicate mean values with significant difference at p < 0.05. The same letter means no significant difference among these data. “ab” signifies no significant difference between “a”/“b” and “ab.” “bc” indicates no significant difference between “b”/“c” and “bc.” “cd” represents no significant difference between “c”/“d” and “cd.” The sensitive enzyme activity index of oxidative stress induced by microcystin-LR and ammonia was identified by Pearson’s correlation analyses. Statistical analyses were performed using SigmaPlot v. 12.5.
Results
Life-cycle parameters of rotifers
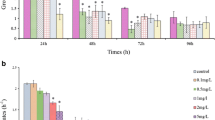
Microcystin-LR and ammonia affected the life-cycle parameters of Brachionus, and the two toxicants had remarkable synergistic effects on the rotifer reproductive performance, as reflected in the parameters of R0 and rm (two-way ANOVA, p < 0.001; Table 1). Microcystin-LR and ammonia negatively affected T. The two toxicants had no interactive effects on T (two-way ANOVA, p = 0.06 > 0.05; Table 1). Ammonia comparisons within certain microcystin-LR doses indicated that R0 increased by 9% compared with N0M0 treatment at low ammonia concentration (740 µg L−1) in the single ammonia solution (Fig. 1a). At 0–200 µg L−1 microcystin-LR, T of rotifers was shortened by 15–72% with increasing ammonia concentrations (Fig. 2b). Low ammonia concentration positively affected rm. The rotifer rm increased by 18% during N740M0 treatment (p < 0.001), whereas rm decreased by 35% in N1580M0 treatment (p < 0.001, Fig. 1c).
Comparisons of ammonia within certain microcystin-LR concentrations on net reproduction rate (R0) (a), generation time (T) (b), and intrinsic growth rate (rm) (c), and comparisons of microcystin-LR within certain ammonia concentrations on R0 (d), T (e), and rm (f) of B. calyciflorus. Values are expressed as mean ± SE of 12 replicate samples (N = 12). Error bars indicate 1 SE, and different letters denote significant difference (p < 0.05)
Comparisons of ammonia within certain microcystin-LR concentrations on reactive oxygen species (ROS) levels (a), and comparisons of microcystin-LR within certain ammonia concentrations on ROS levels (b) of B. calyciflorus. Values are expressed as mean ± SE of three replicate samples (N = 3). Error bars indicate 1 SE, and different letters denote significant difference (p < 0.05)
Microcystin-LR comparisons within certain ammonia doses revealed the reproductive performance of B. calyciflorus inhibited at 200 µg L−1 microcystin-LR. The rotifer R0 increased by 21–71% during N0M10, N0M50, and N0M100 treatments (p < 0.05), but decreased by 38% in N0M200 treatment (p < 0.001, Fig. 1d). The rotifer R0 decreased by 4–76% with increasing microcystin-LR dose under 740–1580 µg L−1 ammonia (Fig. 1d). Increasing the microcystin-LR concentration inhibited T under 0–1580 µg L−1 ammonia (Fig. 1e). High microcystin-LR dose negatively affected rm when Brachionus were exposed to single microcystin-LR solutions. rm of rotifers increased by 6–30% during N0M10, N0M50, and N0M100 treatments, but decreased by 29% in N0M200 treatment (p < 0.001, Fig. 1f). Under ammonia doses of 740 and 1580 µg L−1, rm decreased by 22–77% with increasing microcystin-LR concentrations (Fig. 1f).
ROS levels
Microcystin-LR and ammonia had synergistic effects on ROS production (two-way ANOVA, p = 0.01 < 0.05; Table 1). Microcystin-LR and ammonia enhanced ROS production in B. calyciflorus. ROS levels increased by 6–32% compared with N0M0 treatment with increasing ammonia concentrations under 0–200 µg L−1 microcystin-LR (Fig. 2a). For certain ammonia concentrations, the ROS levels were positively correlated with the doses of microcystin-LR. The additive effect of microcystin-LR promoted ROS formation under 0–1580 µg L−1 ammonia (Fig. 2b).
Na + /K + –ATPase activity
Na+/K+–ATPase activity was inhibited under all microcystin-LR and ammonia concentrations in a dose-dependent manner. Moreover, the two toxicants demonstrated an antagonistic effect on Na+/K+–ATPase activity (two-way ANOVA, p = 0.64 > 0.05; Table 1), which was negatively correlated with the ROS levels (p < 0.01; Table 2). The Na+/K+–ATPase activity decreased with increasing ammonia concentrations under 0–200 µg L−1 microcystin-LR and was reduced at ammonia doses of 740 and 1580 µg L−1 (p < 0.05; Fig. 3a). The Na+/K+–ATPase activity decreased by 8–74% compared with N0M0 treatment with increasing microcystin-LR doses at ammonia concentrations of 0–1580 µg L−1 and was greatly reduced at 10–200 µg L−1 microcystin-LR (p < 0.05; Fig. 3b).
Comparisons of ammonia within certain microcystin-LR concentrations on Na+/K+–adenosine triphosphatase (Na+/K+–ATPase) activity (a), and comparisons of microcystin-LR within certain ammonia concentrations on Na+/K+–ATPase activity (b) of B. calyciflorus. Values are expressed as mean ± SE of three replicate samples (N = 3). Error bars indicate 1 SE, and different letters denote significant difference (p < 0.05)
GSH-related antioxidant enzyme activities
Microcystin-LR and ammonia exhibited a synergistic effect on enzymatic activities of GSH (p = 0.002 < 0.05), GR (p < 0.001), and GST (p = 0.007 < 0.05), but demonstrated an antagonistic effect on the enzymatic activity of GPx (p = 0.06 > 0.05) (two-way ANOVA; Table 1). GSH, GPx, GR, and GST activities were negatively correlated with ROS levels (p < 0.01; Table 2), whereas GSH-related enzyme activities were positively correlated with Na+/K+–ATPase activity (p < 0.01; Table 2). In single ammonia solutions, GSH, GPx, and GST activities increased by 4–6% in N740M0 treatment, but decreased by 12–33% in N1580M0 treatment (p < 0.05; Fig. 4a, b, d). GR activity decreased by 23–31% in N740M0 and N1580M0 treatments (p < 0.001; Fig. 4c). The additive ammonia inhibited the activities of GSH, GPx, GR, and GST, which decreased by 3–78% in 10–200 µg L−1 microcystin-LR (Fig. 4a–d).
Comparisons of ammonia within certain microcystin-LR concentrations on the enzymatic activities of glutathione (GSH) (a), glutathione peroxidase (GPx) (b), glutathione reductase (GR) (c), and glutathione S-transferase (GST) (d), and comparisons of microcystin-LR within certain ammonia concentrations on the enzymatic activities of GSH (e), GPx (f), GR (g), and GST (h) of B. calyciflorus. Values are expressed as mean ± SE of three replicate samples (N = 3). Error bars indicate 1 SE, and different letters denote significant difference (p < 0.05)
Microcystin-LR comparisons within certain ammonia doses indicated that low microcystin-LR concentrations (10–100 µg L−1) positively affected GSH, GPx, and GST activities in single microcystin-LR solutions. The enzymatic activities of GSH, GPx, and GST increased by 3–23% during N0M10, N0M50, and N0M100 treatments (Fig. 4e, f, h). GR activity decreased by 21–74% in single microcystin-LR solutions (p < 0.001; Fig. 4g). In mixtures of microcystin-LR and ammonia, the enzymatic activities of GSH, GPx, GR, and GST were inhibited under ammonia doses of 740 and 1580 µg L−1, and the adverse effect was correlated with microcystin-LR concentrations (Fig. 4e, f, g, h).
Discussion
Microcystin-LR and ammonia demonstrated combined effects on the life-cycle parameters of Brachionus. The two toxicants were harmful to the GSH-related antioxidant defense system in terms of GSH, GPx, GR, and GST enzyme activities. Notably, the two toxicants were toxic to rotifer reproduction. Microcystin-LR combined with ammonia caused greater life-cycle shortening in rotifer T than single factor microcystin-LR or ammonia treatment. Microcystin-LR and ammonia enhanced the ROS formation, but inhibited the Na+/K+–ATPase activity. Low doses of microcystin-LR or ammonia positively affected the activities of GSH, GPx, and GST when rotifers were exposed to single solutions, whereas microcystin-LR in combination with ammonia inhibited these enzyme activities. GR activity decreased with increasing microcystin-LR or ammonia doses. The combined effects of the two toxicants on the levels of R0, rm, and ROS, and the activities of GSH, GR, and GST were determined. Microcystin-LR and ammonia exhibited an antagonistic effect on T, Na+/K+–ATPase, and GPx activities. Results demonstrated that microcystin-LR and ammonia exhibit interactive toxic effects on the life-cycle parameters of rotifers and the combination of the two toxicants is detrimental to GSH-related antioxidant defense system in B. calyciflorus.
Effects of microcystin-LR and ammonia on the life-cycle parameters of rotifers
Microcystin-LR and ammonia have a well-documented toxicity to rotifers (Liang et al. 2018). B. calyciflorus is highly tolerant to microcystin-LR at doses lower than 200 µg L−1 (Huang et al. 2012). An ammonia dose of 2500 ± 200 µg L−1 causes serious harm to Daphnia magna reproduction (EPA 1999). Ammonia doses under lethal concentrations (> 1000 µg L−1) negatively affect the longevity and reproductive performance of Daphnia carinata (Leung et al. 2011). In the present study, single solutions of 200 µg L−1 microcystin-LR and 1580 µg L−1 ammonia at high doses were harmful to the reproduction of B. calyciflorus. Low microcystin-LR (10–100 µg L−1) and ammonia (740 µg L−1) doses induced stimulating effects on the reproduction parameters of rotifers, such as R0 and rm. The improvement in reproductive performance at low doses of the toxicants is considered hormesis (Lave 2001). Brachionus has no gills or a respiratory system, and gas exchange is performed through its body wall (Snell and Joaquim-Justo 2007). These characteristics reduce the toxic effects on rotifers in solutions containing low microcystin-LR/ammonia concentrations (Liang et al. 2018). The ammonia dose for 24 h LC50 and 48 h LC50 to rotifers reached 12,300 and 6700 µg L−1, respectively (Chen et al. 2015). The toxic ammonia dose to B. calyciflorus in terms of R0 and rm reached 1580 µg L−1. This dose was lower than that in the aforementioned study. One reason for this finding is that the ammonia doses in the present study (0–1580 µg L−1) were lower than those in the study of Chen et al. (2015) (0–30,000 µg L−1). Another reason is that the previous authors used different rotifer strains, indicating that the differences in tolerance of zooplanktons to ammonia are due to the experimental strains used (Yang et al. 2017). The life-cycle parameters of B. calyciflorus were inhibited by microcystin-LR and ammonia mixtures at increasing doses of these toxicants. The rotifer T decreased with every treatment of microcystin-LR and ammonia. The two toxicants are dangerous to rotifers in terms of survival time. Microcystin-LR and ammonia disrupt the physiological functions of organisms, thereby further affecting their life-cycle parameters (Sun et al. 2012). The reduction in R0, rm, and T of rotifers exposed to different mixtures of microcystin-LR and ammonia was the result of organism damage correlated with oxidative stress induced by the two toxicants. This result was indicated by the changes in GSH-related enzyme activities.
Effects of microcystin-LR and ammonia on the ROS levels and Na+/K+–ATPase activity of rotifers
Na+/K+–ATPase provides energy for ion transport and facilitates active exchange between K+ and Na+ to maintain ion homeostasis (Kopecka-Pilarczyk 2010; Li et al. 2014). The reduction in Na+/K+–ATPase activity suggested that microcystin-LR and ammonia disrupted the ion exchange and negatively affected the energy supplement in rotifers. In B. calyciflorus exposed to microcystin-LR and ammonia, the ROS levels increased with increasing toxicant concentrations. Na+/K+–ATPase activity was negatively correlated with the ROS levels. Therefore, decreased Na+/K+–ATPase activity in every treatment was partly related to high ROS levels in rotifers. Excessive ROS inhibited the Na+/K+–ATPase activity. This phenomenon adversely affected the life-cycle parameters and GSH-related enzyme activity of rotifers. A study confirmed that Na+/K+–ATPase activity is negatively correlated with high levels of ROS under heavy metal stress (Kim et al. 2016). Therefore, we speculated that ATPase is a biomarker for oxidative stress damage due to exposure to microcystin-LR and ammonia.
Effects of microcystin-LR and ammonia on the GSH-related antioxidant defense system of rotifers
In the present study, GSH-related enzyme activities exhibited different responses to microcystin-LR and ammonia. GR activity decreased with every treatment of microcystin-LR and ammonia, thereby suggesting that GR was more sensitive to Microcystis metabolites than the other three antioxidant enzymes. GSH, GPx, and GST activities increased at low concentrations of microcystin-LR (10–100 µg L−1) or ammonia (740 µg L−1), but decreased at high doses of microcystin-LR (200 µg L−1) or ammonia (1580 µg L−1) and mixtures of the two toxicants. This trend is similar to that of R0 and rm, thereby suggesting that changes in GSH, GPx, and GST activities affected the reproduction parameters of rotifers. GSH is essential for protecting the function of animal tissues against the deleterious action of metabolites (Yilmaz et al. 2009). A decline in GSH level could affect the physiological function and cellular redox homoeostasis network in organisms (Couto et al. 2016). GST is responsible for the detoxification of electrophilic compounds, for example, ROS, metabolites, and heavy metal ions by conjugating with the –thiol group and rendering them harmless (Yim et al. 2015). Microcystin-LR combined with ammonia reduced the GSH, GPx, and GST activities, which might cause the dysfunction of the GSH-related antioxidant defense system in B. calyciflorus. GSH, GPx, GR, and GST play an important role in protecting cells through the detoxification of active species and/or repair of injury and are useful as potential molecular biomarkers for the monitoring of microcystin-LR and ammonia toxicity in aquatic environments (Wang et al. 2015).
GSH-related enzyme activities were negatively correlated with the ROS levels, but were positively correlated with Na+/K+–ATPase activity. Under the stress of microcystin-LR and ammonia, the reduction in Na+/K+–ATPase activity reduced the energy supply. This condition was harmful to the redox reaction. The reduction in the redox reaction accumulated ROS in the rotifer body. This phenomenon negatively influenced enzyme activities (Chen et al. 2015). Decreased GR activity in every treatment of microcystin-LR and ammonia was related to the reduction in Na+/K+–ATPase activity and increasing ROS levels. In one toxicant solution, GSH, GPx, and GST activities improved at low doses of microcystin-LR or ammonia. GSH-related enzyme activities were enhanced by slight oxidative stress because of the antioxidant response to low doses of Microcystis metabolites. Severe oxidative stress had negative effects on the activities of the three GSH-related enzymes due to damage in the GSH-related antioxidant defense system under high doses of microcystin-LR and ammonia (Sun et al. 2012). Microcystin-LR combined with ammonia negatively affects the activities of GSH, GPx, and GST. In addition, the inhibition effect was exacerbated by increasing the doses of these toxicants, thereby resulting in the poor efficiency of GSH, GPx, and GST in removing high ROS levels. Microcystin-LR in combination with ammonia inhibited the GSH-related enzyme activities. This phenomenon resulted in excessive ROS accumulation in the rotifer body. High levels of ROS caused oxidative damage, which negatively affected the life-cycle parameters of rotifers. GSH-related antioxidant enzymes were important in detoxifying ROS and in preventing the disruption of the peroxidase/glutathione reductase redox cycle balance in animals (Yilmaz et al. 2009; Park et al. 2017).
Conclusions
This study shows that the threat to Brachionus presented by Microcystis degradation is not from the toxin microcystin-LR but from ammonia in a eutrophic water environment. Microcystin-LR and ammonia affected the growth and reproductive performance of rotifers through oxidative stress on the GSH-related antioxidant defense system in rotifers. Microcystin-LR in combination with ammonia results in stress responses of rotifers, which are characterized by reduced Na+/K+–ATPase activity and varied GSH-related enzyme activities in B. calyciflorus. The synergistic effects of microcystin-LR and ammonia were observed on R0, rm, ROS levels, and activities of GSH, GR, and GST. By contrast, microcystin-LR and ammonia displayed antagonistic effects on T, Na+/K+–ATPase, and GPx activity. These findings indicated that GSH-related enzyme activities are involved in cellular protection as antioxidant defense systems against microcystin-LR and ammonia-induced oxidative stress. The GSH-related enzymes are potential biomarkers in response to microcystin-LR and ammonia. Microcystin-LR may be combined with some common toxicants in eutrophic waters. This combination affects the zooplankton physiological and ecological responses. As cyanobacterial blooms become increasingly serious worldwide, these results provide a reference for studying the effects of exposure to other toxic Microcystis metabolites on the sensitivity responses of aquatic animals.
References
ASTM (2001) Standard guide for acute toxicity tests with the rotifer Brachionus. Annual book of ASTM standards, water and environmental technology, vol. 1105. Biological effects and environmental fates. American Society for Testing and Materials, West Conshohocken
Bláha L, Babica P, Maršálek B (2009) Toxins produced in cyanobacterial water blooms–toxicity and risks. Interdiscip Toxicol 2:36–41
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cao H, Lyu K, Xiang F, Yang Z (2014) Life history responses of Daphnia similoides simultaneously exposed to microcystin-LR and ammonia and their postexposure recovery. Environ Toxicol Chem 33:2497–2505
Chen Y, Gu R, Wang X, Nie Z, Xu G, Lu J, Huang H (2015) Acute toxicity of ammonia nitrogen to Brachionus calyciflorus and its antioxidant physiological response. J Anhui Agric Sci 43:81–84
Couto N, Wood J, Barber J (2016) The role of glutathione reductase and related enzymes on cellular redox homoeostasis network. Free Radic Bio Med 95:27–42
Dao TS, Do-Hong LC, Wiegand C (2010) Chronic effects of cyanobacterial toxins on Daphnia magna and their offspring. Toxicon 55:1244–1254
Emerson K, Russo RC, Lund RE, Thurston RV (1975) Aqueous ammonia equilibrium calculations: effect of pH and temperature. J Fish Res Board Can 32:2379–2383
EPA US (1999) Update of ambient water quality criteria for ammonia. EPA-822/R-99-014 Office of Water, Washington, DC, pp 1–153
Ge J, Li J, Zhang J, Yang Z (2012) Time-dependent oxidative stress responses of submerged macrophyte Vallisneria natans seedlings exposed to ammonia in combination with microcystin under laboratory conditions. Bull Environ Contam Toxicol 89:67–72
Guo N, Xie P (2011) A study on the effects of food quantity and quality on glutathione S-transferase (GST) activity and growth rate parameters of Daphnia carinata varying in age. Aquat Ecol 45:63–73
Huang L, Xi Y, Xu X, Wen X (2012) Responses in population growth and reproduction of the freshwater rotifer Brachionus calyciflorus to microcystin-LR at different temperatures. Ann Limnol Int J Lim 48:383–390
Kim IS, Nguyen GH, Kim SJ, Jang A (2015) Qualitative analysis of the most toxic and abundant microcystin variants (LR, RR, and YR) by using LCMS–IT–TOF. J Ind Eng Chem 29:375–381
Kim H, Lim B, Kim BD, Lee YM (2016) Effects of heavy metals on transcription and enzyme activity of Na+/K+–ATPase in the monogonont rotifer, Brachionus koreanus. Toxicol Environ Health Sci 8:128–134
Kopecka-Pilarczyk J (2010) Effect of exposure to polycyclic aromatic hydrocarbons on Na+–K+–ATPase in juvenile gilthead seabream sparus aurata. J Fish Biol 76:716–722
Krebs CJ (1994) Ecology: the experimental analysis of distribution and abundance, 4th edn. Harper Collins College Publishers, New York
Lave LB (2001) Hormesis: implications for public policy regarding toxicants. Annu Rev Public Health 22:63–67
Lee YH, Kim DH, Kang HM, Wang M, Jeong CB, Lee JS (2017) Adverse effects of methylmercury (MeHg) on life parameters, antioxidant systems, and MAPK signaling pathways in the rotifer Brachionus koreanus and the copepod Paracyclopina nana. Aquat Toxicol 190:181–189
Leung J, Kumar M, Glatz P, Kind K (2011) Impacts of un-ionized ammonia in digested piggery effluent on reproductive performance and longevity of Daphnia carinata and Moina australiensis. Aquaculture 310:401–406
Li JJ, Wang J, Yang L, Chen Y, Yang Z (2014) Changes in plasma osmolality and Na+/K+ATPase activity of juvenile obscure puffer Takifugu obscurus following salinity challenge. Biochem Syst Ecol 56:111–117
Liang Y, Chen X, Lu X, Jin S, Min Y, Yang J (2017) Combined effects of microcystin and nitrite on the growth, lipid peroxidation, and antioxidant responses of the freshwater rotifer Brachionus calyciflorus. Aquat Toxicol 192:78–88
Liang Y, Lu X, Min Y, Liu L, Yang J (2018) Interactive effects of microcystin and ammonia on the reproductive performance and phenotypic traits of the rotifer Brachionus calyciflorus. Ecotox Environ Safe 147:413–422
López-López E, Sedeño-Díaz JE, Soto C, Favari L (2011) Responses of antioxidant enzymes, lipid peroxidation, and Na+/K+–ATPase in liver of the fish Goodea atripinnis exposed to Lake Yuriria water. Fish Physiol Biochem 37:511–522
Lyu K, Zhu X, Wang Q, Chen Y, Yang Z (2013) Copper/zinc-superoxide dismutase from the Cladoceran Daphnia magna: Molecular cloning and expression in response to different acute environmental stressors. Environ Sci Technol 47:8887–8893
Lyu K, Meng Q, Zhu X, Dai D, Zhang L, Huang Y, Yang Z (2016) Changes in iTRAQ-based proteomic profiling of the cladoceran Daphnia magna exposed to microcystin-producing (MP) and microcystin-free (MF) Microcystis aeruginosa. Environ Sci Technol 50:4798–4807
Mowe MAD, Mitrovic SM, Lim RP, Furey A, Yeo DCJ (2015) Tropical cyanobacterial blooms: a review of prevalence, problem taxa, toxins and influencing environmental factors. J Limnol 74:205–224
Pannala VR, Bazil JN, Camara AKS, Dash RK (2013) A biophysically based mathematical model for the catalytic mechanism of glutathione reductase. Free Radic Biol Med 65:1385–1397
Pantelić D, Svirčev Z, Simeunović J, Vidović M, Trajković I (2013) Cyanotoxins: characteristics, production and degradation routes in drinking water treatment with reference to the situation in Serbia. Chemosphere 91:421–441
Park JC, Han J, Lee MC, Seo JS, Lee JS (2017) Effects of triclosan (TCS) on fecundity, the antioxidant system, and oxidative stress-mediated gene expression in the copepod Tigriopus japonicas. Aquat Toxicol 189:16–24
Rocha S, Gomes D, Lima M, Bronze-Da-Rocha E, Santos-Silva A (2015) Peroxiredoxin 2, glutathione peroxidase, and catalase in the cytosol and membrane of erythrocytes under H2O2-induced oxidative stress. Free Radic Res 49:990–1003
Snell TW, Janssen CR (1995) Rotifers in ecotoxicology: a review. Hydrobiologia 313(314):231–247
Snell TW, Joaquim-Justo C (2007) Workshop on rotifers in ecotoxicology. Hydrobiologia 593:227–232
Stanier RY, Kunisawa R, Mandel M, Cohen-Bazire G (1971) Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol Rev 35:171–205
Sun H, Yang W, Chen YF, Yang Z (2011) Effect of purified microcystin on oxidative stress of silver carp Hypophthalmichthys molitrix larvae under different ammonia concentrations. Biochem Syst Ecol 39:536–543
Sun H, Lü K, Minter EJA, Chen Y, Yang Z, Montagnes DJS (2012) Combined effects of ammonia and microcystin on survival, growth, antioxidant responses, and lipid peroxidation of bighead carp Hypophthalmythys nobilis larvae. J Hazard Mater 221(222):213–219
Sun H, Wang W, Geng L, Chen Y, Yang Z (2013) In situ studies on growth, oxidative stress responses, and gene expression of juvenile bighead carp (Hypophthalmichthys nobilis) to eutrophic lake water dominated by cyanobacterial blooms. Chemosphere 93:421–427
Sun Y, Xu W, Gu Q, Chen Y, Zhou Q, Zhang L, Gu L, Huang Y, Lyu K, Yang Z (2019) Small-sized microplastics negatively affect rotifers: changes in the key life-history traits and rotifer-Phaeocystis population dynamics. Environ Sci Technol 53:9241–9251
Thirumavalavan M, Hu YL, Lee JF (2013) Evaluation of analytical approaches linked to high performance liquid chromatography for analysis of microcystin-LR in natural water systems: effects of column and mobile phase gradient. Toxicol Environ Chem 95:221–231
Wang H, Tang X, Sha J, Chen H, Sun T, Wang Y (2015) The reproductive toxicity on the rotifer Brachionus plicatilis induced by BDE-47 and studies on the effective mechanism based on antioxidant defense system changes. Chemosphere 135:129–137
Xia H, Song T, Wang L, Jiang L, Zhou Q, Wang W, Liu L, Yang P, Zhang X (2018) Effects of dietary toxic cyanobacteria and ammonia exposure on immune function of blunt snout bream (Megalabrama amblycephala). Fish Shellfish Immun 78:383–391
Yang Z, Xiang F, Minter EJA, Lü K, Chen Y, Montagnes DJS (2011) The interactive effects of microcystin and nitrite on life-history parameters of the cladoceran Daphnia obtusa. J Hazard Mater 190:113–118
Yang J, Zhang X, Xie Y, Song C, Sun J, Zhang Y, Giesy JP, Yu H (2017) Ecogenomics of zooplankton community reveals ecological threshold of ammonia nitrogen. Environ Sci Technol 51:3057–3064
Yilmaz Ö, Keser S, Tuzcu M, Güvenc M, Çetintas B, Irtegün S, Tastan H, Sahin K (2009) A practical HPLC method to measure reduced (GSH) and oxidized (GSSG) glutathione concentrations in animal tissues. J Anim Vet Adv 8:343–347
Yim B, Kim H, Jung MY, Lee YM (2015) Cadmium modulates the mRNA expression and activity of glutathione S-transferase in the monogonont rotifer Brachionus koreanus. Toxicol Environ Health Sci 7:217–223
Zhang X, Chen C, Ding J, Hou A, Li Y, Niu Z, Su X, Xu Y, Laws EA (2010) The 2007 water crisis in Wuxi, China: Analysis of the origin. J Hazard Mater 182:130–135
Zhu X, Wang Q, Zhang L, Liu J (2015) Offspring performance of Daphnia magna after short-term maternal exposure to mixtures of microcystin and ammonia. Environ Sci Pollut Res 22:2800–2807
Zong W, Wang Q, Zhang S, Teng Y, Du Y (2018) Regulation on the toxicity of microcystin-LR target to protein phosphatase 1 by biotransformation pathway: effectiveness and mechanism. Environ Sci Pollut Res 25:26020–26029
Zweerus NL, Sommer S, Fontaneto D, Ozgul A (2017) Life-history responses to environmental change revealed by resurrected rotifers from a historically polluted lake. Hydrobiologia 796:121–130
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant Numbers: 31772458 and 31272388), Science Foundation for Young Scientists of Jiangsu in China (Grant Number: 20171093), the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (Grant Number: 19KJB180020), and the Startup Foundation for Introducing Talent of NUIST (Grant Number: 131061901013).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Télesphore Sime-Ngando.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liang, Y., Shao, L., Jiang, Q. et al. Changes in the life-cycle parameters and glutathione-related antioxidant defense system of rotifer Brachionus calyciflorus under the combined stress of microcystin-LR and ammonia. Aquat Ecol 54, 243–256 (2020). https://doi.org/10.1007/s10452-019-09739-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10452-019-09739-8