Abstract
This study investigates the effects of copper and mercury on growth rate, chlorophyll a content, superoxide dismutase (SOD) activity, SOD mRNA gene expression, and frustule morphology of the benthic freshwater diatom Halamphora veneta (Kützing) Levkov and the potential utility of each for toxicity assessment in aquatic habitats. Results showed the following: (1) Compared to mercury, exposure to copper resulted in greater growth inhibition of H. veneta even at low concentrations and after short durations of exposure; (2) high accumulation of chlorophyll a in H. veneta is a stress response to the presence of heavy metals; (3) SOD activity and SOD gene expression varied in H. veneta according to the concentration, exposure time, and type of heavy metal; and (4) exposure to mercury resulted in deformity in the shape and an increase in size of the frustule of H. veneta. Growth rate, chlorophyll a content, SOD activity and gene expression, and frustule morphology of H. veneta are all potential candidates for the toxicological assessment of copper and mercury in aquatic habitats.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metals of pollutants of global importance are derived from numerous sources including industrial processes, mining, transport, agricultural, and urban activities (Ouyang et al. 2017). The toxicity of heavy metals to aquatic animals is well documented (Mzimela et al. 2003; Zhang et al. 2005), and in recent years, increasing attention is being paid to the development of protocols for environmental risk assessment of water environments (Fang et al. 2016; Marks et al. 2017; Zhang et al. 2017).
Copper (Cu) is a necessary micronutrient, which plays important functional roles in the biochemistry of aquatic organisms, but becomes toxic at high concentrations (Ahsan et al. 2007; Castruita et al. 2011). Mercury is considered one of the most dangerous metals in the environment (Goyer et al. 1995), mainly because it can accumulate in biological food chains and ultimately affected human health (Campbell et al. 2005; MacDougal et al. 1996; Raimundo et al. 2014). Copper and mercury contamination in the aquatic environment is mostly derived from industrial processes and anthropogenic activities (Ciji and Bijoy Nandan 2014; Nriagu and Pacyna 1988). The development of rapid and efficient biological monitoring technology to evaluate the effects of heavy metal contamination is an urgent scientific problem needed to be solved (Dai et al. 2014; Torres et al. 2008). It is showed that the nontarget organisms especially microalgae were frequently useful in providing information on the physicochemical characteristics of the aquatic environment (Celekli et al. 2017).
Microalgae exhibit various physiological and biochemical responses to changes in water quality (Leung et al. 2017). These include inhibition of growth and damage to photosynthetic pigments including chlorophyll a. It has long been known that such changes can be used as indicators of environmental stress (Allen et al. 1983). In addition, the heavy metal pollutants can induce oxidative stress by generating reactive oxygen species (ROS) in microalgae. For example, Choudhary et al. (2007) reported that heavy metal stress is closely associated with the induction of oxidative stress biomarkers in Spirulina. Pollutants in the environment can lead to the inducement or inhibition of the antioxidant enzyme activity in aquatic organisms; therefore, such enzymes can be used as biomarkers of the toxicity of these pollutants (Lionetto et al. 2003; Monserrat et al. 2007). Superoxide dismutase (SOD) is an enzyme which can be induced by exogenous pollutants such as heavy metals and pesticide and has been used as a biomarker to detect oxidative stress in plants (Ibrahim et al. 2014; Piotrowska-Niczyporuk et al. 2012). Furthermore, morphological changes in algae can also be used as indicators of environmental pollution (Adshead-Simonsen et al. 1981; Cattaneo et al. 2004).
Diatoms are abundant in terrestrial, freshwater, and marine habitats and are a predominant group of microalgae (Torres et al. 2008). They play important roles in the food chain as primary producers, thereby sustaining food webs (Armbrust et al. 2004). Due to their cosmopolitan distribution, short life cycles, and rapid response to environmental perturbations, they are commonly employed in ecotoxicological and environmental studies for evaluating the effects of chemicals and other stressors in the marine environment (Falasco et al. 2009; Guo et al. 2013; Pandey et al. 2014).
Regarding the diatoms’ toxic responses to a variety of heavy metals, there are several reports available from physiological endpoints such as growth, photosynthetic efficiency, and antioxidant enzyme activity. For example, cadmium exposure can lead to inhibition of cell growth in Phaeodactylum tricornutum (Torres et al. 2000). Increasing heavy metal concentrations can result in a decrease in chlorophyll and induce cellular superoxide dismutase in Odontella mobiliensis, Nitzschia palea, and Chaetoceros calcitrans (Anu et al. 2016; Branco et al. 2010; Manimaran et al. 2012). At the molecular level, it has been reported that exposure to heavy metals including copper and nickel can induce the stress-associated biomarker HSP70/90 in Ditylum brightwellii (Guo et al. 2013). Diatoms subjected to heavy metal stress also exhibit morphological changes, including changes in valve outline, striae pattern, costae and septae modification of the raphe, and raphe canal pattern (Gautam et al. 2017). These morphological changes can be excellent specific indicators of metal contamination (Cattaneo et al. 2004). Benthic diatoms in particular have been used in a number of monitoring studies and for a variety of different stressors (Bellinger et al. 2006; Szczepocka and Szulc 2009). However, information regarding the interacting effect of copper or mercury on benthic diatoms is still limited.
Halamphora veneta is a widely distributed freshwater benthic diatom that can be easily cultured in the laboratory (Dedić et al. 2015; Novais et al. 2014). In this study, H. veneta was used as a test organism to elucidate the toxicity response of copper and mercury exposure under different treatment concentrations. An integrative measurement of growth, photosynthetic efficiency, enzyme activity, molecular responses, and morphological change was employed to reveal the biochemical defense mechanisms used by this diatom to cope with heavy metal stress. We also attempted to evaluate the best potential biomarkers in diatoms for risk assessment in aquatic ecosystems.
Materials and methods
Diatom species and culture conditions
H. veneta was obtained from the Institute of Hydrobiology, Chinese Academy of Sciences and cultured by the Aquatic Laboratory, Harbin Normal University, China, as follows: Cells of H. veneta were inoculated into 500 mL sterile flasks containing 200 mL modified CSI medium (Watanabe et al. 1988) and incubated at 20 ± 1 °C with 12:12 h light-dark photoperiod using a cool-white fluorescent light (4000 lx/cm2). The composition of nitrate, phosphate, silicate, trace metals, and vitamins used in the CSI medium is presented in Table 1. All the flasks were shaken three times per day to keep the diatoms under good growth conditions (Foster et al. 2008). Diatoms in the exponential growth phase were harvested for the following tests.
Toxicity treatments
H. veneta cells were exposed to Cu (0, 0.1, 0.2, 0.5, 1, and 2 mg L−1) and Hg (0, 0.1, 0.5, 1, 2, and 5 mg L−1), with each dose series being tested at four time intervals (24, 48, 72, and 96 h). Each treatment was performed in triplicate in 500-mL flasks. During the acute toxicity experiment, the growth inhibition rates, chlorophyll a content, SOD activity, and gene expression of H. veneta were measured at each time interval.
Growth rate test
Samples of 250 mL were taken at each time interval (24, 48, 72, and 96 h). Optical density values were measured using UV-1700 UV/vis spectrophotometer (Unico Instrument, Shanghai, China) at 540 nm. The growth rate was calculated by the logistic equation (Wang et al. 2010):
where μ i-j is the average growth rate from time i to j, X i is the optical density at time i, and X j is the optical density at time j.
Determination of chlorophyll a content
Measurements of chlorophyll a (Chl a) content were applied as previously described (Harborne 1998). In brief, triplicates of 8 mL blended cultures were centrifuged at 4000×g for 10 min every 24 h. After the supernatant was removed, 5 mL acetone was added to the retentate, and the mixture was ultrasonically disrupted for about 30 min and placed in the dark at 4 °C for 24 h. The methanol-extracted samples were centrifuged at 4000×g for 10 min and the supernatants were transferred into tubes. The absorbance value at 646 and 663 nm were measured. The Chl a content was computed by the following equation:
where A 663 is the absorbance value at 646 nm and A 646 is the absorbance value at 646 nm.
Assay of total superoxide dismutase content
Total superoxide dismutase (T-SOD) activity (including Mn-SOD and CuZn-SOD) was determined according to Ji (1991), using an enzymatic method by using the SOD assay kit (Nanjing Jiancheng, China). At each time interval and each treatment with heavy metals, 200 mL of cultured diatoms was centrifuged at 6000×g for 5 min, and then diatoms were washed in 0.1 mol/L PBS buffer. Assay conditions were 65 μmol phosphate buffer, pH 7.8, 1 μmol hydrochloric hydroxylamine, 0.75 μmol xanthine, and 2.3 × 10−3 IU xanthine dismutase. One hundred microliters of the supernatant was incubated in the system for 40 min at 37 °C, and the reaction was terminated with 2 mL 3.3 g L−1 p-aminobenzene sulfonic acid and 10 g L−1 naphthylamine. An SOD unit is defined as the amount of enzyme that inhibits the superoxide-induced oxidation (monitored at 550 nm) by 50%.
The SOD activity was calculated as the following equation:
Quantitative analysis of the messenger RNA expression of superoxide dismutase enzymes by real-time PCR
After exposure to heavy metals, cells of the treated and control groups were harvested, and the total RNA was extracted from the diatoms by TranZol Up (TransGen Biotech, Beijing, China) according to the manufacturer’s protocol. The total RNA integrity was confirmed using 1% formaldehyde/agarose gel with ethidium bromide (EtBr) staining and UV transilluminator (Bio-Rad, USA). The quality of RNA samples was determined by using Beckman DU800 spectrophotometric measurements of the ratio of absorbance 260 and 280 nm (A 260/280) in a range from 1.8 to 2.0. The cDNA was synthesized from 1 μg of the total RNA, using a kit of Transcript One-Step gDNA Removal and cDNA Synthesis Supermix (TransGen Biotech, Beijing, China). Real-time PCR assays (25 μL) of each individual sample were run in triplicate wells using UltraSYBR Mixture (with ROX) on an Applied Biosystems 7500 Fast Real-Time PCR System (ABI 7500, USA). The PCR cycling conditions comprised an initial polymerase activation step of 95 °C for 3 min, followed by 40 cycles of 95 °C for 30 s and 60 °C for 30 s, 72 °C for 30 s, and 72 °C for 10 min. Primers of sod and 18s are shown in Table 2. The housekeeping gene 18s was used as an internal standard. To confirm the primer specificity, melting curve analysis of amplification products was performed at the end of each PCR reaction to ensure that only one PCR product was amplified and detected. The relative expression levels of different genes were calculated using the 2−ΔΔCT method (Livak and Schmittgen 2001).
Scanning electron microscopy
To study the morphological changes induced by heavy metals, H. veneta was exposed to copper and mercury at concentrations of 1.5 and 4.8 mg L−1 (i.e. their own 96 h EC50), respectively. The diatoms were cleaned by acid treatment, then cells were harvested by centrifugation at 2500×g for 5 min, and frustules were washed by centrifugation several times with ultrapure water. The samples were sputter-coated with gold/palladium and viewed under a Hitachi S-4800 scanning electron microscope at 20 kV.
Statistical analysis
All experiments were performed in triplicate and results were presented as mean value ± standard deviation (SD). The experimental data was analyzed by SPSS version 18.0. The EC50 values with 95% confidence intervals were calculated by probit analysis. One-way ANOVA with Tukey’s test was used to determine whether the outcomes were significantly different from the control treatments without heavy metals (p < 0.05).
Results
Influence of heavy metal on H. veneta growth rates
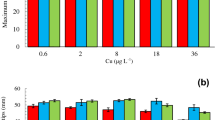
Exposure of H. veneta to copper and mercury induced changes in growth rate during the assay. Figure 1a shows the effects of copper on the growth rates of H. veneta. A clear dose-response trend was observed during the 96-h exposure. Compared with controls, growth rates were significantly lower in all tested treatment concentrations at 24 and 48 h of exposure (p < 0.05). In 0.1, 0.2, 0.5, 1, and 2 mg L−1 copper treatments, growth rates decreased by 28.2, 41.6, 43.8, 45.0, and 70.1% at 24 h and by 47.9, 58.0, 46.6, 47.1, and 64.8% at 48 h compared with the control group, respectively. However, after 48 h exposure, the growth rate of H. veneta was significantly lower in the treatments with low concentrations relative to the controls (0.1, 0.2 mg L−1), and there was no significant difference between the other concentrations at 48 and 96 h (p < 0.05). Figure 1b illustrates the effects of mercury on the growth rates of H. veneta. In 2 and 5 mg L−1 mercury treatments, growth of H. veneta was lower compared with the controls at 24 h exposure (p < 0.05). There was no significant difference between the controls and the treatments with mercury at 48 h exposure (p < 0.05). After 72 h exposure, mercury significantly inhibited the growth of H. veneta in all treatment concentrations (p < 0.05).
Effects of heavy metal on chlorophyll a content
The effects of copper and mercury on the chlorophyll a content differed in H. veneta. As shown in Fig. 2a, the Chl a content of H. veneta was significantly increased at higher concentrations of copper (0.2, 0.5, and 1 mg L−1), at 24 and 96 h exposure, compared to the control group. The Chl a content was 3.6, 3.12, and 2.65 mg L−1 in 0.2, 0.5, and 1 mg L−1 copper treatments, respectively, which were higher by 68.2, 45.8, and 19.2%, respectively, compared with that of the controls at 24 h exposure. In addition, Chl a content was 3.93 mg L−1 in 0.2 mg L−1 copper, significantly increased by 47.2% compared with that of the controls at 96 h exposure (Fig. 2a, p < 0.05). Meanwhile, the Chl a content of H. veneta was significantly lower at higher concentrations of copper (e.g., 1 mg L−1) at 48 and 72 h. Figure 2b indicates that the Chl a content of H. veneta was obviously affected by mercury at higher concentration during 96 h exposure relative to the controls. But the Chl a content in 0.1 mg L−1 mercury treatment was significantly higher than that of the controls at 72 and 96 h exposure. The Chl a content in 0.1 mg L−1 mercury treatment was significantly higher, i.e., by 33.1 and 69.3%, compared with that of the controls at 72 and 96 h exposure, respectively (Fig. 2b, p < 0.05).
Effects of heavy metal on SOD enzyme activities
SOD activities increased gradually with increasing treatment concentrations (Fig. 3). For the 0.5 and 2 mg L−1 copper treatments, SOD activity was higher by 92.5 and 57.6%, respectively, compared to the control at 24 h exposure (Fig. 3a, p < 0.05). At 48 h, SOD activity was higher in all the tested concentrations, i.e., by 95.4, 110.1, 97, 91, and 105.8% compared with the control at 0.1, 0.2, 0.5, 1, and 2 mg L−1 copper, respectively. SOD activity in H. veneta did not increase significantly in copper treatments at 72 h (p < 0.05) but was significantly higher at 96 h, i.e., by 70.7 and 57.8% in 0.1 and 0.2 mg L−1 copper treatments (p < 0.05), respectively, relative to the controls (Fig. 3a). As shown in Fig. 3b, mercury caused a significant increase of SOD activity, by 32.7, 36.9, and 38.9% in 0.1, 0.5, and 5 mg L−1, respectively, at 24 h; by 39.8, 79.3, and 51.3% in 0.1, 0.5, and 2 mg L−1, respectively, at 48 h; and by 54.7 and 37.3% in 0.1 mg L−1 at 72 and 96 h exposure, respectively (p < 0.05, Fig. 3b). By contrast, SOD activity was significantly lower, i.e., by 73.4 and 55.6% in 5 mg L−1, at 72 and 96 h mercury exposure, respectively (p < 0.05, Fig. 3b).
Messenger RNA relative expression levels of SOD
The effects of copper on SOD messenger RNA (mRNA) expression were significantly decreased in H. veneta during 96 h exposure, whereas SOD expression level increased in lower concentrations of mercury with increasing times of exposure (Fig. 4). SOD mRNA levels decreased rapidly and were statistically lower than controls after 96 h of Cu exposure (p < 0.01), except the concentration of 0.5 mg L−1 (Fig. 4a). In contrast to copper treatment, the SOD mRNA levels after 96 h of Hg exposure at different time points under different concentrations did not show a trend of change (Fig. 4b). At 24 h from the beginning of the exposure, mRNA accumulation was temporarily higher than controls at 0.5, 2, and 5 mg L−1 (p < 0.01). Then, SOD mRNA levels significantly increased over the controls after 48 h of Hg exposure at the lower concentration of 0.1 mg L−1 (p < 0.01). In addition, significantly decreased SOD mRNA levels compared to the controls after 48 h of Hg exposure were observed at 0.5, 1, and 5 mg L−1 (p < 0.01). At 72 h exposure, mRNA accumulation was temporarily higher than controls at 1 and 2 mg L−1, lower than the controls at 5 mg L−1 (p < 0.01). At 96 h exposure, the mRNA level was significantly higher than the controls at 2 mg L−1 (p < 0.01).
Morphological changes after heavy metal treatments
The H. veneta cells in control cultures had a semilanceolate, or slightly concave, shape with both ends narrowly rounded or slightly curved inward (Fig. 5). Cells treated with copper were not conspicuously deformed (Fig. 6). However, after mercury treatment, the frustules were enlarged and irregularly deformed with the dorsal margin bulging, the ventral margin concave, and both ends slightly rounded (Fig. 7).
Discussion
Because heavy metals can affect the growth rate of diatoms, it has been suggested that this function can be used as an indicator of heavy metal pollution in water (Kim et al. 2013; Leung et al. 2017). Copper and mercury are common pollutants in many aquatic environments, especially those which are surrounded by densely populated areas (Anu et al. 2016; Mico et al. 2006; Nolde 2007). The toxicity of these two heavy metals to microalgae, such as the marine diatom (C. calcitrans) and the green algae (Tetraselmis chuii and Chlorella sorokiniana), has previously been reported (Anu et al. 2016; Davarpanah and Guilhermino 2015; Gomez-Jacinto et al. 2015). However, there are relatively few data on the acute effects of copper and mercury to freshwater benthic diatoms although Johnson et al. (2007) found that the growth of Nitzschia closterium was acclimated in culture medium containing 5 or 25 μg L−1 copper. In the present study, the growth rate of H. veneta showed differing responses to copper and mercury. It is noteworthy that the concentration of copper in this study was in the range 0.1–2 mg L−1, which was much greater than that used previously for acclimating N. closterium (Johnson et al. 2007). One reason for this was that H. veneta could not be fully adapted to copper stress in the present work. Furthermore, the sensitivity of H. veneta to copper varied with the duration of exposure, the growth rate being significantly lower after 24 and 48 h compared with the control group. This means that copper, at initial treatment time, will give rise to a greater growth inhibition compared with those exposed for longer durations, thereby disrupting the function of the diatoms from the outset. This finding is consistent with of Anu et al. (2016), who reported that the growth rate of C. calcitrans was significantly inhibited by copper at concentrations ≥ 0.18 mg L−1 (Anu et al. 2016). However, it is of no significant effect in H. veneta at 96 h of copper exposure in this study. It has been established that toxic effects induced by copper depend on many factors, including the species used for the test, initial cell density, light illumination, temperature, media used, and exposure time (Manimaran et al. 2012). Our findings are consistent with the fact that exposure time is one of the most important factors in the impact of heavy metals on diatoms. In addition, our results may indicate that benthic diatoms H. veneta may develop some tolerance for copper with the passage of time.
In contrast to copper, mercury inhibits the growth rate of H. venta most at concentrations of greater than 2 mg L−1 (not the lower concentrations), at 24 h of exposure. At 48 h exposure, there was no significant decrease in the growth rate of H. venta (except for 5 mg L−1 mercury treated). In addition, very low concentrations of mercury were found to inhibit the growth rate of H. veneta at 72 h and 96 h exposure. This is consistent with Horvatić and Peršić (2007) who reported that the growth of the marine diatom P. tricornutum was inhibited by mercury at low concentrations of 0.01 mg L−1. In a study of another marine diatom, Thalassiosira weissflogii, growth rate gradually decreased from the concentration of 5 to 500 nM under mercury treatments over a 6-day exposure period (Morelli et al. 2009). Furthermore, our results showed that mercury stress has a similar trend at 72 and 96 h on diatom growth rates. Some reports have indicated that diatom growth does not change significantly with time during chronic mercury exposure experiments (Horvatić and Peršić (2007); Morelli et al. 2009). This phenomenon might be attributed to a modest allocation of cellular components in diatom which might be integrated into other processes leading to a reduction in damage to cell and enhanced resistance (Calabrese 2015).
Another parameter that has been used in plants and algae as an indicator of environmental pollutants is chlorophyll a content (Lau and Lane 2002). It is noteworthy that, in the present study, the chlorophyll a content of H. veneta was higher in the copper and mercury treatments than in the controls. The increase in chlorophyll content following exposure to copper contrasted with previous reports. Dao and Beardall (2016), for example, found that Chl a content in Scenedesmus acutus and Chlorella sp. declined with increasing heavy metal concentrations at 24 h exposure. Chen et al. (2012) and Branco et al. (2010) both reported that the content of photosynthetic pigments, including Chl a, Chl b, and carotenoids, increased in algae exposed to cadmium and titanium dioxide, respectively. These findings show that the response of chlorophyll content to metal stress varies among different species and with metal concentration. Furthermore, it has also been reported that in algae exposed to toxic compounds, other pigments can convert to Chl a leading to an increase in Chl a content (Fang et al. 1998). Chen et al. (2012) suggested that it might be due to increased ROS which was caused by exogenous substances such as exposure to contaminants. In the present study, we also observed higher Chl a levels in H. veneta exposed to copper and mercury compared to the control group, which might be caused by resistance response of diatoms to heavy metals. Knowledge on the tolerance and response of microalgae to heavy metals is important in order to determine the potential utility of microalgae for pollution assessment. Although several previous studies have focused on the biochemical responses of microalgae to heavy metals, the effects of copper and mercury on antioxidant enzyme activity coupled with gene expression levels are poorly known (Glanemann et al. 2003). It is established that ROS such as superoxide, hydroxyl radicals, and hydrogen peroxide are produced in microalgae on being exposed to heavy metal contamination (Dao and Beardall 2016; Li et al. 2006). Although ROS play an important role in host defense, overproduction and residuals can cause oxidative damage (Sharma et al. 2012). SOD is an important component in preventing oxidative damage in microalgae and is widely used as an indicator of environmental stress (Blaise and Menard 1998; Peterson and Stauber 1996). In the present study, SOD activity in H. veneta was induced by both copper and mercury treatments. This finding is consistent with several previous studies including Li et al. (2006) and Verlecar et al. (2007) for the marine dinoflagellate Pavlova viridis and Choudhary et al. (2007) for the cyanobacterium Spirulina platensis. Based on the findings of the present study, we concluded that SOD activity in the benthic diatom H. veneta is a reliable biomarker of oxidative stress.
The present study also investigated the expression pattern of the SOD gene in H. veneta at different concentrations of copper and mercury. Previous studies have demonstrated changes in expression of the SOD gene in the ciliate Euplotes vannus induced by exposure to nitrofurazone (Hong et al. 2015) and in the copepod Tigriopus japonicus induced by exposure to copper, zinc, and mercury (Kim et al. 2015). By contrast, SOD gene expression in T. japonicus was not affected by exposure to triphenyltin chloride (Yi et al. 2014). Lauritano et al. suggested that SOD gene expression is related both to the pollutant and to the duration of exposure (Lauritano et al. 2012). The present study showed that SOD gene expression was suppressed by exposure to copper at all concentrations except at 0.5 mg L−1. By contrast, SOD gene expression levels were significantly induced at lower concentrations of mercury (0.1, 0.5, 1, and 2 mg L−1) at 96 h. It has been suggested that the differences in gene expression might be explained by the structural differences between the various intracellular targets of these contaminants (Lauritano et al. 2012). In addition, the increased SOD gene expression level in H. veneta when exposed to mercury might imply that the cells have developed defense systems consisting of several antioxidant enzymes as protective mechanisms (Kim et al. 2013).
Previous studies have shown that morphological abnormalities in diatom frustules can be used as an effective tool for biomonitoring of heavy metal pollution in water bodies (Falasco et al. 2009; Pandey et al. 2014; Morin et al. 2012). Studies of raphe-bearing diatoms including Gomphonema parvulum and Pinnularia conica showed deformities at elevated concentrations of heavy metals (Duong et al. 2008; Victoria and Gomez 2010). These findings are consistent with the present study, that is heavy metal stress results in deformation of the frustule in H. veneta. Pandey et al. (2014) observed modifications of the raphe in diatoms exposed to Cu. In the present study, the morphological changes in H. veneta were more pronounced in cells exposed to mercury than to copper, showing a deformity of shape and increase in size of the frustule. We speculated that mercury could directly interact with the microalgal cells surface, leading to physical effects, such as cell membrane disruption. In a previous study, Branco et al. (2010) reported that a higher concentration than control of cadmium was found loosely bound to the frustule, and concluded that this was evidence of an effective metal-exclusion mechanism that binds the metal to the extracellular structures preventing its entrance into the cell. Morphological changes were far less pronounced in cells exposed to copper. Analysis of the frustule will be required in order to demonstrate the presence of copper and the possible existence of a mechanism for excluding it from the cells of H. veneta.
Conclusions
To the best of our knowledge, this is the first study of the effects of copper and mercury on a benthic diatom. Based on our findings, we infer the following conclusions:
-
1.
Copper will lead to a greater growth inhibition, destroying diatom and ecology at the very beginning, and exposure time is one of the important factors.
-
2.
High accumulation of chlorophyll a in the cell is indicative of resistance of the diatom to heavy metals.
-
3.
SOD activity and gene expression are reliable biomarkers of oxidative stress in H. veneta.
-
4.
Change in the morphology of the frustule of H. veneta is a potential indicator of mercury contamination in water.
References
Adshead-Simonsen PC, Murray GE, Kushner DJ (1981) Morphological changes in the diatom, Tabellaria flocculosa, induced by very low concentrations of cadmium. Bull Environ Contam Toxicol 26:745–748
Ahsan N, Lee DG, Lee SH, Kang KY, Lee JJ, Kim PJ, Yoon HS, Kim JS, Lee BH (2007) Excess copper induced physiological and proteomic changes in germinating rice seeds. Chemosphere 67:1182–1193
Allen HE, Blatchley C, Brisbin TD (1983) An algal assay method for determination of copper complexation capacities of natural waters. Bull Environ Contam Toxicol 30:448–455
Anu PR, Bijoy Nandan S, Jayachandran PR, Don Xavier ND (2016) Toxicity effects of copper on the marine diatom, Chaetoceros calcitrans. Reg Stud Mar Sci 8(Part 3):498–504
Armbrust EV, Berges JA, Bowler C et al (2004) The genome of the diatom Thalassiosira pseudonana: ecology, evolution, and metabolism. Science 306:79–86
Bellinger BJ, Cocquyt C, O’Reilly CM (2006) Benthic diatoms as indicators of eutrophication in tropical streams. Hydrobiologia 573:75–87
Blaise C, Menard L (1998) A micro-algal solid-phase test to assess the toxic potential of freshwater sediments, 33. National Water Research Institute, Burlington 19 pp
Branco D, Lima A, Almeida SF, Figueira E (2010) Sensitivity of biochemical markers to evaluate cadmium stress in the freshwater diatom Nitzschia palea (Kutzing) W. Smith. Aquat Toxicol 99:109–117
Calabrese EJ (2015) Hormesis: principles and applications. Homeopathy J Fac Homeopathy 104:69–82
Campbell LM, Norstrom RJ, Hobson KA, Muir DC, Backus S, Fisk AT (2005) Mercury and other trace elements in a pelagic Arctic marine food web (Northwater Polynya, Baffin Bay). Sci Total Environ 351-352:247–263
Castruita M, Casero D, Karpowicz SJ, Kropat J, Vieler A, Hsieh SI, Yan W, Cokus S, Loo JA, Benning C, Pellegrini M, Merchant SS (2011) Systems biology approach in Chlamydomonas reveals connections between copper nutrition and multiple metabolic steps. Plant Cell 23:1273–1292
Cattaneo A, Couillard Y, Wunsam S, Courcelles M (2004) Diatom taxonomic and morphological changes as indicators of metal pollution and recovery in Lac Dufault (Québec, Canada). J Paleolimnol 32:163–175
Celekli A, Kapi E, Soysal C, Arslanargun H, Bozkurt H (2017) Evaluating biochemical response of filamentous algae integrated with different water bodies. Ecotoxicol Environ Saf 142:171–180
Chen L, Zhou L, Liu Y, Deng S, Wu H, Wang G (2012) Toxicological effects of nanometer titanium dioxide (nano-TiO2) on Chlamydomonas reinhardtii. Ecotoxicol Environ Saf 84:155–162
Choudhary M, Jetley UK, Abash Khan M, Zutshi S, Fatma T (2007) Effect of heavy metal stress on proline, malondialdehyde, and superoxide dismutase activity in the cyanobacterium Spirulina platensis-S5. Ecotoxicol Environ Saf 66:204–209
Ciji PP, Bijoy Nandan S (2014) Toxicity of copper and zinc to Puntius parrah (Day, 1865). Mar Environ Res 93:38–46
Dai YJ, Jia YF, Chen N, Bian WP, Li QK, Ma YB, Chen YL, Pei DS (2014) Zebrafish as a model system to study toxicology. Environ Toxicol Chem 33:11–17
Dao LH, Beardall J (2016) Effects of lead on growth, photosynthetic characteristics and production of reactive oxygen species of two freshwater green algae. Chemosphere 147:420–429
Davarpanah E, Guilhermino L (2015) Single and combined effects of microplastics and copper on the population growth of the marine microalgae Tetraselmis chuii. Estuar Coast Shelf Sci 167(Part A):269–275
Dedić A, Plenković-Moraj A, Borojević Koraljka K, Hafner D (2015) The first report on periphytic diatoms on artificial and natural substrate in the karstic spring Bunica, Bosnia and Herzegovina. Acta Botanica Croatica, pp. 393
Duong TT, Morin S, Herlory O, Feurtet-Mazel A, Coste M, Boudou A (2008) Seasonal effects of cadmium accumulation in periphytic diatom communities of freshwater biofilms. Aquat Toxicol 90:19–28
Falasco E, Bona F, Badino G, Hoffmann L, Ector L (2009) Diatom teratological forms and environmental alterations: a review. Hydrobiologia 623:1–35
Fang Z, Bouwkamp J, Solomos T (1998) Chlorophyllase activities and chlorophyll degradation during leaf senescence in nonyellowing mutant and wild type of Phaseolus vulgaris. J Exp Bot 49:503–510
Fang H, Huang L, Wang J, He G, Reible D (2016) Environmental assessment of heavy metal transport and transformation in the Hangzhou Bay, China. J Hazard Mater 302:447–457
Foster S, Thomson D, Maher W (2008) Uptake and metabolism of arsenate by anexic cultures of the microalgae Dunaliella tertiolecta and Phaeodactylum tricornutum. Mar Chem 108:172–183
Gautam S, Pandey LK, Vinayak V, Arya A (2017) Morphological and physiological alterations in the diatom Gomphonema pseudoaugur due to heavy metal stress. Ecol Indic 72:67–76
Glanemann C, Loos A, Gorret N, Willis LB, O’Brien XM, Lessard PA, Sinskey AJ (2003) Disparity between changes in mRNA abundance and enzyme activity in Corynebacterium glutamicum: implications for DNA microarray analysis. Appl Microbiol Biotechnol 61:61–68
Gomez-Jacinto V, Garcia-Barrera T, Gomez-Ariza JL, Garbayo-Nores I, Vilchez-Lobato C (2015) Elucidation of the defence mechanism in microalgae Chlorella sorokiniana under mercury exposure. Identification of Hg-phytochelatins. Chem Biol Interact 238:82–90
Goyer R, Klaassen CD, Waalkes MP (1995) Metal toxicology. Academic, San Diego, pp 35–37
Guo R, Lee MA, Ki JS (2013) Different transcriptional responses of heat shock protein 70/90 in the marine diatom Ditylum brightwellii exposed to metal compounds and endocrine-disrupting chemicals. Chemosphere 92:535–543
Harborne JB (1998) Phytochemical methods. A guide to modern techniques of plant analysis. Chapman & Hall, London
Hong Y, Liu S, Lin X, Li J, Yi Z, Al-Rasheid KAS (2015) Recognizing the importance of exposure-dose-response dynamics for ecotoxicity assessment: nitrofurazone-induced antioxidase activity and mRNA expression in model protozoan Euplotes vannus. Environ Sci Pollut Res Int 22:9544–9553
Horvatić J, Peršić V (2007) The effect of Ni2+, Co2+, Zn2+, Cd2+ and Hg2+ on the growth rate of marine diatom Phaeodactylum tricornutum Bohlin: microplate growth inhibition test. Bull Environ Contam Toxicol 79:494–498
Ibrahim OM, Dogru M, Matsumoto Y, Igarashi A, Kojima T, Wakamatsu TH, Inaba T, Shimizu T, Shimazaki J, Tsubota K (2014) Oxidative stress induced age dependent meibomian gland dysfunction in Cu, Zn-superoxide dismutase-1 (Sod1) knockout mice. PLoS One 9:e99328
Ji JP (1991) An ultramicroanalytic and rapid method for determination of superoxide dismutase activity. J Nanjing Railway Med Coll 10:27–30
Johnson HL, Stauber JL, Adams MS, Jolley DF (2007) Copper and zinc tolerance of two tropical microalgae after copper acclimation. Environ Toxicol 22:234–244
Kim S, Park JE, Cho YB, Hwang SJ (2013) Growth rate, organic carbon and nutrient removal rates of Chlorella sorokiniana in autotrophic, heterotrophic and mixotrophic conditions. Bioresour Technol 144:8–13
Kim BM, Lee JW, Seo JS, Shin KH, Rhee JS, Lee JS (2015) Modulated expression and enzymatic activity of the monogonont rotifer Brachionus koreanus Cu/Zn- and Mn-superoxide dismutase (SOD) in response to environmental biocides. Chemosphere 120:470–478
Lau SS, Lane SN (2002) Biological and chemical factors influencing shallow lake eutrophication: a long-term study. Sci Total Environ 288:167–181
Lauritano C, Procaccini G, Ianora A (2012) Gene expression patterns and stress response in marine copepods. Mar Environ Res 76:22–31
Leung PT, Yi AX, Ip JC, Mak SS, Leung KM (2017) Photosynthetic and transcriptional responses of the marine diatom Thalassiosira pseudonana to the combined effect of temperature stress and copper exposure. Mar Pollut Bull. https://doi.org/10.1016/j.marpolbul.2017.03.038
Li M, Hu C, Zhu Q, Chen L, Kong Z, Liu Z (2006) Copper and zinc induction of lipid peroxidation and effects on antioxidant enzyme activities in the microalga Pavlova viridis (Prymnesiophyceae). Chemosphere 62:565–572
Lionetto MG, Caricato R, Giordano ME, Pascariello MF, Marinosci L, Schettino T (2003) Integrated use of biomarkers (acetylcholinesterase and antioxidant enzymes activities) in Mytilus galloprovincialis and Mullus barbatus in an Italian coastal marine area. Mar Pollut Bull 46:324–330
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408
MacDougal KC, Johnson MD, Burnett KG (1996) Exposure to mercury alters early activation events in fish leukocytes. Environ Health Perspect 104:1102–1106
Manimaran K, Karthikeyan P, Ashokkumar S, Ashok Prabu V, Sampathkumar P (2012) Effect of copper on growth and enzyme activities of marine diatom, Odontella mobiliensis. Bull Environ Contam Toxicol 88:30–37
Marks B, Peters A, McGough D (2017) Aquatic environmental risk assessment of manganese processing industries. Neurotoxicology 58:187–193
Mico C, Recatala L, Peris M, Sanchez J (2006) Assessing heavy metal sources in agricultural soils of an European Mediterranean area by multivariate analysis. Chemosphere 65:863–872
Monserrat JM, Martinez PE, Geracitano LA, Amado LL, Martins CM, Pinho GL, Chaves IS, Ferreira-Cravo M, Ventura-Lima J, Bianchini A (2007) Pollution biomarkers in estuarine animals: critical review and new perspectives. Comp Biochem Physiol Toxicol Pharmacol 146:221–234
Morelli E, Ferrara R, Bellini B, Dini F, Di Giuseppe G, Fantozzi L (2009) Changes in the non-protein thiol pool and production of dissolved gaseous mercury in the marine diatom Thalassiosira weissflogii under mercury exposure. Sci Total Environ 408:286–293
Morin S, Cordonier A, Lavoie I et al (2012) Consistency in diatom response to metal-contaminated environments. In: Guasch H, Ginebreda A, Geiszinger A (eds) Emerging and priority pollutants in rivers: bringing science into river management plans. Springer, Berlin, pp 117–146
Mzimela HM, Wepener V, Cyrus DP (2003) Seasonal variation of selected metals in sediments, water and tissues of the groovy mullet, Liza dumerelii (Mugilidae) from the Mhlathuze Estuary, South Africa. Mar Pollut Bull 46:659–664
Nolde E (2007) Possibilities of rainwater utilisation in densely populated areas including precipitation runoffs from traffic surfaces. Desalination 215:1–11
Novais M, Mm M, Rosado J, Ls D, Hoffmann L, Ector L (2014) Diatoms of temporary and permanent watercourses in southern Europe (Portugal). River Res Appl 30:1216–1232
Nriagu JO, Pacyna JM (1988) Quantitative assessment of worldwide contamination of air, water and soils by trace metals. Nature 333:134–139
Ouyang Y, Parajuli PB, Li Y, Leininger TD, Feng G (2017) Identify temporal trend of air temperature and its impact on forest stream flow in Lower Mississippi River Alluvial Valley using wavelet analysis. J Environ Manag 198:21–31
Pandey LK, Kumar D, Yadav A, Rai J, Gaur JP (2014) Morphological abnormalities in periphytic diatoms as a tool for biomonitoring of heavy metal pollution in a river. Ecol Indic 36:272–279
Peterson SM, Stauber JL (1996) New algal enzyme bioassay for the rapid assessment of aquatic toxicity. Bull Environ Contam Toxicol 56:750–757
Piotrowska-Niczyporuk A, Bajguz A, Zambrzycka E, Godlewska-Zylkiewicz B (2012) Phytohormones as regulators of heavy metal biosorption and toxicity in green alga Chlorella vulgaris (Chlorophyceae). Plant Physiol Biochem 52:52–65
Raimundo J, Pereira P, Vale C, Canário J, Gaspar M (2014) Relations between total mercury, methylmercury and selenium in five tissues of Sepia officinalis captured in the south Portuguese coast. Chemosphere 108:190–196
Sharma P, Jha AB, Dubey RS, Pessarakli M (2012) Reactive oxygen species, oxidative damage and antioxidative defense mechanism in plants under stressful conditions. J Bot 2012:1–6. https://doi.org/10.1155/2012/217037
Szczepocka E, Szulc B (2009) The use of benthic diatoms in estimating water quality of variously polluted rivers. Oceanol Hydrobiol Stud 38:17–26
Torres E, Cid A, Herrero C, Abalde J (2000) Effect of cadmium on growth, ATP content, carbon fixation and ultrastructure in the marine diatom Phaeodactylum tricornutum Bohlin. Water Air Soil Pollut 117:1–14
Torres MA, Barros MP, Campos SC, Pinto E, Rajamani S, Sayre RT, Colepicolo P (2008) Biochemical biomarkers in algae and marine pollution: a review. Ecotoxicol Environ Saf 71:1–15
Verlecar XN, Jena KB, Chainy GBN (2007) Biochemical markers of oxidative stress in Perna viridis exposed to mercury and temperature. Chem Biol Interact 167:219–226
Victoria SM, Gomez N (2010) Assessing the disturbance caused by an industrial discharge using field transfer of epipelic biofilm. Sci Total Environ 408:2696–2705
Wang L, Min M, Li Y, Chen P, Chen Y, Liu Y, Wang Y, Ruan R (2010) Cultivation of green algae Chlorella sp. in different wastewaters from municipal wastewater treatment plant. Appl Biochem Biotechnol 162:1174–1186
Watanabe MM, Kasai F, Sudo R (1988) NIES-collection list of strains second edition 1988 microalgae and protozoa. Microbial Culture Collection, the National Institute for Environmental Studies, Tsukuba, 148 pp
Yi AX, Han J, Lee J-S, Leung KMY (2014) Ecotoxicity of triphenyltin on the marine copepod Tigriopus japonicus at various biological organisations: from molecular to population-level effects. Ecotoxicology 23:1314–1325
Zhang F-S, Nriagu JO, Itoh H (2005) Mercury removal from water using activated carbons derived from organic sewage sludge. Water Res 39:389–395
Zhang H, Jiang Y, Ding M, Xie Z (2017) Level, source identification, and risk analysis of heavy metal in surface sediments from river-lake ecosystems in the Poyang Lake, China. Environ Sci Pollut Res Int. https://doi.org/10.1007/s11356-017-9855-y
Funding
This research was supported by The National Natural Science Foundation of China (31470308, 31601866) and the Program of Natural Science of Heilongjiang Province of the People’s Republic of China (C2015021).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Mu, W., Jia, K., Liu, Y. et al. Response of the freshwater diatom Halamphora veneta (Kützing) Levkov to copper and mercury and its potential for bioassessment of heavy metal toxicity in aquatic habitats. Environ Sci Pollut Res 24, 26375–26386 (2017). https://doi.org/10.1007/s11356-017-0225-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-0225-6