Abstract
Cyanobacterial blooms aggravate with increasing temperature, and the increased concentrations of toxicants such as cyanotoxins and nitrite during bloom decay adversely affect the growth of aquatic animals. Heat-shock proteins (Hsps) are induced by a wide range of environmental stressors, including temperature and toxicants. In this study, Brachionus calyciflorus Pallas was exposed to different combined solutions of microcystin-LR (0, 10, 30, and 100 µg L−1) and nitrite (0, 1, 3, and 5 mg L−1) to evaluate their effects on the rotifer lifespan, the reproductive rate (R), and the responses of four Hsp genes at 20 °C, 25 °C, and 30 °C. Results revealed that single high doses of microcystin-LR (100 µg L−1) and nitrite (5 mg L−1) were harmful to the lifespan and reproduction of rotifers. Hormesis was induced by low doses of microcystin-LR (10–30 µg L−1) and nitrite (1–3 mg L−1). At different toxicant concentrations, the expression levels of Hsp40, Hsp60, Hsp70, and Hsp90 fluctuated, whereas reactive oxygen species (ROS) levels increased regardless of temperature. The two toxicants induced high levels of ROS production, which negatively affected the lifespan, R, and Hsp gene expression at 30 °C (p < 0.05). Microcystin-LR and nitrite exerted synergistic effects on the lifespan, R, ROS levels, and Hsp gene expression levels at 20 °C and 25 °C (p < 0.05) but had antagonistic effects on Hsp40 and Hsp60 expression levels at 30 °C (p > 0.05). Temperature, microcystin-LR, and nitrite had interactive effects on the lifespan, R, ROS levels, and Hsp gene expression levels (p < 0.05). The expression levels of Hsp genes are useful biomarkers of high-temperature exposure, and Hsp-mediated heat shock responses are important in microcystin-LR and nitrite stress tolerance of B. calyciflorus.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Temperature has a fundamental effect on organisms, and this influence exerts ecosystem-wide effects as the life-history strategies of individual species differ in response to temperature (Zhang et al. 2011; Henning-Lucass et al. 2016). As one prominent factor, temperature influences toxicity effects of contaminants on aquatic animals (Willming et al. 2013). Toxic cyanobacterial blooms have become increasingly common in freshwater ecosystems, mainly due to eutrophication and climate warming (Mowe et al. 2015). High water temperatures facilitate cyanobacterial blooms and the release of cyanotoxins (Lürling et al. 2017). The rapid degradation of toxic Microcystis with increasing temperature during daytime promotes the release of toxicants, such as cyanotoxins, resulting in severe water quality problems (Zhang et al. 2017). In aquatic ecosystems, temperature magnifies the effects of toxicants on zooplankton (Viñuela et al. 2011; Huang et al. 2012).
Microcystin-LR is one of the secondary metabolites of Microcystis, which can accumulate in the food chain and negatively affect organisms (Lahti et al. 1997). Microcystin-LR causes cellular damage by directly inhibiting the serine/threonine protein phosphatases PP1 and PP2A (Campos and Vasconcelos 2010). The hyperphosphorylation of PP2A by microcystin-LR induces a cascade of negative effects on cellular functions, including the regulation of phosphoproteins (e.g., P53 and MAPKs) and the creation of reactive oxygen species (ROS) (Mclellan and Manderville 2017). In general, the concentration of microcystin-LR in natural waters is below 200 µg L−1, but it may rise to 1800 µg L−1 during bloom decay (Jones and Orr 1994; Lahti et al. 1997).
Nitrite is a natural component of the nitrogen cycle in ecosystems, and its level can increase during severe cyanobacterial blooms (Lyu et al. 2013). Nitrite production is the process of a noncomplete oxidation of N-degrading products into nitrate due to the high consumption of oxygen during cyanobacterial decay. The toxic effects of nitrite include reducing extracellular chloride concentrations and muscle potassium content, and inducing lipid peroxidation and protein denaturation (Jensen 2003; Kroupová et al. 2016). The concentration of nitrite is below 50 μg L−1 in unpolluted waters, but it can reach as high as 46 mg L−1 or more due to eutrophication (Philips et al. 2002). The concentrations of nitrite and microcystin-LR reach 2.5 mg L−1 and 10–15 μg L−1, respectively, after the collapse of dense Microcystis blooms in certain areas of Lake Taihu, China (Zhang et al. 2010).
Microcystin-LR and nitrite can impair the growth of aquatic animals (Jiang et al. 2012; Liang et al. 2017). Two exposure routes exist for microcystin-LR during blooms. One is ingestion of toxin containing prey. The other route is via dissolved toxin exposure. Zooplanktons typically have a higher tolerance to dissolved microcystin-LR than to ingested microcystin-LR. During cyanobacterial decay, increased concentrations of dissolved microcystin-LR cause increased exposure in zooplankton and fish, resulting in adverse effects on the fitness and life-history traits of these animals (Yang et al. 2011; Zhang et al. 2011). Acute toxicity is the dose that causes lethal effects (mortality) short term (usually 1–2 days, measured with LD50/LC50, and determines if the organism survives or not). By contrast, chronic toxicity refers to doses that cause sublethal effects over long periods of time, with reproductive and life-history effects (measured with EC50 and determines if the organism grows/reproduces) (Ger et al. 2009; Lyu et al. 2013). Few studies have linked the toxic effects of microcystin-LR or nitrite on zooplankton in general (Jensen 2003; Huang et al. 2012; Kroupová et al. 2016). The reduced lifespan and impaired reproduction of cladoceran Daphnia obtuse with increased microcystin and nitrite concentrations have been confirmed (Yang et al. 2011). Molecular studies have been useful to obtain mechanistic insights into the tolerance of Daphnia to toxic Microcystis aeruginosa (Lyu et al. 2016, 2018) and estimate the genetic responses of copepod Acartia tonsa to heat shock (Petkeviciute et al. 2015).
Heat-shock proteins (Hsps) are useful biomarkers in the stress responses (e.g., high temperatures, altered pH, oxidative stress, toxicants, starvation, oxygen, and water deprivation) of organisms (Mukhopadhyay et al. 2003; Smith et al. 2012). Toxic substances in water induce the production of ROS (Kim et al. 2014), which lead to cytoskeletal modifications, general oxidative damages, lipid and protein damages, DNA damages, and apoptosis (Mclellan and Manderville 2017). Heat shock response is the coordinated activation of Hsp gene expression, which is an ubiquitous adaptation mechanism in organisms ranging from bacteria to mammals (Yang et al. 2014). Hsp40 targets proteins for proteasomal degradation in the cytosol by preventing their aggregation in mammalian cells (Fan et al. 2004). Hsp60 prevents protein denaturation under heat stress and is involved in stress protection in the mitochondria of eukaryotes (Song et al. 2016). Hsp70 is present in subcellular compartments and primarily binds to target proteins to modulate protein folding, transport, and repair in all animals (Mukhopadhyay et al. 2003). Hsp90 participates in the folding and maintenance of structural integrity and the proper regulation of a subset of cytosolic proteins in organisms, including aquatic animals (Sun et al. 2015). Although the effects of microcystin-LR and nitrite on zooplankton have been reported (Yang et al. 2011; Lyu et al. 2014), few literature used Hsp genes as markers to evaluate the interactive effects of these toxicants on rotifers. The combined impact of microcystin-LR and nitrite on the heat shock responses of rotifers is not yet fully understood.
As a dominant group of zooplankton, rotifers play a mediating role in the food web of aquatic ecosystems (Shah et al. 2015). Rotifers are sensitive to chemicals and environmental changes, making them useful as toxicological test models (Olah et al. 2017). The combined effects of microcystin-LR and nitrite on rotifers are rarely reported (Liang et al. 2017). The rotifer Brachionus calyciflorus, one of the major zooplankton groups in freshwater communities, can be utilized as an ecotoxicological test model for evaluating the risks of chemicals due to their short lifespan and rapid reproduction (Snell and Janssen 1995). Several studies have reported on the interactions between rotifers and cyanobacteria and focused on the life-history traits of rotifers in response to toxic Microcystis (Soares et al. 2010; Zhang and Geng 2012; Ger et al. 2016). However, the molecular mechanism behind the observed life-history effects of microcystin-LR and nitrite on rotifers is unknown.
This study evaluated Hsp gene expression in B. calyciflorus to characterize the ecotoxicological effects on rotifer life expectancy and reproduction in response to microcystin-LR and nitrite at variable temperatures. This investigation is necessary because increased temperatures exacerbate cyanobacterial blooms, which are the source of microcystins and nitrites in surface waters worldwide (Peng et al. 2018). The following hypotheses were tested: (1) temperature, microcystin-LR, and nitrite interaction affects the lifespan, reproduction, and heat shock responses of rotifers; (2) microcystin-LR and nitrite promote ROS production, thereby inducing Hsp gene expression; and (3) changes in the expression levels of Hsp genes are correlated with the life-history parameters of rotifers at different temperatures.
Materials and methods
Test organism
Rotifers B. calyciflorus Pallas 1766 were originally collected from Moon Lake, where toxic Microcystis blooms broke out, in Nanjing, China (32° 6′35.24″ N, 118° 54′32.71″ E) and continually cultured in the laboratory. Test animals were obtained by hatching eggs in the experiments. The advantage of resting eggs in neonate rotifers is that they can hatch under uniform physiological conditions (Snell and Janssen 1995). The tested rotifers monitored in the laboratory were used for acute toxicity tests, not chronic toxicity tests with long-term exposure coexistence. Neonates (< 2 h old) were collected directly from cultures in freshwater Environmental Protection Agency (EPA) medium, which was prepared using the formula from ASTM (2001): 96 mg of NaHCO3, 60 mg of CaSO4·H2O, 123 mg of MgSO4, and 4 mg of KCl in 1 L of deionized water at 25 °C and pH 7.8. Chlorella pyrenoidosa Chick, 1903 (3 × 106 cells mL−1) was used as rotifer feed. The alga was cultured in Bold’s Basal Medium in 5 L bags. The natural temperature range of the rotifers is from 10 to 30 °C, and the optimal culture temperature is approximately 25 °C. Rotifers and alga were cultured at 25 °C under fluorescent illumination at 2000 lx with 12 h:12 h light:dark photoperiod.
Experimental design
Pure microcystin-LR was obtained from Express Biotechnology Co., Ltd., Beijing, China. The purchased microcystin-LR (250 µg, purity ≥ 95% by high-performance liquid chromatography) was first diluted with 1 mL of distilled water to a stock solution of 250 µg mL−1 and then diluted further to the desired concentrations by using EPA medium. Nitrite was purchased from Kemiou Chemical Reagent Co., Ltd., Tianjin, China. NaNO2 was weighed to 1, 3, and 5 mg by using an electronic balance and then dissolved into 1 L of EPA medium to obtain the desired concentrations. The test solutions were stocked in small sealed glass bottles, and the effect of oxygen was not considered. The prepared concentrations of microcystin-LR and nitrite were mixed separately.
Microcystin-LR and nitrite concentrations were 0, 10, 30, and 100 µg L−1 (M0, M10, M30, and M100) and 0, 1, 3, and 5 mg L−1 (N0, N1, N3, and N5), respectively. The concentrations of microcystin-LR and nitrite were prepared during the experiments. The toxicant concentrations were set according to the observed dissolved concentrations of nitrite and microcystin-LR during the degradation of cyanobacterial blooms and on the 24 h LC50 values of the two toxicants (microcystin-LR LC50: 56.2 µg L−1, nitrite LC50: 4.6 mg L−1) for B. calyciflorus with reference to our previous measurements (Liang et al. 2017). These set concentrations of microcystin-LR and nitrite exist in seriously eutrophic waters during the collapse of highly toxic blooms (Lahti et al. 1997; Zhang et al. 2010).
Three temperatures (20 °C, 25 °C, and 30 °C) were set to determine the rotifer lifespan and reproduction, ROS levels, and Hsp gene mRNA expression. These temperatures were reported to increase rotifer sensitivity to toxicants and affect the physiological state, population growth, and reproduction of B. calyciflorus (Huang et al. 2012). Room temperature of 25 °C is the optimal and critical temperature of this rotifer species. Sixteen treatment combinations of N × M were used at 20 °C, 25 °C, and 30 °C successively. The control treatment contained EPA medium, that is, N0M0 treatment was considered the control for each temperature in all the experiments. The test solutions were replaced every 24 h. Microcystin-LR and nitrite concentrations were quantified before replacing the medium. In every treatment, 24 replicates were conducted for estimating the lifespan and reproductive performance of rotifers. Three replicates were performed to evaluate the ROS levels and Hsp gene mRNA expression at each temperature.
B. calyciflorus is small (< 0.5 mm), which allows it to be cultured in microliter volumes (Snell and Janssen 1995). Thus, 1 mL of the test solution was added into each well of 24-well microplates to evaluate the lifespan and reproduction of rotifers at 20 °C, 25 °C, and 30 °C. At a given temperature, one female rotifer (< 2 h old, 1 individual mL−1) was used in each N × M treatment and was placed into each well of 24-well microplate containing a total volume of 1 mL of test solutions to determine the lifespan and reproduction of B. calyciflorus through individual-based experiments. The lifespan of rotifers was calculated as the time from birth to death. Reproductive performance was evaluated according to reproductive rate (R), which is calculated using Eq. 1 (Snell 1980).
where Nt = the number of females at the exposure time of t; N0 = 1; t = the duration of the experiment (d); t0 = 0; and R = the mean number of female offspring/day.
Each treatment was conducted in 24 replicates at each temperature to estimate the lifespan and R of rotifers (N = 24; N replicates denote the wells of microplates; a replicate here refers to a combination of nitrite and microcystin-LR concentrations). Resting egg hatchlings were used to initiate experiments. All test animals were amictic females. Rotifers were fed with Chlorella pyrenoidosa (3 × 106 cells mL−1) at 12 h intervals, and the test solutions were changed every 24 h. The original rotifers were monitored, and newborn neonates were recorded and removed systematically from the test solutions every 6 h. The original females were transferred daily into freshly prepared test solutions containing 3 × 106 cells mL−1 of Chlorella until all the experimental animals were dead. Approximately 10 µL of the test solution, which included the original rotifer, were transferred by micropipette. Handling-related mortalities or damaged test animals were discarded during the individual-based experiments.
Considering the death of B. calyciflorus during cultivation, the initial culture density of 600–800 females in each treatment was exposed to temperatures of 20 °C, 25 °C, and 30 °C. The rotifers received the same volume of C. pyrenoidisa (3 × 106 cells mL−1) at 12 h, and the feeding alga was nearly consumed within 12 h. A total of 400 rotifers were selected from the initial females and homogenized in each N × M treatment to evaluate the ROS levels and mRNA expression of Hsp genes after 24 h of exposure. The rotifers were collected using sieves with a mesh size of 37.4 µm into 1.5 mL tubes with a micropipette after 24 h. All test animals were attached to the bottom of the tubes, which were inserted into ice after centrifugation (6000 rpm, 15 min, 4 °C). The rotifers were then carefully isolated by removing the test solutions with a sterile syringe with a needle. Each treatment was performed in three replicates at a given temperature (N = 3; N denotes the biological replicates), that is, three samples were obtained from each of the three homogenates to measure the ROS levels and mRNA expression of Hsp genes.
Small-molecular Hsp genes (16–30 kDa), namely, Hsp40, Hsp60, Hsp70, and Hsp90, which are commonly studied, are sensitive to oxidation stress and essential to the enhancement of stress resistance during the growth and aging of rotifers (Jung and Lee 2012; Yang et al. 2014). The specific protein homeostatic functions of small Hsp genes were reported to extend the lifespan of organisms (Vos et al. 2016). The mRNA expression of Hsp genes was measured to determine the correlation between Hsp gene expression and B. calyciflorus lifespan and reproduction under the stress of temperature, microcystin-LR, and nitrite. Thus, four Hsp genes were selected in this study. The combined treatments of microcystin-LR, nitrite, and different temperatures were identical in all experiments.
Measurement of ROS levels
About 400 rotifers were separated into a 1.5 mL centrifuge tube (400 individuals tube−1). The weight (W) of 400 rotifers was calculated to be the difference between the weight of the tube containing 400 individuals (W1) and empty tube (W2) (W = W1 − W2), which were weighed with an electronic balance. We omitted the influence of feed source Chlorella (consumed by rotifers within 12 h) and the weight of test solutions (removed from the tubes after centrifugation). The 400 rotifers were homogenized in nine volumes (v/w) of 0.1 mol L−1 cold phosphate buffer (pH 7.3) solutions. The homogenized 400 rotifers were centrifuged (3000 rpm, 10 min, 4 °C). The supernatants were transferred into 1.5 mL centrifuge tubes and then stored at − 80 °C for rotifer ROS measurements. Total ROS levels in the supernatants were measured using a reactive oxygen species assay kit (Nanjing Jiancheng Bioengineering Institute, China) according to the manual’s instructions. ROS assay was performed within the linear range of the standard curve, and ROS values were calculated based on the protein content of rotifers.
Determination of the mRNA expression levels of Hsp genes
Approximately 400 females based on a mean concentration of the culture were homogenized in 1000 µL of TRIzol reagent (Thermo Fisher Scientific, Waltham, USA). RNA was extracted from each treatment at 20 °C, 25 °C, and 30 °C after 24 h of exposure. The extracted RNA (268.5 ng µL−1) was reverse-transcribed to cDNA with oligo-dT primers and an aM-MLV RTase cDNA Synthesis Kit (TaKaRa, Shiga, Japan) according to the manufacturer’s protocol. The mRNA expression levels of Hsp genes were determined through real-time quantitative polymerase chain reaction (RT-qPCR) by using a CFX96 RT-PCR (Bio-Rad, Hercules, CA, USA). RT-qPCR was performed in 25 μL volume with the SYBR Premix Ex Taq™ Kit (TaKaRa, Shiga, Japan), 1 μL of cDNA, and 2 μM of each gene specific primer (Table 1). All primers were designed in accordance with the study of Yang et al. (2014). RT-qPCR analysis was conducted at 94 °C for 4 min, followed by 35 cycles of 94 °C for 30 s, 58 °C for 30 s, and 72 °C for 30 s. Three genes were selected from UniGene data as alternative reference genes. β-actin, the most stably expressed housekeeping gene, was used as the internal standard for relative expression quantification. The expression of β-actin could be influenced during individual developmental stages as well as experimental conditions (Heckmann et al. 2006; Lyu et al. 2014). In this study, the expression of β-actin was checked by RT-qPCR at microcystin-LR concentrations of 0 (control), 10, 30, and 100 µg L−1. No significant difference in the expression levels of β-actin was observed, which proved that microcystin-LR did not influence β-actin expression. Gene expression was calculated with 2−ΔΔCT method according to the study of Livak and Schmittgen (2001).
Statistical analysis
Data distribution and homogeneity of variance were tested using Kolmogorov–Smirnov and Levene’s tests, respectively. Data feasibility analysis was suitable for ANOVA. Tukey’s and Duncan’s multiple range tests were conducted, which revealed no differences in the data trends. At a given temperature, the effects of microcystin-LR and nitrite on rotifer lifespan, R, ROS levels, and Hsp gene expression levels were evaluated using two-way ANOVA, followed by Tukey’s multiple range test. The interactive effects of temperature, microcystin-LR, and nitrite were assessed using three-way ANOVA, followed by Tukey’s multiple range test. The correlation between Hsp gene expression levels and ROS levels was identified using Pearson’s correlation analyses. All data were shown as mean, and all analyses were conducted in SigmaPlot 12.5.
Results
Lifespan and reproduction of rotifers
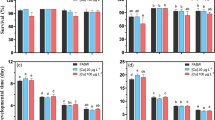
Single factor (temperature, microcystin-LR, or nitrite) and two factors (temperatures × microcystin-LR/nitrite; microcystin-LR × nitrite) negatively affected the lifespan and reproduction of rotifers (p < 0.001, Table 2). Three factors (temperature × microcystin-LR × nitrite) had interactive effects on the lifespan and R of rotifers (three-way ANOVA, p < 0.05, Table 2). Considering the effects of temperature as a single factor, the lifespan and R of rotifers exposed to N0M0 treatment were improved by 6%–17% at 20 °C but reduced by 13–52% at 30 °C compared with those at 25 °C (Figs. 1 and S1). Considering one toxicant, the rotifer lifespan (Figs. 1a–c and S1a–c) and R (Figs. 1d–f and S1d–f) improved by 2–19% compared with the control in N1M0, N3M0, N0M10, and N0M30 treatments but reduced by 3–35% in N5M0 and N0M100 treatments at 20 °C, 25 °C, and 30 °C. Microcystin-LR and nitrite had synergistic effects on the lifespan and reproduction of rotifers at the three temperatures (two-way ANOVA, p < 0.05, Table 3). For microcystin-LR and nitrite mixtures, the rotifer lifespan (Figs. 1a–c and S1a–c) and R (Figs. 1d–f and S1d–f) reduced by 7%–68% in N5M10, N5M30, N1M100, N3M100, and N5M100 treatments at 20 °C and 25 °C and by 2%–46% with increasing toxicant concentrations at 30 °C (p < 0.001).
ROS levels
The ANOVA showed that single factor (temperature, microcystin-LR, or nitrite) and two factors (temperatures × microcystin-LR; microcystin-LR × nitrite) promoted ROS production in rotifers (p < 0.001, Table 2). Three factors (temperature × microcystin-LR × nitrite) had interactive effects on ROS production (three-way ANOVA, p < 0.05, Table 2). Considering the effects of temperature as a single factor, the ROS levels of rotifers exposed to N0M0 treatment increased by 5–13% at 20 °C and 30 °C compared with those at 25 °C (Figs. 2 and S2). Microcystin-LR and nitrite had synergistic effects on the ROS levels at 20 °C, 25 °C, and 30 °C (two-way ANOVA, p < 0.05, Table 3). Dose-dependent enhancements in ROS levels were observed in single solutions and mixtures of the two test toxicants at each temperature (Figs. 2a–c and S2a–c). The ROS levels increased by 4–29% at 20 °C (Figs. 2a and S2a), 6–32% at 25 °C (Figs. 2b and S2b), and 3–33% at 30 °C (Figs. 2c and S2c) compared with the control at a given temperature.
mRNA expression levels of Hsp genes
Single factor (temperature, microcystin-LR, or nitrite) and two factors (temperatures × microcystin-LR/nitrite; microcystin-LR × nitrite) induced Hsp gene expression (p < 0.05, Table 2). Three factors (temperature × microcystin-LR × nitrite) had interactive effects on Hsp gene expression (three-way ANOVA, p < 0.05, Table 2). Microcystin-LR and nitrite had synergic effects on Hsp gene expression at 20 °C and 25 °C (p < 0.05) but had antagonistic effects on Hsp40 and Hsp60 at 30 °C (two-way ANOVA, p > 0.05, Table 3). The expression of Hsp40 (p < 0.01), Hsp60 (p < 0.01), and Hsp90 (p < 0.05) showed negative correlations with ROS levels at 30 °C (Table 4).
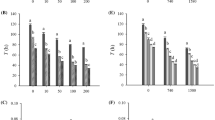
Considering one toxicant, the expression levels of Hsp40 (Figs. 3a, b and S3a, b) and Hsp60 (Figs. 3d, e and S3d, e) increased by 18%–280% in N1M0, N3M0, N0M10, and N0M30 treatments compared with those of the control at 20 °C and 25 °C (p < 0.001). For mixtures of microcystin-LR and nitrite, the expression levels of Hsp40 (Figs. 3a, b and S3a, b) and Hsp60 (Figs. 3d, e and S3d, e) increased by 86–445% in N1M10, N3M10, N1M30, and N3M30 treatments, but these levels decreased by 22–76% in N1M100, N3M100, and N5M100 treatments at 20 °C and 25 °C (p < 0.001). The expression levels of Hsp40 (Figs. 3c and S3c) and Hsp60 (Figs. 3f and S3f) decreased by 23–80% with increasing toxicant concentrations at 30 °C.
Considering the effects of nitrite as a single factor, the expression levels of Hsp70 (Figs. 4a–c and S4a–c) and Hsp90 (Figs. 4d–f and S4d–f) increased by 14–557% in N1M0 and N3M0 treatments at 20 °C, 25 °C, and 30 °C (p < 0.05). Considering the effects of microcystin-LR as a single factor, the expression level of Hsp70 increased by 58%–360% in N0M10 and N0M30 treatments (Figs. 4a–c and S4a–c), while that of Hsp90 increased by 90–553% in N0M10, N0M30, and N0M100 treatments at the three temperatures (Figs. 4d–f and S4d–f). For the mixtures of the two toxicants, the expression levels of Hsp70 (Figs. 4a, b and S4a, b) and Hsp90 (Figs. 4d, e and S4d, e) increased by 96–522% in N1M10, N3M10, N1M30, and N3M30 treatments, whereas the Hsp70 expression decreased by 41–69% in N1M100, N3M100, and N5M100 treatments at 20 °C and 25 °C (p < 0.001). The expression levels of Hsp70 (Figs. 4c and S4c) and Hsp90 (Figs. 4f and S4f) increased by 58–187% in N1M10, N3M10, N5M10, N1M30, N3M30, and N5M30 treatments, but these levels decreased by 26–85% in N3M100 and N5M100 treatments at 30 °C.
Discussion
In this study, the lifespan and reproductive performance of rotifers were improved at low doses of microcystin-LR (10–30 µg L−1) and nitrite (1–3 mg L−1). Hormesis is a dose–response relationship characterized by low-dose stimulation and high-dose inhibition (Calabrese 2008). The improved lifespan and reproductive performance of B. calyciflorus at low microcystin-LR and nitrite exposures were considered as hormesis. Hormesis was not observed in the toxicants used in this study previously. Mild stress has been shown to protect and improve the lifespan performance of goldfish Carassius auratus and nematode Caenorhabditis elegans, indicating that hormesis is a common response in aquatic ecosystems (Berry and López-Martínez 2020; Kim and Park 2020). The hormetic response in rotifers provides a broad range of toxicologically based exposure options, which permit a consideration for avoiding harm from microcystin-LR (≤ 30 µg L−1) and nitrite (≤ 3 mg L−1). High doses of microcystin-LR (100 µg L−1) and nitrite (5 mg L−1) had negative effects on the life-history parameters of rotifers. The lifespan and reproductive performance were significantly suppressed in the N5M100 treatment. Rotifers have short-term stress effects that improve their lifespan and reproduction, which was correlated with the high expression of Hsp genes under low-dose exposure to microcystin-LR and nitrite. These results support the hypotheses stated in the introduction.
High doses above the LC50 levels of the two test toxicants resulted in downregulated Hsp gene expression, which was detrimental to the life-history parameters of rotifers. Previous studies reported similar effects on other zooplankton species. More than 140 µg L−1 dissolved microcystin-LR had chronic effects on the survival and reproduction of the copepods Eurytemora affinis (the 48 h LC50 and LC10 values were 1550 and 140 µg L−1) and Pseudodiaptomus forbesi (the 48 h LC50 and LC10 values were 520 and 210 µg L−1) (Ger et al. 2009). The EC50 values of survival time and total offspring per female for Daphnia similis were 8.1 and 3.1 mg L−1 nitrite, respectively (Lyu et al. 2013). These studies suggest that the tolerance of zooplankton to microcystin-LR and nitrite is species specific and is related to maintenance conditions and/or the potency of the toxicants (Yang et al. 2011).
The lifespan and reproduction of B. calyciflorus were improved at a low temperature (20 °C) but adversely affected at a high temperature (30 °C) in N0M0 treatment. This result indicated that temperature, as a single factor, affected the growth of rotifers. The rotifer lifespan reduction and reproductive impairment occurred at high doses of microcystin-LR and nitrite at 20 °C and 25 °C and in the mixtures of two toxicants at 30 °C. Hence, temperature, microcystin-LR, and nitrite had interactive effects on the survival and reproduction of zooplankton. Microcystin-LR concentrations lower than 200 µg L−1 were reported to increase the population growth rate but decrease the ovigerous/non-ovigerous female ratio and the mictic rate of B. calyciflorus at 30 °C (Huang et al. 2012). The toxic effect of microcystin-LR on Danio rerio was enhanced at 32 °C (Zhang et al. 2011). High temperatures possibly increased the toxicity of microcystin-LR and nitrite, which had negative effects on the lifespan and reproduction of rotifers.
ROS levels were positively correlated with microcystin-LR and nitrite concentrations at 20 °C, 25 °C, and 30 °C. The genes implied in ROS scavenging enzymes, such as catalase, manganese superoxide dismutase, and copper and zinc superoxide dismutase, were evaluated in a previous study (Yang et al. 2013). Moreover, significant increases in ROS levels revealed the occurrences of oxidative stress caused by microcystin-LR and nitrite, which affected the growth of rotifers (Liang et al. 2017). In this study, ROS levels were negatively correlated with Hsp gene expression, indicating that increased ROS levels could suppress the expression levels of Hsp genes under the stress of temperature, microcystin-LR, and nitrite. Microcystin-LR and nitrite promoted ROS production, and excessive ROS levels had negative effects on the Hsp gene expression levels at 100 µg L−1 microcystin-LR and 5 mg L−1 nitrite. High concentrations of microcystin-LR and nitrite induced oxidative stress in rotifers together with the inhibition of protein phosphatases and the MAPK single pathway, which was considered the main mechanisms that lead to toxic responses (Mclellan and Manderville 2017). High ROS levels destroyed protein structures and functions, and damaged proteins aggregated in the rotifers, thereby inhibiting Hsp gene expression. The rotifer lifespan and R were reduced at high doses of microcystin-LR and nitrite, and this reduction was correlated with oxidative stress-mediated Hsp gene expression in B. calyciflorus.
At high temperature, the Hsp40 and Hsp60 expression levels were downregulated in every treatment, and the lowest Hsp70 and Hsp90 expression levels appeared in the mixtures of the high doses of microcystin-LR and nitrite. Hsp90 showed higher expression levels than Hsp40, Hsp60, and Hsp70 at 30 °C, indicating that Hsp90 was more sensitive to high temperature than the three other Hsp genes under the stress of microcystin-LR and nitrite. Comprehensive modulations of Hsp40, Hsp60, Hsp70, and Hsp90 reflected the involvement of a strong defense strategy of B. calyciflorus in response to temperature, microcystin-LR, and nitrite. Each Hsp gene has different mechanisms, functions, and pathways involved; therefore, they react differently (Kim et al. 2014). Hsp40, which plays an important role in protein homeostasis, simulates the ATPase activity of Hsp70, which is involved in protein translation, folding, translocation, and degradation (Qiu et al. 2006). Hsp60 is a mitochondrial chaperonin that is typically responsible for the transportation and refolding of proteins from the cytoplasm into the mitochondrial matrix (Song et al. 2016). The downregulation of Hsp40 and Hsp60 was observed in the mixtures of microcystin-LR and nitrite at 30 °C and at high doses of two toxicants at 20 °C and 25 °C. Hsp-mediated cellular damage occurred under warming and nitrite-enriched conditions. MC-LR exposure promoted the expression of apoptosis-related genes (p53, bax, and bcl-2), leading to the death of cells (Campos and Vasconcelos 2010). The downregulation of Hsp genes was associated with mitochondrial dysfunction or cell apoptosis at high doses of toxicants (Jung and Lee 2012), which were harmful to the lifespan and reproduction of B. calyciflorus.
Hsp70 and Hsp90 are the primary sensors of misfolded proteins and play crucial roles in proteasome-mediated protein degradation systems and against stress-induced cellular damage (Mukhopadhyay et al. 2003; Padmini and Rani 2011). High temperatures stimulate the expression levels of Hsp70 and Hsp90 in the rotifer Brachionus manjavacas (Smith et al. 2012). The expression levels of Hsp70 and Hsp90 were actively modulated by microcystin-LR and nitrite at the three tested temperatures, indicating that temperature and toxicant exposure had a combined effect on Hsp gene expression in B. calyciflorus. Low expression levels of Hsp70 and Hsp90 were observed in the high-concentration mixtures of microcystin-LR and nitrite, demonstrating that severe cyanobacterial blooms affected the Hsp gene expression of B. calyciflorus. Cyanobacteria release numerous potentially toxic compounds that may have different effects on Hsp gene expression in aquatic animals. The mRNA levels of Hsp90 were shown to increase in bream Megalobrama amblycephala after nitrite exposure (Sun et al. 2015). The presence of microcystin-LR may also inhibit Hsp70 expression, which contributes to the microcystin tolerance of carp Cyprinus carpio (Jiang et al. 2012). Our results agree with the above literature. This study is the first to report the combined effects of microcystin-LR, nitrite, and temperature on the heat shock responses of zooplankton.
Hsp-mediated heat shock responses are important to the survival and adaptation of rotifers. In the present study, the Hsp gene expression levels were induced by temperature pressure and toxicant exposure, thereby confirming that Hsp are “stress proteins” (Jung and Lee 2012; Yang et al. 2014). Stress proteins were implicated in the lifespan of Drosophila (Vos et al. 2016). Low concentrations of toxicants promoted the Hsp gene expression levels at 20 °C and 25 °C. The upregulated Hsp gene expression was part of the cellular stress response. Increased Hsp gene expression mediated the damaged proteins that accumulated in cells (Kim et al. 2014). However, low Hsp gene expression levels were observed at high concentrations of microcystin-LR and nitrite, affecting the lifespan and reproduction of organisms, as observed in B. calyciflorus. The results suggested that at low microcystin-LR and nitrite concentrations, rotifers are able to respond to stress by increasing Hsp gene expression but not able to do so at high toxicant concentrations. High toxicant concentrations inhibit the ability of rotifers to minimize the effects of heat stress. The variations in the response of rotifers to microcystin-LR, nitrite, and temperature provide a reference in evaluating other toxicant exposure limits on the general sensitivity of aquatic animals and a comprehensive understanding of the robustness of zooplankton communities in eutrophic waters.
Conclusions
Microcystin-LR and nitrite had synergetic effects on rotifer lifespan, reproductive performance, ROS levels, and Hsp gene expression at 20 °C and 25 °C. The two toxicants had antagonistic effects on the expression levels of Hsp40 and Hsp60 at 30 °C. High temperature increased microcystin-LR and nitrite toxicity, which adversely influenced the lifespan, reproduction, and Hsp gene expression of rotifers. The interactive effects among temperature, microcystin-LR, and nitrite on the lifespan, reproduction, ROS levels, and Hsp gene expression of rotifers were detected. This study indicated that microcystin-LR and nitrite induced the oxidative stress-mediated Hsp gene expression levels at different temperatures, thereby affecting rotifer life-history parameters. Hsp-mediated heat shock responses play a functional role in microcystin-LR and nitrite stress tolerance of B. calyciflorus. Therefore, Hsp genes could be involved in protecting rotifers against oxidative stress under eutrophic conditions due to temperature changes. Hsp genes could be used as markers for heat stress or toxin exposure in assessing the toxicity of environmental pollutants. Considering that thermal regimes and eutrophication of water bodies affect plankton community structures, heat tolerance mechanisms that affect the growth of potential zooplankton species under toxicants and environmental temperatures should be investigated.
References
ASTM (2001) Standard guide for acute toxicity tests with the rotifer Brachionus. Annual book of ASTM standards, water and environmental technology. Biological effects and environmental fates, vol 1105. American Society for Testing and Materials, West Conshohocken
Berry R, López-Martínez G (2020) A dose of experimental hormesis: when mild stress protects and improves animal performance. Comp Biochem Physiol A Mol Integr Physiol 242:110658
Calabrese EJ (2008) Hormesis: why it is important to toxicology and toxicologists. Environ Toxicol Chem 27:1451–1474
Campos A, Vasconcelos V (2010) Molecular mechanisms of microcystin toxicity in animal cells. Int J Mol Sci 11:268–287
Fan CY, Lee S, Ren HY, Cyr DM (2004) Exchangeable chaperone modules contribute to specification of type I and type II Hsp40 cellular function. Mol Biol Cell 15:761–773
Ger KA, Teh SJ, Goldman CR (2009) Microcystin-LR toxicity on dominant copepods Eurytemora affinis and Pseudodiaptomus forbesi of the upper San Francisco Estuary. Sci Total Environ 407:4852–4857
Ger KA, Urrutia-Cordero P, Frost PC, Hansson LA, Sarnelle O, Wilson AE, Lürling M (2016) The interaction between cyanobacteria and zooplankton in a more eutrophic world. Harmful Algae 54:128–144
Heckmann LH, Connon R, Hutchinson TH, Maund SJ, Sibly RM, Callaghan A (2006) Expression of target and reference genes in Daphnia magna exposed to ibuprofen. BMC Genomics 7:175
Henning-Lucass N, Cordellier M, Streit B, Schwenk K (2016) Phenotypic plasticity in life-history traits of Daphnia galeata in response to temperature—a comparison across clonal lineages separated in time. Ecol Evol 6:881–891
Huang L, Xi Y, Xu X, Wen X (2012) Responses in population growth and reproduction of the freshwater rotifer Brachionus calyciflorus to microcystin-LR at different temperatures. Ann Limnol Int J Lim 48:383–390
Jensen FB (2003) Nitrite disrupts multiple physiological functions in aquatic animals. Comp Biochem Physiol A Mol Integr Physiol 135:9–24
Jiang J, Shi Y, Shan Z, Yang L, Wang X, Shi L (2012) Bioaccumulation, oxidative stress and HSP70 expression in Cyprinus carpio L. exposed to microcystin-LR under laboratory conditions. Comp Biochem Physiol C Toxicol Pharmacol 155:483–490
Jones GJ, Orr PT (1994) Release and degradation of microcystin following algicide treatment of a Microcystis aeruginosa bloom in a recreational lake, as determined by HPLC and protein phosphatase inhibition assay. Water Res 28:871–876
Jung MY, Lee YM (2012) Expression profiles of heat shock protein gene families in the monogonont rotifer Brachionus koreanus—exposed to copper and cadmium. Toxicol Environ Health Sci 4:235–242
Kim BK, Park SK (2020) Phosphatidylserine modulates response to oxidative stress through hormesis and increases lifespan via DAF-16 in Caenorhabditis elegans. Biogerontology 21:231–244
Kim BM, Rhee JS, Jeong CB, Seo JS, Park GS, Lee YM, Lee JS (2014) Heavy metals induce oxidative stress and trigger oxidative stress-mediated heat shock protein (hsp) modulation in the intertidal copepod Tigriopus japonicus. Comp Biochem Physiol C Toxicol Pharmacol 166:65–74
Kroupová HK, Valentová O, Svobodová Z, Šauer P, Máchová J (2016) Toxic effects of nitrite on freshwater organisms: a review. Rev Aquacult 10:1–18
Lahti K, Rapala J, Färdig M, Niemelä M, Sivonen K (1997) Persistence of cyanobacteria hepatotoxin microcystin-LR in particulate material and dissolved in lake water. Water Res 31:1005–1012
Liang Y, Chen X, Lu X, Jin S, Min Y, Yang J (2017) Combined effects of microcystin and nitrite on the growth, lipid peroxidation, and antioxidant responses of the freshwater rotifer Brachionus calyciflorus. Aquat Toxicol 192:78–88
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 25:402–408
Lürling M, van Oosterhout F, Faassen E (2017) Eutrophication and warming boost cyanobacterial biomass and microcystins. Toxins 9:64
Lyu K, Wang Q, Chen R, Lu Q, Yang Z (2013) Inter-specific differences in survival and reproduction of cladocerans to nitrite gradient and the ecological implications. Biochem Syst Ecol 48:151–156
Lyu K, Zhu X, Chen R, Chen Y, Yang Z (2014) Molecular cloning of manganese superoxide dismutase gene in the cladoceran Daphnia magna: effects of microcystin, nitrite, and cadmium on gene expression profiles. Aquat Toxicol 148:55–64
Lyu K, Meng Q, Zhu X, Dai D, Zhang L, Huang Y, Yang Z (2016) Changes in iTRAQ-based proteomic profiling of the cladoceran Daphnia magna exposed to microcystin-producing and microcystin-free Microcystis aeruginosa. Environ Sci Technol 50:4798–4807
Lyu K, Gu L, Wang H, Zhu X, Zhang L, Sun Y, Huang Y, Yang Z (2018) Transcriptomic analysis dissects the mechanistic insight into the Daphnia clonal variation in tolerance to toxic Microcystis. Limnol Oceanogr 9999:1–12
Mclellan NL, Manderville RA (2017) Toxic mechanisms of microcystins in mammals. Toxicol Res 6:391–405
Mowe MAD, Mitrovic SM, Lim RP, Furey A, Yeo DCJ (2015) Tropical cyanobacterial bloom: a review of prevalence, problem taxa, toxins and influencing environmental factor. J Limnol 74:205–224
Mukhopadhyay I, Nazir A, Saxena DK, Chowdhuri DK (2003) Heat shock response: hsp70 in environmental monitoring. J Biochem Mol Toxic 17:249–254
Olah Z, Bush AI, Aleksza D, Galik B, Ivitz E, Macsai L, Janka Z, Karman Z, Kalman J, Datki Z (2017) Novel in vivo experimental viability assays with high sensitivity and throughput capacity using a bdelloid rotifer. Ecotoxicol Environ Saf 144:115–122
Padmini E, Rani MU (2011) Heat-shock protein 90 alpha (HSP90α) modulates signaling pathways towards tolerance of oxidative stress and enhanced survival of hepatocytes of Mugil cephalus. Cell Stress Chaperon 16:411–425
Peng G, Martin RM, Dearth SP, Sun X, Boyer GL, Campagna SR, Lin S, Wilhelm SW (2018) Seasonally relevant cool temperatures interact with N chemistry to increase microcystins produced in lab cultures of Microcystis aeruginosa NIES-843. Environ Sci Technol 52:4127–4136
Petkeviciute E, Kania PW, Skovgaard A (2015) Genetic responses of the marine copepod Acartia tonsa (Dana) to heat shock and epibiont infestation. Aquacult Rep 2:10–16
Philips S, Laanbroek HJ, Verstraete W (2002) Origin, causes and effects of increased nitrite concentrations in aquatic environments. Rev Environ Sci Biotechnol 1:115–141
Qiu XB, Shao YM, Miao S, Wang L (2006) The diversity of the DnaJ/Hsp40 family, the crucial partners for Hsp70 chaperones. Cell Mol Life Sci 63:2560–2570
Shah JA, Pandit AK, Shah GMA (2015) Research on rotifers of aquatic ecosystems of Kashmir Himalaya for documentation and authentication. Proc Natl Acad Sci India Sect B Biol Sci 85:13–19
Smith HA, Burns AR, Shearer TL, Snell TW (2012) Three heat shock proteins are essential for rotifer thermotolerance. J Exp Mar Biol Ecol 413:1–6
Snell TW (1980) Blue-green algae and selection in rotifer populations. Oecologia 46:343–346
Snell TW, Janssen CR (1995) Rotifers in ecotoxicology: a review. Hydrobiologia 313–314:231–247
Soares MCS, Lürling M, Huszar VLM (2010) Responses of the rotifer Brachionus calyciflorus to two tropical toxic cyanobacteria (Cylindrospermopsis raciborskii and Microcystis aeruginosa) in pure and mixed diets with green algae. J Plankton Res 32:999–1008
Song E, Tang S, Xu J, Yin B, Bao E, Hartung J (2016) Lenti-siRNA Hsp60 promote bax in mitochondria and induces apoptosis during heat stress. Biochem Biophys Res Commun 481:125–131
Sun SM, Zhu J, Ge XP, Zhang CF, Miao LH, Jiang XJ (2015) Cloning and expression analysis of a heat shock protein 90 β isoform gene from the gills of Wuchang bream (Megalobrama amblycephala Yih) subjected to nitrite stress. Genet Mol Res 14:3036–3051
Viñuela A, Snoek LB, Riksen JA, Kammenga JE (2011) Gene expression modifications by temperature-toxicants interactions in Caenorhabditis elegans. PLoS ONE 6:e24676
Vos MJ, Carra S, Kanon B, Bosveld F, Klauke K, Sibon OCM, Kampinga HH (2016) Specific protein homeostatic functions of small heat-shock proteins increase lifespan. Aging Cell 15:217–226
Willming MM, Qin G, Maul JD (2013) Effects of environmentally realistic daily temperature variation on pesticide toxicity to aquatic invertebrates. Environ Toxicol Chem 32:2738–2745
Yang Z, Xiang F, Minter EJA, Lü K, Chen Y, Montagnes DJS (2011) The interactive effects of microcystin and nitrite on life-history parameters of the cladoceran Daphnia obtuse. J Hazard Mater 190:113–118
Yang J, Dong S, Jiang Q, Kuang T, Huang W, Yang J (2013) Changes in the expression of manganese superoxide dismutase, copper and zinc superoxide dismutase and catalase in Brachionus calyciflorus during the aging process. PLoS ONE 8:e57186
Yang J, Mu Y, Dong S, Jiang Q, Yang J (2014) Changes in the expression of four heat shock proteins during the aging process in Brachionus calyciflorus (rotifera). Cell Stress Chaperon 19:33–52
Zhang X, Geng H (2012) Effect of Microcystis aeruginosa on the rotifer Brachionus calyciflorus at different temperatures. Bull Environ Contam Toxicol 88:20–24
Zhang X, Chen C, Ding J, Hou A, Li Y, Niu Z, Su X, Xu Y, Laws EA (2010) The 2007 water crisis in Wuxi, China: analysis of the origin. J Hazard Mater 182:130–135
Zhang X, Ji W, Zhang H, Zhang W, Xie P (2011) Studies on the toxic effects of microcystin-LR on the zebrafish (Danio rerio) under different temperatures. J Appl Toxicol 31:561–567
Zhang L, Gu L, Wei Q, Zhu X, Wang J, Wang X, Yang Z (2017) High temperature favors elimination of toxin-producing Microcystis and degradation of microcystins by mixotrophic Ochromonas. Chemosphere 172:96–102
Acknowledgements
This study was supported by the National Natural Science Foundation of China (grant number: 31772458, 31272388), the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (grant number: 19KJB180020), the Research Start-up Fund of NUIST (grant number: 2018r040), and the Fund of Fujian Provincial Key Laboratory of Marine Ecological Conservation and Restoration (grant number: EPR2020004).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liang, Y., Gao, T., Shao, L. et al. Effects of microcystin-LR and nitrite on the lifespan, reproduction, and heat shock responses of rotifer Brachionus calyciflorus at different temperatures. Aquat Sci 82, 74 (2020). https://doi.org/10.1007/s00027-020-00748-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00027-020-00748-6