Abstract
The harmful dinoflagellate Karenia mikimotoi is responsible for the mortality of aquatic animals. However, the mechanism behind these toxic effects has not been fully determined. Herein, the toxic effects of K. mikimotoi on the growth performance, antioxidative responses, physiological activities, and energetic substance contents of rotifer Brachionus plicatilis were assessed. Rotifers were exposed to Nannochloropsis salina (Eustigmatophyceae), K. mikimotoi, and a mixture of N. salina and K. mikimotoi with biomass ratio proportions of 3:1, 1:1, and 1:3, respectively. Results indicated that K. mikimotoi negatively affected the population growth, survival, and specific growth rates of rotifers within 24 h. The level of reactive oxygen species (ROS), the content of malondialdehyde, and the activity of amylase increased. However, the total antioxidant capacity level, pepsase, cellulase, alkaline phosphatase, xanthine oxidase, and lactate dehydrogenase activities, and glycogen and protein contents decreased with increasing proportions of K. mikimotoi. The mixture of 50% N. salina and 50% K. mikimotoi promoted the increase in glutamic–pyruvic transaminase activity and triglyceride content. These findings underscore ROS-mediated antioxidative responses, physiological responses, and energetic substance content changes in B. plicatilis work together to affect population dynamics inhibition of rotifers by K. mikimotoi.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The ichthyotoxic dinoflagellate Karenia mikimotoi is distributed worldwide and capable of inhibiting the growth and reproduction of aquatic animals (Higo et al. 2017; Niu et al. 2021). K. mikimotoi bloom promotes the release of toxicants into the surrounding seawater, causing harmful effects on local fisheries and aquatic ecosystem health (Lin et al. 2015). The cell density of K. mikimotoi could reach 1 × 105 cells mL−1 in the peak stage of blooms (Higo et al. 2017; Kok and Leong 2019; Chen et al. 2021). In 2012, large-scale K. mikimotoi blooms (2 × 103–1.18 × 105 cells mL−1) caused mass mortality of pufferfish Takifugu rubripes in Japan (Basti et al. 2015). All throughout the K. mikimotoi blooms, the extracellular toxins released, and the intracellular cytotoxins inside could deteriorate the population growth of zooplankton (Yang et al. 2011; Li et al. 2019). K. mikimotoi has low nutrient content and produces hemolytic toxin, which induces the mortality of organisms (Li et al. 2020). K. mikimotoi can secrete toxic substances onto the algae cell surface, and direct contact with its body plays a major role in causing toxicity in zooplankton (Li et al. 2018). The influence of K. mikimotoi on zebrafish Danio rerio larval behavior has been investigated, in which a low concentration (102–103 cells mL−1) of K. mikimotoi did not cause a lethal effect but showed enzyme activity toxicity on organisms (Niu et al. 2021).
K. mikimotoi at high cell density blooms can negatively affect several surrounding organisms not only through reactive oxygen species (ROS)-mediated oxidative stress but also via its hemolytic activity (Cho et al. 2022). The total antioxidant capacity (T-AOC) indicates the antioxidation defense level of the antioxidant enzymes and antioxidants to remove ROS inside animal bodies (Jia et al. 2015; Cheng et al. 2021). Toxic substances induce ROS increase in organisms, leading to oxidative damage and activating the antioxidant system (Wang et al. 2015). A high level of ROS promotes the reaction of lipid peroxidation, thus increasing the malondialdehyde (MDA) content, which is the end-product of cell membrane lipid peroxidation (Coggins et al. 2017). Oxidative stress affects physiological activities within organisms exposed to toxic substances (Cheng et al. 2021). The evaluation of physiological activity is useful for monitoring fluctuating toxicant exposures and can act as a tool to assess the population growth dynamics of zooplankton in ecotoxicological tests (Liang et al. 2021a, 2021b). Enzyme activity measurement is necessary to assess the physiological response characteristics of animals under toxicant stress (Cheng et al. 2019).
Digestive and metabolic physiological activities reveal the metabolic function of animals, which are useful indices to evaluate toxicant stress (Conlin et al. 2018; Abdel-Tawwab et al. 2021), including hydrolytic enzymes (e.g., glycoside hydrolases, polysaccharide hydrolases, and proteolytic enzymes) (Liang et al. 2017). Digestive enzyme is an important index that can be used to assess the nutritional situation (Durigona et al. 2019). Amylase (AMS), pepsase (PEP), and cellulase (CL) are important indicators about the digestive physiological function and are correlated with the growth and healthiness of animals (Xu et al. 2020). In addition, organisms have many metabolic enzymes, including alkaline phosphatase (ALP), glutamic–pyruvic transaminase (GPT), xanthine oxidase (XOD), and lactate dehydrogenase (LDH) (Dube et al. 2014). ALP is important in the metabolic process of phosphate groups in organisms (Kanta et al. 2021). GPT is a transaminase secreted by the liver (Yu et al. 2020). XOD converts hypoxanthine into xanthine and then into uric acid to protect organisms from oxidative damage (Dube et al. 2014). LDH, an important metabolic enzyme, participates in glycolysis in animals (Sun et al. 2018). The evaluation of the changes in these crucial digestive and metabolic enzyme activities are effective for investigating the potential toxic mechanism of K. mikimotoi (Li et al. 2019, 2020).
The toxicants released from harmful algae might also affect the physiological activities and nutrient absorption of zooplankton. Glycogen (Glc), protein (Pro), and triglyceride (TG) are the main energetic substances in aquatic animals. The grazing intensity of rotifer Brachionus calyciflorus on toxic cyanobacterium Microcystis aeruginosa affects the Glc, Pro, and TG contents of the rotifers. The Glc and Pro contents are sensitive to the toxicity of cyanobacteria (Liang et al. 2017). Extracellular toxins released by K. mikimotoi have toxic effects on the survival and behavior of marine animals, such as fish, shellfish, and shrimps (Niu et al. 2021), and intracellular cytotoxins mainly inhibit enzyme activities and induce oxidative stress after zooplankton graze harmful algae (Li et al. 2018, 2020). The toxic effects of K. mikimotoi on the energetic substance contents of zooplankton have not been reported.
Rotifers, as a marine zooplankton indicator, have a short lifespan and are sensitive to toxicant pollutants and environmental changes. Accordingly, rotifers are useful subjects for evaluating the toxic effects of harmful algae (Snell and Janssen 1995). The rotifer Brachionus plicatilis Müller adjusts the growth performance, population abundance, and physiological responses to enhance its fitness in eutrophic waters, which has been extensively used in ecotoxicology in terms of the measuring ecosystem impacts of harmful algal blooms (Lin et al. 2016; Li et al. 2020; Liang et al. 2020). Toxicants released from harmful algae during bloom decay can accumulate in the food chain and reduce the survival and growth of zooplankton (Lin et al. 2015). Although the toxic effects of K. mikimotoi on the ecological characteristics of aquatic animals have been determined, relatively little is known about the potential mechanism of K. mikimotoi on the growth performance of rotifers (Dang et al. 2015; Li et al. 2017; Niu et al. 2021).
To assess whether the toxic effect of K. mikimotoi on the population growth of B. plicatilis is correlated with the antioxidative responses, physiological activities, and energetic substance contents within the rotifer organism, this work hypothesizes that (1) different proportions of K. mikimotoi solutions negatively affect the growth performance of B. plicatilis and (2) changes in the key biological enzyme activities and energetic substance contents of B. plicatilis that are related to K. mikimotoi influence its population growth, survival, and specific growth. The growth performance, antioxidant, digestive, and metabolic enzyme activities, and energetic substance absorption of B. plicatilis under K. mikimotoi stress were determined to evaluate the release of toxins for some mechanisms that affect rotifer population growth.
Materials and methods
Algal and rotifer culture
The chlorophyte Nannochloropsis salina was a single axenic clone and originally isolated from the coastal waters of Haikou, Hainan Province, China, in 2019. N. salina was obtained from the School of Marine Science and Engineering, Nanjing Normal University, Nanjing, China. The dinoflagellate K. mikimotoi (K01) was originally isolated from the Johor Strait between Singapore and Malaysia in 2018 (1°25′59″N, 103°53′52″E) and continuously cultured at the Algal Culture Center of the Third Institute of Oceanography, Ministry of Natural Resources, Xiamen, China. K. mikimotoi was axenic and was not contaminated by bacteria that affected the rotifers’ growth and metabolisms. This K. mikimotoi strain was confirmed to have hemolytic toxicants according to a previous study of Li et al. (2018). N. salina and K. mikimotoi were cultivated in a 5000 mL flask containing autoclaved seawater (salinity 32, aeration and dissolved oxygen >6.0 mg L−1) with f/2 medium. The rotifer B. plicatilis Müller was initially collected from a breeding farm located on the coast of the Yellow Sea and was provided by Professor Jiaxin Yang (Nanjing Normal University, Nanjing, China). The rotifers were continuously cultivated in a 500 mL flask with autoclaved seawater (salinity 32, aeration and dissolved oxygen >6.0 mg L−1) in the laboratory for more than 3 years prior to this work. The identity of the rotifer species was confirmed via morphological analysis and sequencing of the mitochondrial cytochrome oxidase I gene (Mills et al. 2017). The experimental N. salina, K. mikimotoi, and B. plicatilis were cultured in an illuminated incubator and maintained at 25 ± 1 °C under fluorescent light at 45 μmol photons m−2 s−1 with a 12 h:12 h light:dark photoperiod.
Experimental design
The initial cell densities of K. mikimotoi and N. salina were about 1 × 105 and 6 × 104 cells mL−1, respectively. Treatment groups were set according to the biomass ratio: (1) 100% N:100% N. salina, (2) 75% N + 25% K (3:1):75%N. salina + 25% K. mikimotoi, (3) 50% N + 50% K (1:1):50% N. salina + 50% K. mikimotoi, (4) 25% N + 75% K (1:3):25% N. salina + 75% K. mikimotoi, and (5) 100% K:100% K. mikimotoi. N. salina is the preferred diet for B. plicatilis in seawater. Thus, 100% N. salina was treated as the control treatment in all experiments. The combinations were set to the same volume in all cases. The total carbon content per unit volume of each treatment group was maintained at 1.27 ± 0.13 µg C mL−1 to enable maintaining an equal amount of carbon food supply for the rotifers. All the treatments were kept in the same carbon level. The carbon equivalent contents of N. salina and K. mikimotoi were used for each treatment. The carbon contents of K. mikimotoi (12.68 ± 1.58 pg C cell−1) and N. salina (2.13 ± 0.42 pg C cell−1) were measured in the laboratory with an element analyzer (Vario EL III, Elementar Analysensysteme GmbH, Hanau, Germany) (Graff et al. 2012). The linear regressions of visible light absorption at A680 nm in a spectrophotometer (Ultraspec 2100, Biochrom Ltd., Cambridge, UK) versus the carbon contents of each algae were pre-established (Liang et al. 2017). The N. salina and K. mikimotoi solutions were prepared by diluting a stock culture according to the linear regressions. To avoid algae settling at the bottom of the test containers, the tests were conducted by using horizontal mechanical shakers in all experiments.
Growth performance of B. plicatilis
In population growth experiments, ten rotifer neonates ( < 2 h old) were placed into each well of a six-well microplate containing different proportions of K. mikimotoi. The rotifers were observed every 6 h under a stereomicroscope (Zeiss, SteREO Discovery V8, Germany), and the numbers of original rotifers alive and neonates produced were recorded. The newborn neonates were removed from the test solutions. The rotifers were fed with different proportions of K. mikimotoi every 12 intervals. The rotifers cannot consume K. mikimotoi. However, they can consume N. salina, which has a cell size that is within the edible cellular size range of brachionid rotifers (Cho et al. 2022). Population experiments were not ended until all the initial mothers had died.
The growth performance was evaluated based on the population growth rate (r), survival rate (S), and specific growth rate (SGR) of rotifers. In B. plicatilis, lorica growth stops after 24–36 h, and the maternal rotifers produced some neonates at the exposure time of 24 h. Accordingly, the r, S, and SGR were calculated every 6 h during the exposure time of 24 h. Each treatment had 20 replicates to evaluate the r and S of the rotifers (n = 20). r and S were calculated using Eqs. 1 and 2, respectively (Krebs 1994).
where Nt is the population density of the rotifers at the exposure time of t (ind. mL–1), N0 is the initial growth density of the rotifers (ind. mL–1), nt is the number of surviving original rotifers at the exposure time of t (ind. mL–1), n0 is the initial number of rotifers (ind. mL–1), and t is the duration of the experiment.
SGR was estimated according to the dry weight of rotifers. The dry weight of rotifers was determined as previously described by the study of Liang et al. (2021a). Each treatment had 20 replicates to evaluate the SGR of B. plicatilis (n = 20). A few individuals from each treatment were fixed at lower intervals (6 h) until the age at maturity (24 h) to determine the rotifer somatic growth. SGR was calculated based on the dry weight of a subsample of B. plicatilis at the beginning and end of the experiment by using Eq. 3 (Lampert and Trubetskova 1996).
where dw0 and dwt are the mean dry weights of the B. plicatilis at the beginning and end of the experiment, respectively; and d is the duration of the experiment.
Determination of the total ROS levels, T-AOC level, MDA content, enzyme activities, and energetic substance contents in B. plicatilis
In the biochemical experiments, 600 B. plicatilis were cultured in a 50 mL beaker containing 40 mL of autoclaved seawater. B. plicatilis were fed with each alga combination every 12 h, and the rotifers were cultured for 24 h. During the experiments, beakers containing microalgae were shaken slightly twice at set times, and sufficient oxygen supply was ensured to prevent K. mikimotoi from dying within 12 h (Li et al. 2018). As detected under microscopy, N. salina cells can be consumed by rotifers. A total of 300 rotifers were chosen from the initial cultured 600 rotifers after 24 h and transferred to 1.5 mL centrifuge tubes by using precision test sieves with a mesh size of 50 µm. The 300 individuals of B. plicatilis were used for the extraction of detection targets for each treatment group. The number of rotifers matched the numbers required by the testing kits. After centrifugation (3500 × g, 15 min, and 4 °C), the rotifers were collected and homogenized in nine volumes (v/w) with phosphate-buffered saline (PBS, pH 7.5). The B. plicatilis homogenates were centrifuged at 3500 × g for 15 min at 4 °C, and the supernatants with different treatment groups were stored at −80 °C for the measurement of each biochemical parameter in B. plicatilis. Three biological replicates were employed for each treatment, indicating that three test samples were obtained from three rotifer homogenates with a treatment group (n = 3).
Biochemical parameters were assessed by using kits (Jiancheng Bioengineering Institute, Nanjing, China) in accordance with the protocol given in the kit’s manual. The T-AOC level (kit code: A015-1-1), MDA content (kit code: A003-1-2), enzymatic activities of AMS (kit code: C016-2-1), PEP (kit code: A080-1-1), CL (kit code: A138-1-1), ALP (kit code: A059-1-1), GPT (kit code: C009-1-1), XOD (kit code: A002-1-1), and LDH (kit code: A020-1-2), and contents of Glc (kit code: A043-1-1), Pro (kit code: A045-2-2), and TG (kit code: A110-2-1) were determined with a microplate reader (SpectraMax M5, Molecular Devices, San Jose, CA, USA). The supernatants prepared from different treatment groups for the measurement of the biochemical parameters of B. plicatilis lacked any color reaction. The absorption wavelengths of the biochemical parameters in the absorptiometric method are as follows: T-AOC (520 nm), MDA (532 nm), AMS (540 nm), PEP (660 nm), CL (550 nm), ALP (520 nm), GPT (505 nm), XOD (530 nm), LDH (440 nm), Glc (620 nm), Pro (595 nm), and TG (510 nm).
The enzyme activities were calculated in the linear range according to the protein content in the rotifers. The T-AOC level and MDA content were determined as described by Jia et al. (2015) and Cheng et al. (2019). The activities of ALP, GPT, XOD, and LDH were measured as described by Dube et al. (2014). Meanwhile, the activities of AMS, PEP, and CL and the contents of Glc, Pro, and TG were determined as described by Liang et al. (2017, 2021b). The total ROS levels (O2–, H2O2, and ·OH) of B. plicatilis were determined. The fluorescent probe 2′,7′-dichlorodihydrofluorescein diacetate acetyl ester (DCFH-DA, St. Louis, MO, USA) was used to measure the total ROS levels of the rotifers in accordance with the protocol included with the kit (kit code: E004-1-1) and the study of Wang et al. (2021).
Determination of the ROS levels generated in K. mikimotoi
K. mikimotoi in the exponential growth phase was grown in enriched f/2 media to the concentration of 1 × 105 cells mL−1 to determine the ROS levels in the tested K. mikimotoi strain. Algal intracellular ROS level was analyzed by using DCFH-DA as the fluorescent dye. K. mikimotoi cells were collected through centrifugation at 2000 × g for 10 min at 4 °C. The collected K. mikimotoi cells were washed with PBS (pH 7.4–7.6) and stained with 10 µM DCFH-DA for 30 min at 22 °C. Thereafter, the algal cells were washed and resuspended in PBS, and ROS production was detected in reference to the studies of Chen et al. (2016) and Kim et al. (2019). Three biological replicates were employed for ROS determination.
Statistical analysis
The normally distributed data were evaluated by Kolmogorov–Smirnov test, and homogeneity was assessed by Levene’s test. All data were suitable for normal distributions and satisfied with testing by analysis of variance (ANOVA). The r, S, and SGR of the rotifers within 24 h were assessed by two-way ANOVA, followed by Tukey’s multiple range test. The enzyme activities and energetic substance contents of the rotifers were analyzed by using one-way ANOVA according to Fisher’s LSD test. The differences were considered significant at p < 0.05. The data were presented as mean ± 1 standard error (SE). The statistical analysis and graphs were developed using OriginPro 2021 and SigmaPlot 12.5.
Results
Growth performance of rotifers
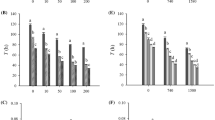
K. mikimotoi with different proportions significantly inhibited the population growth, survival, and specific growth of B. plicatilis within 24 h (two-way ANOVA, p < 0.001, Table 1). The S of maternal rotifers exposed to the treatments with different proportions of K. mikimotoi was <100%. Under these treatments, the rotifers that remained alive could produce offspring and K. mikimotoi was harmful to reproduction, thus reducing the r value to less than 1. Toxicity evaluation using the S, r, and SGR of B. plicatilis can comprehensively reflect the ecotoxicological effect of K. mikimotoi on the rotifers in this study. The rotifer r decreased by 8–83%, S decreased by 10–87%, and SGR decreased by 24–89% compared with the control treatment (100% N) when the proportions of K. mikimotoi and the exposure time increased (Fig. 1). The significant decline in the r, S, and SGR of B. plicatilis was exposed in the 25, 50, 75, and 100% K. mikimotoi at exposure times of 6, 12, 18, and 24 h (p < 0.05, Fig. 1a–c). The minimum r (0.17 ± 0.03 h–1), S (12.5 ± 3.44%), and SGR (10.6 ± 1.82%) were observed after the rotifers were exposed to treatment with 100% K and at the exposure time of 24 h (Fig. 1).
Changes in the (a) population growth rate (r), (b) survival rate (S), and (c) specific growth rate (SGR) of the rotifers within different treatments during the exposure time of 24 h. All treatments were compared with 100% N. salina (100% N). N: N. salina, K: K. mikimotoi. The values are expressed as the mean + SE of 20 replicate samples, and the error bars indicate one SE. The different letters denote the significant difference at a certain exposure time (p < 0.05)
Antioxidative responses of rotifers and the generation of ROS in K. mikimotoi
K. mikimotoi inhibited the total antioxidant capacity T-AOC level (p < 0.05) but promoted the ROS production (p < 0.05), and the end-product of lipid peroxide MDA content (p < 0.001) increased (one-way ANOVA, Fig. 2). The T-AOC level, an indicator of the capacity of antioxidation response in rotifers, decreased by 27–38% when the proportion of K. mikimotoi increased (p < 0.05, Fig. 2a). The high proportion of K. mikimotoi enhanced the toxicity on antioxidation response in the rotifers in terms of the T-AOC level, and the minimum T-AOC level was observed under the 100% K treatment (Fig. 2a). The total ROS levels and MDA content of the rotifers increased by 17–24% (p < 0.05) and 80–141% (p < 0.01), respectively, when the proportion of K. mikimotoi was increased (Fig. 2b, c). The K. mikimotoi strain produced a large amount of ROS. The ROS level generated in the test K. mikimotoi was 26082 ± 585 fluorescence intensity mg–1 protein.
Changes in the (a) total antioxidant capacity level (T-AOC), (b) total reactive oxygen species levels (ROS), and (c) malondialdehyde content (MDA) of the rotifers within different treatments. All treatments were compared with 100% N. salina (100% N). N: N. salina, K: K. mikimotoi. The values are expressed as the mean + SE of three replicate samples. The error bars indicate one SE, and the different singles denote a significant difference (*p < 0.05, **p < 0.01)
Digestive physiological activities of rotifers
The AMS, PEP, and CL activities were evaluated to estimate the digestive physiological activities of the rotifers under the stress imposed by different proportions of K. mikimotoi. The K. mikimotoi solution and the mixture of K. mikimotoi and N. salina improved the rotifer AMS activity (p < 0.05) but negatively affected the activities of PEP (p < 0.05) and CL (p < 0.05, one-way ANOVA, Fig. 3). The AMS activity increased by 50–117% with increasing K. mikimotoi proportions, but the activities of PEP and CL decreased by 2–26% (Fig. 3). The higher proportions of K. mikimotoi significantly affected the digestive enzyme activities. Significant differences in the changes of AMS, PEP, and CL activities were observed under the 50, 75, and 100% K. mikimotoi treatments (p < 0.05, Fig. 3a–c).
Changes in the (a) amylase activity (AMS), (b) pepsase activity (PEP), and (c) cellulase activity (CL) of the rotifers within different treatments. All treatments were compared with 100% N. salina (100% N). N: N. salina, K: K. mikimotoi. The values are expressed as the mean + SE of three replicate samples. The error bars indicate one SE, and the different singles denote a significant difference (*p < 0.05, **p < 0.01)
Metabolic physiological activities of rotifers
The metabolic enzyme activities were generally suppressed under K. mikimotoi stress (Fig. 4). The activities of ALP (p < 0.05), XOD (p < 0.001), and LDH (p < 0.001) decreased by 8–45%, 2–11%, and 3–9%, respectively, when the proportions of K. mikimotoi increased (one-way ANOVA, Fig. 4a, c and d). The GPT activity increased by 3–21% under the 25 and 50% K. mikimotoi treatments and decreased by 20–33% with the 75 and 100% K. mikimotoi (p < 0.01, Fig. 4b). The peak value of the GPT activity (24.87 ± 0.54 U mg−1 protein) was recorded under the treatment with 50% N. salina + 50% K. mikimotoi, and the minimum GPT activity (13.82 ± 0.49 U mg−1 protein) was observed under the 100% K. mikimotoi treatment (p < 0.01, Fig. 4b).
Changes in the (a) alkaline phosphatase activity (ALP), (b) glutamic–pyruvic transaminase activity (GPT), (c) xanthine oxidase activity (XOD), and (d) lactate dehydrogenase activity (LDH) of the rotifers within different treatments. All treatments were compared with 100% N. salina (100% N). N: N. salina, K: K. mikimotoi. The values are expressed as the mean + SE of three replicate samples. The error bars indicate one SE, and the different singles denote a significant difference (*p < 0.05, **p < 0.01)
Energetic substance contents of rotifers
The contents of Glc (p < 0.001) and Pro (p < 0.05) in the rotifers were decreased by K. mikimotoi (one-way ANOVA, Fig. 5). Meanwhile, the contents of Glc and Pro decreased by 6–34% and 15–44%, respectively, when the proportions of K. mikimotoi increased (p < 0.05, Fig. 5a, b). The TG content slightly increased by 2–3% with the rotifers fed with 25 and 50% K. mikimotoi, but the TG content decreased by 5–7% under the 75 and 100% K. mikimotoi treatments (p < 0.05, Fig. 5c). The minimum values of Glc (11.75 ± 0.33 mg g−1 dry weight), Pro (0.34 ± 0.04 g g−1 dry weight), and TG (28.96 ± 0.45 mg g−1 dry weight) were observed under the 100% K. mikimotoi treatment (Fig. 5).
Changes in the (a) glycogen content (Glc), (b) protein content (Pro), and (c) triglyceride content (TG) of the rotifers within different treatments. All treatments were compared with 100% N. salina (100% N). N: N. salina, K: K. mikimotoi. The values are expressed as the mean + SE of three replicate samples. The error bars indicate one SE, and the different singles denote a significant difference (*p < 0.05, **p < 0.01)
Correlation analysis among the growth performance, antioxidant responses, physiological activities, and energetic substance contents of rotifers
The r and S were positively correlated with the SGR and T-AOC level (p < 0.05, Fig. 6). The r, S, and SGR were negatively correlated with the ROS level, MDA content, and AMS activity (p < 0.05), but had positive correlations with the PEP, CL, ALP, XOD, and LDH activities and Glc and Pro contents (p < 0.05, Fig. 6). The T-AOC level was negatively correlated with the ROS level, MDA content, and AMS activity, but had positive correlations with the PEP, CL, and LDH activities and Pro content (p < 0.05, Fig. 6). The ROS level, MAD content, and AMS activity were negatively correlated with the PEP, CL, ALP, XOD, and LDH activities and Glc and Pro contents (p < 0.05, Fig. 6).
Pearson’s correlation analysis results of the growth performance, antioxidant responses, physiological activities, and energetic substance contents of B. plicatilis at the exposure time of 24 h. *Significant differences at p < 0.05, **significant differences at p < 0.01. r: population growth rate, S survival rate, SGR specific growth rate, T-AOC total antioxidant capacity level, ROS reactive oxygen species level, MDA malondialdehyde content, AMS amylase activity, PEP pepsase activity, CL cellulase activity, ALP alkaline phosphatase activity, GPT glutamic–pyruvic transaminase activity, XOD xanthine oxidase activity, LDH lactate dehydrogenase activity, Glc glycogen content, Pro protein content, TG triglyceride content
Discussion
Effects of K. mikimotoi on the growth performance of rotifers
The toxic K. mikimotoi deteriorated the growth performance of the rotifers, thus affecting the population dynamics of the zooplankton in the seawater ecosystem. The K. mikimotoi cells and their lipophilic extracts showed hemolytic and cytotoxic activity against fish, shellfish, and zooplankton (Dang et al. 2015; Li et al. 2019, 2020). By contrast, the lipophilic extracts of K. mikimotoi showed hypotoxicity, indicating their slight effect on the survival of fish and shellfish (Li et al. 2017). K. mikimotoi causes mortality by reducing the activity of the crucial biological enzymes of rotifers, leading to cell permeability disorder and growth inhibition (Li et al. 2020). To find out which part of K. mikimotoi is toxic, the acute lethal test was applied under cell-free culture, ruptured cells, and cell resuspension, suggesting that the toxicities of K. mikimotoi on the growth of rotifers were correlated with the contacted intact algal cells (Zou et al. 2010; Li et al. 2017; Li et al. 2018). The toxicity of the K. mikimotoi cells was weakened when the cells were ruptured and almost disappeared when the algal cells were removed from the culture by filtration (Li et al. 2019). The importance of r lies partly in its central role in forecasting future population trends (Sibly and Hone 2002). The extracellular mixture of toxins released from the living alga cells mainly caused the toxic effect of K. mikimotoi on the population growth of B. plicatilis (Li et al. 2017). The decreased S and SGR were positively correlated with the decline of population growth of rotifers under the stress of K. mikimotoi.
Morphologically, although K. mikimotoi lacks thecal plates, the diameter of this algal cell is much larger (23–40 µm) than that of N. salina (up to only 4 µm) (Cho et al. 2022). K. mikimotoi cells were not eaten by the rotifers because braconid rotifers avoid a diet size larger than 20 µm. Consequently, the toxic effects of K. mikimotoi on the rotifers are strongly related to the contact with living cells. K. mikimotoi causes lethality in B. plicatilis by direct contact with live cell-mediated hemolytic activity (Zou et al. 2010). Direct contact with living algal cells to promote the release of the extracellular mixture of toxins of K. mikimotoi have played an important role in inhibiting the population growth, survival, and specific growth of the rotifers. The r, S, and SGR reflect the survival and population growth status of B. plicatilis. Rotifers fed with 100% K. mikimotoi had the lowest r, S, and SGR, given that the 100% K. mikimotoi had the highest proportions of extracellular mixtures of toxins, and the rotifers had great opportunities to contact the toxic cells directly, thus negatively affecting the growth of the rotifers. Moreover, B. plicatilis has a simple body structure surrounded by a thin layer of corneum, causing rotifers to become sensitive to K. mikimotoi cells and its toxic effects. The inhibited survival and growth performance had pronounced influences on wider aquatic animal population dynamics. Therefore, the planktonic community structure would be negatively affected by the K. mikimotoi-dominated eutrophic seawater with the decline in the population growth of rotifers.
Effects of K. mikimotoi on the antioxidative responses of rotifers
The negative influences of toxic algae on aquatic animals were related to algal toxicity, mechanical damage, and poor nutritional quality (Liang et al. 2017; Xu et al. 2020). The T-AOC level of B. plicatilis reflected the antioxidant capacity of the organism, including the antioxygenation of the antioxidant enzyme activities and nonenzyme antioxidant. The tested K. mikimotoi strain produced a large amount of ROS. When the rotifers were exposed to 25, 50, 75, and 100% K. mikimotoi, the released toxins and ROS generated by K. mikimotoi increased the total ROS levels of the rotifers. The excessive ROS reacted with the lipids on the cell membranes, thus increasing the lipid peroxide end-product MDA content in the rotifers. K. mikimotoi was hemolytic, cytotoxic, and ROS producing, causing hemolysis, cytolysis, and oxidative damage in aquatic organisms (Li et al. 2019). A recent study showed that K. mikimotoi could threaten copepod Tigriopus japonicus population recruitment, and its adverse effects are associated with oxidative stress (Chen et al. 2022). In B. plicatilis, the decreased T-AOC level and the increased total ROS levels and MDA content indicated that lipid peroxidation was enhanced, and that the antioxidant defense system could not overcome the excessive ROS. The excessive ROS that accumulated in the rotifers induced oxidative stress, which may deteriorate the physiological responses and growth performance of B. plicatilis. The increased ROS level and MDA content had negative effects on the survival and growth performance of the rotifers.
Effects of K. mikimotoi on the digestive and metabolic physiological activities of rotifers
Digestive enzyme activities could be used as endpoints to predict the population growth and survival of organisms (Durigon et al. 2019). The AMS activity increased with the increase in the proportions of K. mikimotoi, indicating that it may contain substances to promote the production of AMS in the rotifers. The PEP and CL activities decreased under the treatments with 25, 50, 75, and 100% K. mikimotoi. Considering that B. plicatilis could not ingest K. mikimotoi, the extracellular mixtures of toxins released by K. mikimotoi mainly caused the altered physiological activity in the rotifers. Moreover, the PEP and CL activities had negative correlations with the ROS level, indicating that digestive enzyme activities were sensitive to oxidative stress, and a high ROS level negatively affected the PEP and CL activities in the rotifers fed with different proportions of K. mikimotoi. The AMS activity negatively affected the r, S, and SGR, whereas the PEP and CL activities had positive correlations with the population growth, survival, and specific growth of rotifers. Changes in the activities of AMS, PEP, and CL were related to the population growth of B. calyciflorus under the combined stress of M. aeruginosa and nitrite (Liang et al. 2021b). The increase in the AMS activity and decline in PEP and CL activities in this study suggested that digestive physiological response might be correlated with the population growth dynamics of B. plicatilis.
The metabolic physiological activities were useful for monitoring the survival and growth of rotifers (Liang et al. 2021b). The activities of ALP, XOD, and LDH decreased with the increased proportions of K. mikimotoi. The GPT activity increased under the 25 and 50% K. mikimotoi treatments but decreased under 75 and 100% K. mikimotoi treatments. Consequently, the ALP, XOD, and LDH activities were more sensitive than the GPT activity to K. mikimotoi. The dietary conditions affected the growth and metabolic enzyme activities in the organisms (Anand et al. 2014; Abdel-Tawwab et al. 2021). The rotifers fed with 50% N. salina and 50% K. mikimotoi had the highest GPT activity, indicating that mixtures with the same proportions of N. salina and K. mikimotoi enhanced the GPT activity. ALP that mainly catalyzes the dephosphorylation of monophosphate is a kind of homodimer metalloproteinases with hydrolytic activity (Zhang et al. 2021). The XOD that promotes uric acid production is one of the main enzymatic sources of ROS in vivo (You et al. 2022). LDH is a stable cytoplasmic enzyme, which activity could be as an effect criterion in toxicity tests with the cladoceran Daphnia magna (Diamantino et al. 2001). This study found that the ALP, XOD, and LDH activities had negative correlations with the ROS level, but had positive correlations with the r, S, and SGR of B. plicatilis.
Oxidative damage might have mainly caused the decrease in metabolic enzyme activities in B. plicatilis, which inhibited the population growth of the rotifers. Metabolic enzyme assay, such as LDH, is an easy and fast method of detecting cell lysis caused by harmful algal species (Zou et al. 2013). K. mikimotoi causes cytolysis in rainbow trout gill, thus reducing cell viability and inducing decreased LDH activity (Dorantes-Aranda et al. 2015). In this work, the decreased ALP, XOD, and LDH activities affected the metabolic processes and nutritional status and were related to the decreased r, S, and SGR of B. plicatilis. K. mikimotoi is known to produce low-molecular weight polyether toxins and hemolytic toxins, cytotoxic polyethers, and ROS, which could inflict cell-mediated hemolytic damage to the growth performance of rotifers (Yamasaki et al. 2004; Neely and Campbell 2006; Li et al. 2019). The metabolic function changes in B. plicatilis were mainly caused by the extracellular toxins released from the K. mikimotoi, which were harmful to the population growth of rotifers (Yang et al. 2011; Li et al. 2020).
Effects of K. mikimotoi on the energetic substance contents of rotifers
The energetic substance analysis was related to the content differentiation affecting the rotifer’s population dynamic alteration under the stress of K. mikimotoi. Digestive and metabolic enzyme activities were directly related to the absorption of energetic substances in the rotifers, thus inhibiting the growth performance of B. plicatilis. Glycogen, protein, and lipids were important energetic substances for the survival of rotifers (Olsen et al. 1993). Dietary lipids affect the growth, survival, and physiological activities in juvenile cuttlefish (Han et al. 2017). The Glc and Pro contents decreased with increasing proportions of K. mikimotoi, and the TG content remarkably decreased under the 75 and 100% K. mikimotoi treatments. The total amount of lipid in organisms was related to the TG content, which is commonly used to evaluate the carbohydrate level in rotifers (Kwiterovich 2000; Liang et al. 2017). The contents of Glc and TG reflected the nutrition levels of carbohydrates and lipids in rotifers. The decreased Glc, Pro, and TG contents were related to the inhibition of digestive and metabolic enzyme activities and oxidative damage in B. plicatilis.
Various lipophilic toxic secondary metabolites, including hemolytic compounds and cytotoxic polyethers, which were produced by K. mikimotoi, might contribute to the poor energetic substance absorption in rotifers. Some other toxins might be involved in the intoxication and affect food digestion and absorption in B. plicatilis. The same toxic effect or nutritional inadequacy was observed in multiple grazers, including the cladoceran Moina mongolica and the copepod Pseudodiaptomus annandalei (Dang et al. 2015). The decreased Glc, Pro, and TG contents contribute to a reduction in energy availability to B. plicatilis. Zooplankton typically has a critical threshold of energy requirement. Below the threshold, the growth performance of zooplankton declines due to the inadequate energy provided by ambient resource (Lyu et al. 2022). With increased proportions of K. mikimotoi, the energy consumed is larger than the energy required for rotifer survival, specific growth, and population growth due to the decreased energetic substance contents. The reduction in growth performance, increase in oxidative stress, and inhibition of crucial enzyme activities in the rotifers supported the hypothesis that changes in the physiological activities and energetic substance contents of the rotifers are correlated with the influence of K. mikimotoi on the population growth of B. plicatilis. The potential toxicity mechanism is related to ROS-mediated antioxidative responses, digestive and metabolic physiological activities, and energetic substance content changes in B. plicatilis that negatively affected population growth, survival, and specific growth rates and resulted in the population growth inhibition of the rotifers under K. mikimotoi stress (Fig. 7).
Conjectured potential toxicity mechanism of how K. mikimotoi induces toxicity in the growth performance of B. plicatilis according to the obtained results. r: population growth rate, S survival rate, SGR specific growth rate, T-AOC total antioxidant capacity level, ROS total reactive oxygen species levels, MDA malondialdehyde content, AMS amylase activity, PEP pepsase activity, CL cellulase activity, ALP alkaline phosphatase activity, GPT glutamic–pyruvic transaminase activity, XOD xanthine oxidase activity, LDH lactate dehydrogenase activity, Glc glycogen content, Pro protein content, TG triglyceride
Conclusions
Our results proved that K. mikimotoi inhibited the survival and growth performance of the rotifers. The ROS level, MDA content, and AMS activity increased, but the T-AOC level, the PEP, CL, ALP, XOD, and LDH activities, and the Glc and Pro contents decreased under the 25, 50, 75, and 100% K. mikimotoi treatments. The GPT activity and TG content increased under the 25 and 50% K. mikimotoi treatments but decreased under the 75 and 100% K. mikimotoi treatments. Accordingly, the K. mikimotoi solutions showed a considerably inhibitory effect on the population growth of B. plicatilis and ROS-mediated physiological activities, and energetic substance content changes were involved in the inhibition of the growth performance of the rotifers. The internal regulatory mechanisms of K. mikimotoi that negatively affected the population growth and physiological responses of aquatic animals under eutrophic conditions should be further studied.
References
Abdel-Tawwab M, Shukry M, Farrag FA, EI-Shafai NM, Dawood MAO, Abdel-Latif HMR (2021) Dietary sodium butyrate nanoparticles enhanced growth, digestive enzyme activities, intestinal histomorphometry, and transcription of growth-related genes in Nile tilapia juveniles. Aquaculture 536:736467. https://doi.org/10.1016/j.aquaculture.2021.736467
Anand PSS, Kohli MPS, Roy SD, Sundaray JK, Kumar S, Sinha A, Pailan GH, Sukham MK (2014) Effect of dietary supplementation of periphyton on growth, immune response and metabolic enzyme activities in Penaeus monodon. Aquacult Res 46:2277–2288. https://doi.org/10.1111/are.12385
Basti L, Nagai S, Go J, Okano S, Nagai K, Watanabe R, Suzuki T, Tanaka Y (2015) Differential inimical effects of Alexandrium spp. and Karenia spp. on cleavage, hatching, and two larval stages of Japanese pearl oyster Pinctada fucata martensii. Harmful Algae 43:1–12. https://doi.org/10.1016/j.hal.2014.12.004
Chen B, Wang K, Guo H, Lin H (2021) Karenia mikimotoi blooms in coastal waters of China from 1998 to 2017. Estuarine Coastal Shelf Sci 249:107034. https://doi.org/10.1016/j.ecss.2020.107034
Chen H, Tang X, Zhou B, Xu N, Wang Y (2016) Mechanism of Deca-BDE-induced apoptosis in Neuro-2a cells: Role of death-receptor pathway and reactive oxygen species-mediated mitochondrial pathway. J Environ Sci 46:241–251. https://doi.org/10.1016/j.jes.2016.02.015
Chen H, Wang J, Zhuang Y, Yu W, Liu G (2022) Reduced fitness and elevated oxidative stress in the marine copepod Tigriopus japonicus exposed to the toxic dinoflagellate Karenia mikimotoi. Antioxidants 11:2299. https://doi.org/10.3390/antiox11112299
Cheng CH, Ma HL, Deng YQ, Feng J, Jie YK, Guo ZX (2021) Oxidative stress, cell cycle arrest, DNA damage and apoptosis in the mud crab (Scylla paramamosain) induced by cadmium exposure. Chemosphere 263:128277. https://doi.org/10.1016/j.chemosphere.2020.128277
Cheng CH, Ma HL, Su YL, Deng YQ, Feng J, Xie JW, Chen XL, Guo ZX (2019) Ammonia toxicity in the mud crab (Scylla paramamosain): The mechanistic insight from physiology to transcriptome analysis. Ecotoxicol Environ Saf 179:9–16. https://doi.org/10.1016/j.ecoenv.2019.04.033
Cho K, Ueno M, Liang Y, Kim D, Oda T (2022) Generation of reactive oxygen species (ROS) by harmful algal bloom (HAB)–forming phytoplankton and their potential impact on surrounding living organisms. Antioxidants 11:206. https://doi.org/10.3390/antiox11020206
Coggins BL, Collins JW, Holbrook KJ, Yampolsky LY (2017) Antioxidant capacity, lipid peroxidation, and lipid composition changes during long-term and short-term thermal acclimation in Daphnia. J Comp Physiol B 187:1091–1106. https://doi.org/10.1007/s00360-017-1090-9
Conlin SM, Tudor MS, Shim J, Gosse JA, Neilson A, Hamlin HJ (2018) Elevated nitrate alters the metabolic activity of embryonic zebrafish. Environ Pollut 235:180–185. https://doi.org/10.1016/j.envpol.2017.12.069
Dang LX, Li Y, Liu F, Zhang Y, Yang WD, Li HY, Liu JS (2015) Chemical response of the toxic dinoflagellate Karenia mikimotoi against grazing by three species of zooplankton. J Eukaryot Microbiol 62:470–480. https://doi.org/10.1111/jeu.12201
Diamantino TC, Almeida E, Soares AMVM, Guilhermino L (2001) Lactate dehydrogenase activity as an effect criterion in toxicity tests with Daphnia magna straus. Chemosphere 45:553–560. https://doi.org/10.1016/S0045-6535(01)00029-7
Dorantes-Aranda JJ, Seger A, Mardones JI, Nichols PD, Hallegraeff GM (2015) Progress in understanding algal bloom-mediated fish kills: the role of superoxide radicals, phycotoxins and fatty acids. PLoS ONE 10:e0133549. https://doi.org/10.1371/journal.pone.0133549
Dube PN, Shwetha A, Hosetti BB (2014) Impact of copper cyanide on the key metabolic enzymes of freshwater fish Catla catla (Hamilton). Biotechnol Anim Husband 30:499–508. https://doi.org/10.2298/BAH1403499D
Durigon EG, Almeida APG, Jerônimo GT, Baldisserotto B, Emerenciano MGC (2019) Digestive enzymes and parasitology of Nile tilapia juveniles raised in brackish biofloc water and fed with different digestible protein and digestible energy levels. Aquaculture 506:35–41. https://doi.org/10.1016/j.aquaculture.2019.03.022
Graff JR, Milligan AJ, Behrenfeld MJ (2012) The measurement of phytoplankton biomass using flow-cytometric sorting and elemental analysis of carbon. Limnol Oceanogr: Methods 10:910–920. https://doi.org/10.4319/lom.2012.10.910
Han Q, Wang Y, Lv T, Han Q, Jiang X (2017) Effects of dietary lipids on the growth performance, survival, and digestive enzymes of juvenile cuttlefish, Sepia lycidas. J World Aquacult Soc 48:963–971. https://doi.org/10.1111/jwas.12446
Higo S, Maung-Saw-Htoo-Thaw Yamatogi T, Ishida N, Hirae S, Koike K (2017) Application of a pulse-amplitude-modulation (PAM) fluorometer reveals its usefulness and robustness in the prediction of Karenia mikimotoi blooms: a case study in Sasebo Bay, Nagasaki, Japan. Harmful Algae 61:63–70. https://doi.org/10.1016/j.hal.2016.11.013
Jia R, Han C, Lei JL, Liu BL, Huang B, Huo HH, Yin ST (2015) Effects of nitrite exposure on haematological parameters, oxidative stress and apoptosis in juvenile turbot (Scophthalmus maximus). Aquat Toxicol 169:1–9. https://doi.org/10.1016/j.aquatox.2015.09.016
Kanta P, Ghosh T, Kaur A, Muthukumarappa T (2021) An innovative and cost-effective way to estimate alkaline phosphatase activity in in vitro cellular model systems. Int J Biochem Mol Biol 12:1–7. https://doi.org/10.1016/j.aquatox.2015.09.016
Kim D, Wencheng L, Matsuyama Y, Cho K, Yamasaki Y, Takeshita S, Yamaguchi K, Oda T (2019) Extremely high level of reactive oxygen species (ROS) production in a newly isolated strain of the dinoflagellate Karenia mikimotoi. Eur J Phycol 54:632–640. https://doi.org/10.1080/09670262.2019.1632936
Kok JWK, Leong SCY (2019) Nutrient conditions and the occurrence of a Karenia mikimotoi (Kareniaceae) bloom within East Johor Straits. Singapore. Reg Stud Mar Sci 27:100514. https://doi.org/10.1016/j.rsma.2019.100514
Krebs CJ (1994) Ecology: the Experimental Analysis of Distribution and Abundance, 4th edn. Harper Collins College Publishers, New York
Kwiterovich PO (2000) The metabolic pathways of high-density lipoprotein, low-density lipoprotein, and triglycerides: a current review. Am J Cardiol 86:5–10. https://doi.org/10.1016/S0002-9149(00)01461-2
Lampert W, Trubetskova I (1996) Juvenile growth rate as a measure of fitness in Daphnia. Funct Ecol 10:631–635. https://doi.org/10.2307/2390173
Li X, Yan T, Lin J, Yu R, Zhou M (2017) Detrimental impacts of the dinoflagellate Karenia mikimotoi in Fujian coastal waters on typical marine organisms. Harmful Algae 61:1–12. https://doi.org/10.1016/j.hal.2016.11.011
Li X, Yan T, Yu R, Zhou M (2019) A review of Karenia mikimotoi: bloom events, physiology, toxicity and toxic mechanism. Harmful Algae 90:101702. https://doi.org/10.1016/j.hal.2019.101702
Li X, Yan T, Zhang Q, Yu R, Zhou M (2020) Inhibition to crucial enzymes in the lethal effects of the dinoflagellate Karenia mikimotoi on the rotifer Brachionus plicatilis. Mar Environ Res 157:104866. https://doi.org/10.1016/j.marenvres.2019.104866
Li Y, Yu J, Sun T, Liu C, Sun Y, Wang Y (2018) Using the marine rotifer Brachionus plicatilis as an endpoint to evaluate whether ROS-dependent hemolytic toxicity is involved in the allelopathy induced by Karenia mikimotoi. Toxins 10:439. https://doi.org/10.3390/toxins10110439
Liang Y, Guo H, Liao Q, Zhang X, Huang K (2020) Growth performance, phenotypic traits, and antioxidant responses of the rotifer Brachionus plicatilis under different proportions of Phaeocystis globosa. Ecotox Environ Safe 202:110963. https://doi.org/10.1016/j.ecoenv.2020.110963
Liang Y, Su Y, Ouyang K, Chen X, Yang J (2017) Effects of microcystin-producing and mucrocystin-free Microcystis aeruginosa on enzyme activity and nitrite content in the rotifer Brachionus calyciflorus. Environ Sci Pollut Res 24:10430–10442. https://doi.org/10.1007/s11356-017-8704-3
Liang Y, Yang X, Wang Y, Liu R, Gu H, Mao L (2021a) Influence of polystyrene microplastics on rotifer (Brachionus calyciflorus) growth, reproduction, and antioxidant responses. Aquat Ecol 55:1097–1111. https://doi.org/10.1007/s10452-021-09885-y
Liang Y, Zhou Y, Wang Y, Liu R, Qi J, Lin Y, Zhang T, Jiang Q (2021b) Use of physiological activities to estimate the population growth of rotifer (Brachionus calyciflorus) under the stress of toxic Microcystis and nitrite. Chemosphere 285:131419. https://doi.org/10.1016/j.chemosphere.2021.131419
Lin J, Yan T, Zhang Q, Zhou M (2016) Impact of several harmful algal bloom (HAB) causing species, on life history characteristics of rotifer Brachionus plicatilis Müller. Chin J Oceanol Limnol 34:642–653. https://doi.org/10.1007/s00343-016-5065-6
Lin JN, Song JJ, Yan T, Zhang QC, Zhou MJ (2015) Large-scale dinoflagellate bloom species Prorocentrum donghaiense and Karenia mikimotoi reduce the survival and reproduction of copepod Calanus sinicus. J Mar Biol Assoc UK 95:1071–1079. https://doi.org/10.1017/S0025315415000533
Lyu K, Yu B, Li D, Gu L, Yang Z (2022) Increased food availability reducing the harmful effects of microplastics strongly depends on the size of microplastics. J hazard Mater 437:129375. https://doi.org/10.1016/j.jhazmat.2022.129375
Mills S, Alcántara-Rodríguez JA, Ciros-Pérez J, Gómez A, Hagiwara A, Galindo KH, Jersabek CD, Malekzadeh-Viayeh R, Leasi F, Lee JS, Mark Welch DB, Papakostas S, Riss S, Segers H, Serra M, Shiel R, Smolak R, Snell TW, Stelzer CP, Tang CQ, Wallace RL, Fontaneto D, Walsh EJ (2017) Fifteen species in one: deciphering the Brachionus plicatilis species complex (Rotifera, Monogononta) through DNA taxonomy. Hydrobiologia 796:39–58. https://doi.org/10.1007/s10750-016-2725-7
Neely T, Campbell L (2006) A modified assay to determine hemolytic toxin variability among Karenia clones isolated from the Gulf of Mexico. Harmful Algae 5:592–598. https://doi.org/10.1016/j.hal.2005.11.006
Niu X, Xu S, Yang Q, Xu X, Zheng M, Li X, Guan W (2021) Toxic effects of the dinoflagellate Karenia mikimotoi on zebrafish (Danio rerio) larval behavior. Harmful Algae 103:101996. https://doi.org/10.1016/j.hal.2021.101996
Olsen Y, Reitan KI, Vadstein O (1993) Dependence of temperature on loss rates of rotifers, lipids and ω3 fatty acids in starved Brachionus plicatilis cultures. Hydrobiologia 255:13–20. https://doi.org/10.1007/BF00025815
Sibly RM, Hone J (2002) Population growth rate and its determinants: an overview. Phil Trans R Soc Lond B 357:1153–1170. https://doi.org/10.1098/rstb.2002.1117
Snell TW, Janssen CR (1995) Rotifers in ecotoxicology: a review. Hydrobiologia 313–314:231–247. https://doi.org/10.1007/BF00025956
Sun S, Fu H, Zhu J, Ge X, Wu X, Qiao H, Jin S, Zhang W (2018) Molecular cloning and expression analysis of lactate dehydrogenase from the oriental river prawn Macrobrachium nipponense in response to hypoxia. Int J Mol Sci 19:1990. https://doi.org/10.3390/ijms19071990
Wang B, Guo Y, Zhou B, Zhang H, Cui X, Sun Y, Wang Y (2021) A possible speculation on the involvement of ROS and lysosomes mediated mitochondrial pathway in apoptosis of rotifer Brachionus plicatilis with BDE-47 exposure. Sci Total Environ 787:147315. https://doi.org/10.1016/j.scitotenv.2021.147315
Wang H, Tang X, Sha J, Chen H, Sun T, Wang Y (2015) The reproductive toxicity on the rotifer Brachionus plicatilis induced by BDE-47 and studies on the effective mechanism based on antioxidant defense system changes. Chemosphere 135:129–137. https://doi.org/10.1016/j.chemosphere.2015.03.090
Xu A, Shang-Guan J, Li Z, Gao Z, Huang YC, Chen Q (2020) Effects of dietary asafoetida (Ferula sinkiangensis K. M. Shen) levels on feeding attraction activity, growth performance, healthiness, and digestive enzyme activity in juvenile Lateolabrax japonicus. Fish Physiol Biochem 46:1991–2003. https://doi.org/10.1007/s10695-020-00849-x
Yamasaki Y, Kim D, Matsuyama Y, Oda T, Honjo T (2004) Production of superoxide anion and hydrogen peroxide by the red tide dinoflagellate Karenia mikimotoi. J Biosci Bioeng 97:212–215. https://doi.org/10.1016/S1389-1723(04)70193-0
Yang W, Zhang N, Cui W, Xu Y, Li H, Liu J (2011) Effects of co-existing microalgae and grazers on the production of hemolytic toxins in Karenia mikimotoi. Chin J Oceanol Limnol 29:1155–1163. https://doi.org/10.1007/s00343-011-0274-5
You S, Wang G, Zhou F, Wu H, Han Y, Xue W, Ma Y, Zhang C, Zhou L, Yan F, Fu C, Dong A (2022) Chapter 8–Antihyperuricemic peptides: A review focused on xanthine oxidase inhibitory activities. Studies Nat Products Chem 74:279–294. https://doi.org/10.1016/B978-0-323-91099-6.00013-X
Yu Z, Wu X, Zheng L, Dai Z, Wu L (2020) Effect of acute exposure to ammonia and BFT alterations on Rhynchocypris lagowski: Digestive enzyme, inflammation response, oxidative stress and immunological parameters. Environ Toxicol Phar 78:103380. https://doi.org/10.1016/j.etap.2020.103380
Zhang H, Ju Q, Pang S, Wei N, Zhang Y (2021) Recent progress of fluorescent probes for the detection of alkaline phosphatase (ALP): A review. Dyes Pigments 194:109569. https://doi.org/10.1016/j.dyepig.2021.109569
Zou Y, Kim D, Yagi M, Yamasaki Y, Kurita J, Iida T, Matsuyama Y, Yamaguchi K, Oda T (2013) Application of LDH-release assay to cellular-level evaluation of the toxic potential of harmful algal species. Biosci Biotechnol Biochem 77:345–352. https://doi.org/10.1271/bbb.120764
Zou Y, Yamasaki Y, Matsuyama Y, Yamaguchi K, Honjo T, Oda T (2010) Possible involvement of hemolytic activity in the contact-dependent lethal effects of the dinoflagellate Karenia mikimotoi on the rotifer Brachionus plicatilis. Harmful Algae 9:367–373. https://doi.org/10.1016/j.hal.2010.01.005
Acknowledgements
The work was supported by the National Natural Science Foundation of China (Grant number: 42206217), the Fund of Fujian Provincial Key Laboratory of Marine Ecological Conservation and Restoration, China (Grant number: EPR2020004), the Open–End Funds of Jiangsu Key Laboratory of Marine Biotechnology, Jiangsu Ocean University, China (Grant number: HS2021001), and the Startup Foundation for Introducing Talent of NUIST, China (Grant numbers: 2018r040 and 2020r062).
Author information
Authors and Affiliations
Contributions
YL: Investigation, Conceptualization, Methodology, Visualization, Writing-original draft, Funding acquisition, Project administration, Supervision, Writing-review & editing. JY: Methodology, Visualization. ZN: Data curation, Software, Validation. JZ: Methodology. HG: Project administration, Supervision, Writing-review & editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
All applicable international, national, and institutional guidelines for the use of animals were followed by the authors.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liang, Y., Yang, J., Ni, Z. et al. Dinoflagellate Karenia mikimotoi on the growth performance, antioxidative responses, and physiological activities of the rotifer Brachionus plicatilis. Ecotoxicology 32, 768–781 (2023). https://doi.org/10.1007/s10646-023-02686-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-023-02686-z