Abstract
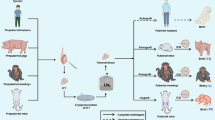
New and improved oncological therapies are now able to cure more than 80% of cancer-affected children in Europe. However, such treatments are gonadotoxic and result in fertility issues, especially in boys who are not able to provide a sperm sample before starting chemo/radiotherapy because of their prepubertal state. For these boys, cryopreservation of immature testicular tissue (ITT) is the only available option, aiming to preserve spermatogonial stem cells (SSCs). Both slow-freezing and vitrification have been investigated to this end and are now applied in a clinical setting for SSC cryopreservation. Research now has to focus on methods that will allow fertility restoration. This review discusses different studies that have been conducted on ITT transplantation, including those using growth factor supplementation like free molecules, or tissue encapsulation with or without nanoparticles, as well as the possibility of developing a bioartificial testis that can be used for in vitro gamete production or in vivo transplantation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Continued improvements in the long-term survival of prepubertal boys affected by cancer give rise to concerns about the future quality of life of these patients, especially in terms of reproductive capacity after gonadotoxic treatments.27,39 Freezing of sperm is not applicable in these young boys in whom spermatogenesis has not yet started. For these patients, cryopreservation of immature testicular tissue (ITT) is increasingly applied to preserve spermatogonial stem cells (SSCs) and, although still experimental, is considered acceptable from an ethical perspective.9,10,29,95,96 A number of patients whose ITT was cryopreserved have now reached the age at which they start to wonder about their fertility, some of them having been diagnosed with azoospermia (personal data from our department). Providing them with fertility restoration options is of paramount importance, since loss of fertility linked to cancer therapy is associated with major psychological trauma.80
The efficiency of ITT cryopreservation by slow-freezing or vitrification has been demonstrated in several animal species, such as mice, pigs, and Japanese quail yielding healthy offspring after transplantation.44,55,63,83 Cryopreservation of human ITT has been achieved using slow freezing or vitrification with similar results when evaluated after transplantation in nude mice.68 Clinical experience with cryopreservation of ITT has been reported45,95 but cryopreserved ITT has to the best of our knowledge not been used for restoring fertility so far. Slow programmed freezing of small fragments (2–4 mm3) with dimethyl sulfoxide as a cryoprotectant is commonly applied and it is accepted to limit the sampling to one testis and less than 5% of the testicular volume.95 However, while cryopreservation techniques allow spermatogonial survival and proliferation, significant loss of the spermatogonial pool and impaired differentiation have been observed in xenotransplantation models.20,67,68,89,90,94,97 Moreover, comparable spermatogonial survival rates with both fresh and cryopreserved tissue suggest that etiologies other than the cryopreservation procedure, namely hypoxia or recipient environment, could be implicated.68
A successful xenografting outcome depends on the speed of connection to the blood circulatory system of the recipient, supplying oxygen, nutrients, and hormones. Indeed, ischemic stress before graft revascularization may induce tissue necrosis or activation of the apoptosis pathway, as reported for testicular tissue.67 Ischemia–reperfusion may therefore cause damage to the SSC niche, consisting of Sertoli cells (SCs), Leydig cells (LCs), peritubular myoid cells, and the interstitial vascular network,81 known to be essential for maintenance of functional SSCs and tissue integrity. An inadequate nutrient and oxygen supply also appears to preclude seminiferous tubule (ST) maturation in some areas of grafted tissue.71 Limiting tissue ischemia and stem cell niche damage by ensuring faster graft reperfusion are therefore vital.
After discussing the state of the art in the field of cryopreserved ITT transplantation, this review looks at possible ways of improving the survival of spermatogonia in ITT transplants, either by free molecule supplementation or tissue engineering techniques based on nanotechnology involving encapsulation in hydrogels. Perspectives on creating a transplantable scaffold to support growth and differentiation of isolated testicular cells or an artificial testis are also discussed.
Testicular Tissue Transplantation
Transplantation of Cryopreserved ITT
Transplantation of testicular tissue pieces has the advantage of preserving an intact SSC niche, hence maintaining cellular cohesion and interactions. Numerous studies have assessed orthotopic or heterotopic grafting of frozen-thawed ITT in many animal species and complete spermatogenesis was achieved after transplantation of slow-frozen ITT in mice,28,59,63,83,98 rabbits,83 and pigs,1,37 as well as vitrified ITT in mice,7 pigs,1,44 and avians.55 Healthy offspring have also been obtained by intracytoplasmic sperm injection (ICSI) using spermatozoa isolated from cryopreserved ITT grafts from mice, avians, pigs, and monkeys.44,55,56,63,83 Details about cryopreserved animal ITT transplantation are summarized in Table 1.
Regarding cryopreserved human ITT, no allo- or autotransplantations have been performed to date, so current knowledge is only based on a few xenotransplantation experiments.67,68,89,90,94,97 Differentiation up to the pachytene spermatocyte stage was found after xenografting to mice testes of frozen-thawed and vitrified human ITT (one piece of 1–6 mm3).68,90,97 In addition, spermatid-like structures were observed by histology and electron microscopy, but no specific markers could be identified by immunohistochemistry, suggesting abnormal differentiation in frozen SSCs transplanted in nude mice.97
While studies on cryopreserved human ITT xenografts demonstrated survival of SSCs and initiation of spermatogenesis,68,94,97 spermatogonial recovery was greatly reduced, showing worsening rates over time (61% at 5 days, 14.5% at 3 weeks, and 3.7% at 6 months of orthotopic xenografting of one fragment of 1–6 mm3 in a nude mouse model).67,94,97 LCs also appeared to be preserved, with maintenance of their steroidegenic activity.68,97 Indeed, cryopreservation methods (automated/controlled or non-controlled slow-freezing or vitrification) have all their individual advantages, but survival of human spermatogonia is always found to be affected after xenografting.44,68,89,90,94,97
Interestingly, transplantation of fresh tissue68 yields similar results to frozen-thawed tissue, suggesting that the grafting procedure itself may be implicated and must therefore be improved. In order to minimize the period of hypoxia associated with avascular grafting, and hence ischemic injury that occurs during the first 5 days,88 different strategies aiming to enhance outcomes after testicular tissue transplantation have been evaluated.
Towards Improvement of Testicular Tissue Transplantation Outcomes
Use of Free Molecule Supplementation
To promote revascularization of testicular tissue grafts, both testicular tissue vessels and host vessels may be targeted, as reperfusion is initiated by outgrowing vessels from the transplanted tissue, which then connect to larger subcutaneous vessels formed by the host.78 Use of vascular endothelial growth factor (VEGF), a potent angiogenic protein, was found to stimulate neoangiogenesis.12,14,62,79 Testicular tissue was therefore pretreated with VEGF before or at the time of transplantation in order to improve graft outcomes, subsequently showing increased numbers of STs containing elongated spermatids in bovines.12,79
Limiting oxidative stress present under hypoxic conditions and responsible for germ cell loss is another option. Indeed, hypoxia induces formation of reactive oxygen species (ROS) that accumulate in cells and cause cell death. To prevent ROS formation or mitigate their deleterious effects, antioxidant molecules like N-acetylcysteine (NAC), vitamin C and/or vitamin E may be added. Systemic administration of antioxidants was found to reduce oxidative stress by enhancing intracellular generation of glutathione, and proved its efficacy in experiments looking to circumvent histopathological damage by cell membrane lipid peroxidation after testicular torsion/distortion.2,15,87 This approach was also used to counterbalance ROS-induced male germ cell damage in case of exposure to toxic agents, where NAC prevented apoptosis in the presence of methoxyacetic acid,70 and vitamin C and E were effective in mitigating the impact of lead exposure in rats by replenishing the antioxidant capacity of germ cells.6 Furthermore, treatment of lead-exposed animals with vitamin C and/or vitamin E resulted in increased number of luminal spermatozoa.6
However, data on testicular tissue transplantation outcomes that also take into account means of improvement are very limited. Indeed, few studies have explored the benefits of antioxidant supplementation. Addition of NAC as an antioxidant molecule did not improve spermatogonial survival in xenografts of frozen-thawed human ITT during the 5-day revascularization period.67 On the other hand, daily intraperitoneal injection of melatonin as a protective drug had a positive impact on the survival and recovery of SSCs and on tissue integrity in grafts of cryopreserved mouse ITT.33,34
Besides local or systemic supplementation of free molecules, tissue to be transplanted can be embedded in hydrogels loaded with growth factors or other molecules, incorporated or not into nanoparticles (NPs) allowing their controlled release. Use of extracellular matrices (ECMs) has also been considered for this purpose.
Hydrogels for Tissue Encapsulation and Drug Delivery
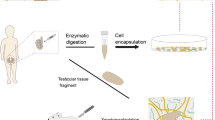
Tissue fragment encapsulation is based on cell encapsulation technology.61 Cell encapsulation relies on immobilization of cells in a biomaterial which allows bidirectional diffusion of nutrients, oxygen, and waste, thus promoting cell interactions. Hydrogels are commonly used for encapsulation. An ideal hydrogel for our purposes must be biocompatible, allow dissemination of vital nutrients and cell-secreted molecules, and favor (subject to application) vascular invasion and tissue integration in the host.24,43,61 Hydrogels can be synthetic or of biological origin, and degradable or non-degradable, depending on the intended use. The two main classes of natural polymers are proteins (collagen, gelatin, fibrinogen, elastin, keratin, actin, and myosin) and polysaccharides (alginate, amylose, dextran, chitin, and glycosaminoglycans). Synthetic polymers make up the largest group of biodegradable polymers. Polylactic acid, polyglycolic acid, and polylactic-co-glycolic acid copolymers are among the most commonly used synthetic polymers in tissue engineering.24,64 The particular physical characteristics of hydrogels and scope for structural modulation are useful for drug delivery applications, especially in the context of tissue transplantation. Indeed, on the one hand, their heavily porous structure can be easily adjusted by controlling the density of crosslinks in the matrix and/or hydrogel affinity in the aqueous environment. This is of particular relevance for neoangiogenesis, as the speed of revascularization and structure of the neovessel network have been shown to depend on chemical, physical, and mechanical properties of the polymer scaffold. Pore size over 100 µm is recommended to facilitate migration of endothelial cells through the hydrogel pores.4
On the other hand, hydrogel porosity also allows incorporation of drugs into the matrix and gradual release of active compounds through the hydrogel network.36 The advantages of using hydrogels for drug delivery therefore lie to a large extent in pharmacokinetics, especially with the possibility of creating depot formulations from which the drugs can be slowly eluted,24,36 maintaining high local concentrations in surrounding tissues for a defined period of time. This is especially pertinent in the context of clinical application in cancer patients, where systemic delivery of angiogenic molecules may be unsafe due to their inherent instability in vivo, hence running the risk of uncontrolled effects at distant sites.62
A number of studies have evaluated the beneficial impact of local delivery of drugs via hydrogels in regenerative medicine. Incorporation of VEGF microspheres into hydrogels has been found to enhance angiogenesis,51 with improved proliferation of endothelial cells and increased formation of new blood vessels. Moreover, sustained release of VEGF was also shown to boost vascularization in various pathologies, like cardiac disease85 and spinal cord lesions,22 as well as in experimental models, like the murine hind limb ischemia model.84 Dual growth factor administration, such as simultaneous delivery of VEGF and platelet-derived growth factor (PDGF), was also reported in several papers. Combined administration of VEGF and PDGF in different hydrogels led to formation of new blood vessels, with improved vessel maturity thanks to the addition of PDGF,5,17,31,32,74 and subsequently enhanced therapeutic outcomes in terms of cardiac function after myocardial infarction.32 To achieve a better control of drug release, encapsulation of the desired bioactive molecules in NPs to be incorporated into hydrogels has been considered.
VEGF Encapsulation in NPs for Controlled Local Delivery
The duration and site of angiogenic factor delivery is a key consideration in the development of effective therapeutic strategies for graft neovascularization,14 especially in the context of clinical application in cancer patients, where controlled local delivery should be favored. Complexation of VEGF with dextran sulfate and chitosan formed aggregates, with the advantages of biodegradability, desirable particle size (± 250 nm), entrapment efficiency (± 85%), controlled release (almost linear for 10 days), and maintenance of high VEGF levels.40 Incorporation of VEGF into chitosan/dextran sulfate NPs, followed by their encapsulation in an alginate hydrogel, ensured sustained and controlled VEGF release for more than a month.22 Furthermore, VEGF-NP-loaded hydrogels induced more pronounced angiogenesis than did free VEGF-loaded hydrogels,23 demonstrating that complexation and encapsulation preserve VEGF activity.
Cell or Tissue Encapsulation and Local Drug Delivery Systems Applied to Reproductive Medicine
Sustained germ cell-SC interactions resulted in gonocyte differentiation up to the round spermatid stage in bulls after encapsulation of dissociated testicular cells in calcium alginate.50 Promising results were also obtained after transplantation of ovarian cells encapsulated in matrices composed of fibrin57,58 or alginate.91,92
Very few studies have been conducted on encapsulation of reproductive tissue: three on ovarian tissue35,48,82 and just one on testicular tissue.21 Papers reporting the combination of tissue encapsulation and localized release of growth factors looking to increase avascular graft survival are summarized in Table 2. In these studies, increased vascular density, evidenced by morphometry21 and immunostaining (CD31 and αSMA),35,48,82 was observed in the first 3–5 days of grafting in groups supplemented with free or entrapped VEGF. Moreover, stromal/interstitial cell proliferation increased during these first few days, then decreased by 3 weeks post-grafting,21,48 suggesting completion of the two phases of establishment of a new vasculature, namely initiation of neoangiogenesis and vasculature stabilization. Regarding testicular tissue transplantation, increased cell proliferation and activation of VEGF receptor 2 were encountered on day 5 in all grafted groups compared to the control group, corresponding to extensive endothelial cell proliferation during neovessel formation. As expected, after 21 days, proliferation rates fell, reaching values similar to the control group, consistent with stabilization of the vascular network.21 These results corroborated previous reports on ovarian tissue transplantation without encapsulation, where induction of vascularization over the first few days was followed by stabilization after 21 days.88 Unfortunately, most studies investigated only one hydrogel, with or without VEGF supplementation, making it difficult to compare different biomaterials (Table 2). Only one study explored two different hydrogels (fibrin and alginate) and found an increase in the spermatogonial survival in the alginate groups, regardless of VEGF supplementation.21 This disparity between matrices may be explained by variations in structure, density, and biodegradability. Indeed, alginate used in this study had a honeycomb structure, with pores of 200 μm in diameter, while fibrin pore size (1 µm) was less favorable for vascular cell invasion and diffusion of nutrients and oxygen. Moreover, the properties of specific matrices, such as the antioxidant activity of oligo- and polysaccharides from seaweed like alginate, which is known to exert a scavenger effect against hydroxyl radicals,93 could also explain the higher survival rates of spermatogonia after transplantation.21
Besides favoring local administration of neoangiogenic factors for tissue transplantation in the context of cancer, a number of other safety issues must be taken into account for fertility restoration in oncological patients. Indeed, in subjects with hematological malignancies or cancers metastasizing through the blood and therefore running the risk of cancer cell contamination, whole testicular tissue transplantation is not an option. Development of bioengineered scaffolds able to support testicular cells that have been sorted to eliminate cancer cells might be a solution to restore their fertility.
Towards Development of an Artificial Testis
Use of Bioengineered Scaffolds
With advances in the field of regenerative medicine thanks to tissue engineering, it is now possible to obtain acellular matrices by decellularization of fresh or frozen tissues.19 This process allows removal of cells and debris while preserving biological activity, biochemical composition, three-dimensional structure and integrity of the ECM.41 Many agents and protocols have been tested in attempts to obtain fully cleared matrices, their efficacy depending on tissue type and exposure time. Agents commonly used can be physical (freeze-thawing, hydrostatic pressure, sonication), chemical (alcohol, hypo/hypertonic solutions, ionic/non-ionic detergents) or biological, associated with enzymatic (trypsin, dispase, nuclease) or non-enzymatic (EDTA) constituents. Many different empirical combinations of these agents have been used for decellularization, but it would be difficult to identify any particular one as best (for review, see Hussein et al. 41).
Repopulation of such decellularized scaffolds with stem cells to create artificial tissues/organs is clearly a promising way of restoring, repairing, maintaining, or improving tissue/organ functions in clinical therapy.11 Indeed, and specifically in the field of reproduction, it offers new avenues for restoration of sexual function and fertility preservation in both men and women. In females, seeding of human cells onto intestinal submucosa scaffolds yielded encouraging outcomes in the treatment of vaginal aplasia due to Mayer-Rokitansky-Küster-Hauser syndrome, with yearly follow-up biopsies showing the triple-layered structure characteristic of vaginal tissue.72 Three-dimensional construction of the uterine cervix with collagen-coated silk sponges38 and the uterus from one’s own ECM13 were also investigated. In males, penis reconstruction, involving muscular and epithelial cell reimplantation in a decellularized penis to form a bioartificial organ, showed promising results in rabbits in terms of ejaculation and pregnancy rates of mated females.16,25
Bioengineering technologies may also be used to restore or enhance functions of organs involved in gamete production. These are complex organs where reproductive cells are organized in their specific environments, and various components are produced to form the ECM, both acting in concert for proper gametogenesis. It is well established that in mammals, the ECM can regulate cell behavior through cell adhesion receptors and defined mechanical characteristics.42 Proteins like integrin mediate cell–matrix adhesion and are responsible for signal transduction. In general terms, the ECM is a mixture of cell-secreted elements forming both basement membranes and the interstitial matrix. Laminin, collagens, nidogen/entactin, and perlecan are examples of proteins constituting basement membranes, each of them associated with cellular receptors required for cell signaling.77 Elements such as proteoglycans, glycoproteins (laminin, fibronectin), and fibrous proteins (collagen, elastin) are important for resistance to compressive forces, adhesion, and tensile strength respectively.77,86 Acellular tissues, with variable degrees of maintenance of ECM components, may be tailored to meet specific needs by use of different decellularizing agents and protocols. Seeding cells onto such scaffolds should eventually lead to the development of bioartifical tissues suitable for both in vitro studies and in vivo tissue reconstruction/transplantation.41 First steps towards an artificial ovary engineering and perspectives for future developments were recently reviewed.3
Role of the ECM in the Testis
The testicular ECM is vital for cell differentiation as it regulates organization of the testicular cords during embryogenesis.66 It was also identified as a key factor in spermatogenesis, with laminin and collagens allowing differentiating germ cells to cross the STs from the basal lamina to the lumen through regulation of junctional restructuring events.18 Moreover, modification in the localization of laminin and collagen IV observed in Sertoli cell-only syndrome, cryptorchidism, and testis atrophy highlight the significance of the ECM for normal functioning of the testis.69 Consequently, we can assume that development of a system allowing completion of spermatogenesis must be one system in which important ECM components are present to help testicular cells to organize and interact in an appropriate manner.
Supporting this hypothesis, a number of teams have favored use of ECM components to achieve germ cell differentiation in vitro. Indeed, it appears that co-culture of SCs and myoid cells dissociated from ITT on plastic dishes formed only squamous monolayers, but resulted in formation of testicular cord-like structures when seeded on Matrigel™, a commercialized coating agent rich in ECM elements like laminin, collagen IV and proteoglycans, thus highlighting the importance of the ECM for proper organization of testicular cells.30 Furthermore, use of collagen/Matrigel™ or a collagen matrix in a three-dimensional culture system allowed induction of germ cell differentiation of rat testicular cells53 and differentiation of human spermatocytes into spermatids52 respectively. Commercially available three-dimensional collagen structures or sponges also proved useful for testicular organogenesis, showing correct attachment, and organization of seeded rat testicular cells.73 However, the three-dimensional structure rather than the inherent nature of ECM components appears to be fundamental to proper spermatogenesis. Indeed, using polylactic-co-glycolic acid as a biocompatible and biodegradable synthetic polymer for a scaffold, attachment, viability, and differentiation of rat germ cells were found to be improved compared to monolayer and organ culture systems. This was probably thanks to the presence of large pore sizes and the number of interconnections promoting nutrient and gas exchange.54
Tissue Scaffolds from Testes
Use of tissue scaffolds as supporting matrices for germ cells that have been sorted to prevent transmission of cancer cells is an innovative approach in the field of fertility restoration from cryopreserved reproductive tissues. Protocols and agents used for decellularization of reproductive tissues are described in Table 3.
Previous analyses have revealed testicular infiltration by malignant cells in 21% of leukemia-affected children.46,47 Since Sadri-Ardekani et al. demonstrated that co-culture of leukemic and testicular cells leads to elimination of cancer cells after 26 days with a specific culture method,76 it may be assumed that once this has been achieved, the amplified testicular cells could be used for seeding in a three-dimensional structure capable of supporting spermatogenesis. Considering the importance of the testicular ECM, decellularized testicular tissue could well provide good support for these isolated testicular cells. Back in 1986, Enders et al. already decellularized rat STs using both enzymes and chemicals, and showed that SCs were able to reattach to the basement membrane of the tubules.26 Since then, only a few studies have investigated the possibility of obtaining a well preserved ECM from testes. Baert et al. recently demonstrated that decellularization of adult cadaveric testicular tissue through immersion and agitation in 1% sodium dodecyl sulfate resulted in satisfactory preservation of the structure and composition of the ECM.8 The scaffold they obtained was not toxic to fibroblasts, suggesting that abundant washing prior to culture was able to eliminate detergents. Unfortunately, the functionality of the seeded testicular cells was not assessed.
Successful development of an artificial reproductive organ capable of supporting SSC differentiation would certainly represent a big step forward in the field of male infertility.
Use of Bioprinting to Build Artificial Scaffolds
Three-dimensional bioprinting is becoming an alternative to develop functional organs or structures, offering the possibility of assembling cells and tissues with a high degree of accuracy (for review, see Murphy et al. 60). Biomaterials used for construction of bioprinted scaffolds often involved hydrogels based on natural (alginate, gelatin, collagen, fibrin, hyaluronic acid, chitosan, and agarose) or synthetic (polyethylene glycol) polymers.99 Decellularized ECM can also be solubilized and used as bioink to build the artificial structure, with the advantage of providing an optimized microenvironment for cell growth.65
Three-dimensional bioprinting has not yet been applied to develop an artificial testis. However, using gelatin-based bioprinting, one team recently developed a scaffold mimicking the architecture of the ovary.75 Both endocrine and exocrine functions of follicles contained in the scaffolds were evidenced, suggesting that bioprinting could be a novel way of developing matrices able to support gamete differentiation. In their study, survival and function of seeded follicles was contingent on angles forming the structure. Indeed, 30°- and 60°-angled pores increased the likelihood of follicles making multiple scaffold contacts, while square (90°) pores did not.
This could constitute a valuable strategy for ST reconstruction. Indeed, unlike common cell culture on collagen, gelatin or Matrigel™-coated dishes, three-dimensional systems can provide spatial arrangements similar to in vivo niches found in STs, where germ cells are embedded in SCs. A better understanding of the structure and factors involved is nevertheless needed to achieve maturation of seeded germ cells. Developing a functional bioartificial testis would offer new fertility restoration options, either for in vitro production of spermatozoa, or transplantation without the risk of cancer cell contamination to restore spermatogenesis in vivo.
Conclusion
Increasing numbers of boys treated with fertility-threatening therapies are now reaching the age at which fertility concerns become an issue. Development of solutions to allow these boys to become fathers from cryopreserved ITT is therefore crucial. Autologous grafting of ITT appears to be a good option for patients who do not run the risk of intratesticular cancer cell infiltration. However, results from animal studies show that this method should be improved in terms of graft revascularization in order to reduce loss of spermatogonia and better preserve the SSC niche. While tissue encapsulation and/or molecule supplementation was found to yield improved transplantation outcomes, further studies must be conducted to achieve better SSC preservation and differentiation.
Another promising approach is development of a bioartificial testis that can be used in vitro to obtain spermatozoa or transplanted in vivo to restore spermatogenesis. At present, three-dimensional matrices, whether simple polymers or decellularized scaffolds, appear to allow colonization and appropriate arrangement of seeded testicular cells, although complete differentiation has yet to be achieved. However, because most available ECMs needed to build artificial matrices that are able to mimic the in vivo environment contain components of animal origin, and hence may possibly be associated with xenogenic pathologies, translation to a clinical setting could be hampered. Use of decellularized testis scaffolds of human origin may also be limited due to availability issues. Nevertheless, with increasing instances of porcine-derived tissues entering clinical practice, the prospect of using scaffold originating from Gal-KO pig testes could be considered.
Continued efforts are therefore required to develop methods of fertility restoration following gonadotoxic treatments that can be applied in a clinical context.
Abbreviations
- ITT:
-
Immature testicular tissue
- SSC:
-
Spermatogonial stem cell
- SC:
-
Sertoli cell
- LC:
-
Leydig cell
- ST:
-
Seminiferous tubule
- ICSI:
-
Intracytoplasmic sperm injection
- VEGF:
-
Vascular endothelial growth factor
- ROS:
-
Reactive oxygen species
- NAC:
-
N-Acetylcystein
- NP:
-
Nanoparticle
- ECM:
-
Extracellular matrix
- PDGF:
-
Platelet-derived growth factor
- EDTA:
-
Ethylenediaminetetraacetic acid
- Gal-KO:
-
α1,3-Galactosyltransferase knockout
References
Abrishami, M., M. Anzar, Y. Yang, and A. Honaramooz. Cryopreservation of immature porcine testis tissue to maintain its developmental potential after xenografting into recipient mice. Theriogenology 73:86–96, 2010.
Aktas, B. K., S. Bulut, S. Bulut, M. M. Baykam, C. Ozden, M. Senes, D. Yucel, and A. Memis. The effects of N-acetylcysteine on testicular damage in experimental testicular ischemia/reperfusion injury. Pediatr. Surg. Int. 26:293–298, 2010.
Amorim, C. A., and A. Shikanov. The artificial ovary: current status and future perspectives. Future Oncol. 12:2323–2332, 2016.
Artel, A., H. Mehdizadeh, Y. C. Chiu, E. M. Brey, and A. Cinar. An agent-based model for the investigation of neovascularization within porous scaffolds. Tissue Eng. Part A 17:2133–2141, 2011.
Awada, H. K., N. R. Johnson, and Y. Wang. Sequential delivery of angiogenic growth factors improves revascularization and heart function after myocardial infarction. J. Control Release 207:7–17, 2015.
Ayinde, O. C., S. Ogunnowo, and R. A. Ogedegbe. Influence of Vitamin C and Vitamin E on testicular zinc content and testicular toxicity in lead exposed albino rats. BMC Pharmacol. Toxicol. 13:17, 2012.
Baert, Y., E. Goossens, D. van Saen, L. Ning, P. In’t Veld, and H. Tournaye. Orthotopic grafting of cryopreserved prepubertal testicular tissue: in search of a simple yet effective cryopreservation protocol. Fertil. Steril. 97:1152–1157, 2012.
Baert, Y., J. B. Stukenborg, M. Landreh, J. De Kock, H. Jornvall, O. Soder, and E. Goossens. Derivation and characterization of a cytocompatible scaffold from human testis. Hum. Reprod. 30:256–267, 2015.
Bahadur, G., R. Chatterjee, and D. Ralph. Testicular tissue cryopreservation in boys. Ethical and legal issues: case report. Hum. Reprod. 15:1416–1420, 2000.
Bahadur, G., and D. Ralph. Gonadal tissue cryopreservation in boys with paediatric cancers. Hum. Reprod. 14:11–17, 1999.
Bonandrini, B., M. Figliuzzi, E. Papadimou, M. Morigi, N. Perico, F. Casiraghi, C. Dipl, F. Sangalli, S. Conti, A. Benigni, A. Remuzzi, and G. Remuzzi. Recellularization of well-preserved acellular kidney scaffold using embryonic stem cells. Tissue Eng. Part A 20:1486–1498, 2014.
Caires, K. C., J. de Avila, and D. J. McLean. Vascular endothelial growth factor regulates germ cell survival during establishment of spermatogenesis in the bovine testis. Reproduction 138:667–677, 2009.
Campbell, G. R., G. Turnbull, L. Xiang, M. Haines, S. Armstrong, B. E. Rolfe, and J. H. Campbell. The peritoneal cavity as a bioreactor for tissue engineering visceral organs: bladder, uterus and vas deferens. J. Tissue Eng. Regen. Med. 2:50–60, 2008.
Cao, Y., A. Hong, H. Schulten, and M. J. Post. Update on therapeutic neovascularization. Cardiovasc. Res. 65:639–648, 2005.
Cay, A., A. Alver, M. Kucuk, O. Isik, M. S. Eminagaoglu, S. C. Karahan, and O. Deger. The effects of N-acetylcysteine on antioxidant enzyme activities in experimental testicular torsion. J. Surg. Res. 131:199–203, 2006.
Chen, K. L., D. Eberli, J. J. Yoo, and A. Atala. Bioengineered corporal tissue for structural and functional restoration of the penis. Proc. Natl Acad. Sci. U.S.A. 107:3346–3350, 2010.
Chen, R. R., E. A. Silva, W. W. Yuen, and D. J. Mooney. Spatio-temporal VEGF and PDGF delivery patterns blood vessel formation and maturation. Pharm. Res. 24:258–264, 2007.
Cheng, C. Y., E. W. Wong, H. H. Yan, and D. D. Mruk. Regulation of spermatogenesis in the microenvironment of the seminiferous epithelium: new insights and advances. Mol. Cell. Endocrinol. 315:49–56, 2010.
Crapo, P. M., T. W. Gilbert, and S. F. Badylak. An overview of tissue and whole organ decellularization processes. Biomaterials 32:3233–3243, 2011.
Curaba, M., J. Poels, A. van Langendonckt, J. Donnez, and C. Wyns. Can prepubertal human testicular tissue be cryopreserved by vitrification? Fertil. Steril. 95:2123.e9–2123.e12, 2011.
de Michele, F., J. Poels, L. Weerens, C. Petit, Z. Evrard, J. Ambroise, D. Gruson, and C. Wyns. Preserved seminiferous tubule integrity with spermatogonial survival and induction of Sertoli and Leydig cell maturation after long-term organotypic culture of prepubertal human testicular tissue. Hum. Reprod. 97:143–150, 2016.
des Rieux, A., P. De Berdt, E. Ansorena, B. Ucakar, J. Damien, O. Schakman, E. Audouard, C. Bouzin, D. Auhl, T. Simon-Yarza, O. Feron, M. J. Blanco-Prieto, P. Carmeliet, C. Bailly, F. Clotman, and V. Preat. Vascular endothelial growth factor-loaded injectable hydrogel enhances plasticity in the injured spinal cord. J Biomed Mater Res A 102:2345–2355, 2013.
des Rieux, A., B. Ucakar, B. P. Mupendwa, D. Colau, O. Feron, P. Carmeliet, and V. Preat. 3D systems delivering VEGF to promote angiogenesis for tissue engineering. J Control Release 150:272–278, 2011.
Dhandayuthapani, B., Y. Yoshida, T. Maekawa, and D. S. Kumar. Polymeric scaffolds in tissue engineering application: a review. Int. J. Polym. Sci. 1–19:2011, 2011.
Eberli, D., R. Susaeta, J. J. Yoo, and A. Atala. A method to improve cellular content for corporal tissue engineering. Tissue Eng. Part A 14:1581–1589, 2008.
Enders, G. C., J. H. Henson, and C. F. Millette. Sertoli cell binding to isolated testicular basement membrane. J. Cell Biol. 103:1109–1119, 1986.
Gatta, G., G. Zigon, R. Capocaccia, J. W. Coebergh, E. Desandes, P. Kaatsch, G. Pastore, R. Peris-Bonet, C. A. Stiller, and EUROCARE Working Group. Survival of European children and young adults with cancer diagnosed 1995–2002. Eur. J. Cancer 45: 992–1005, 2009.
Gerdprasert, O., M. K. O’Bryan, J. A. Muir, A. M. Caldwell, S. Schlatt, D. M. de Kretser, and M. P. Hedger. The response of testicular leukocytes to lipopolysaccharide-induced inflammation: further evidence for heterogeneity of the testicular macrophage population. Cell Tissue Res. 308:277–285, 2002.
Ginsberg, J. P., C. A. Carlson, K. Lin, W. L. Hobbie, E. Wigo, X. Wu, R. L. Brinster, and T. F. Kolon. An experimental protocol for fertility preservation in prepubertal boys recently diagnosed with cancer: a report of acceptability and safety. Hum. Reprod. 25:37–41, 2010.
Hadley, M. A., S. W. Byers, C. A. Suarez-Quian, H. K. Kleinman, and M. Dym. Extracellular matrix regulates Sertoli cell differentiation, testicular cord formation, and germ cell development in vitro. J. Cell Biol. 101:1511–1522, 1985.
Han, F., X. Jia, D. Dai, X. Yang, J. Zhao, Y. Zhao, Y. Fan, and X. Yuan. Performance of a multilayered small-diameter vascular scaffold dual-loaded with VEGF and PDGF. Biomaterials 34:7302–7313, 2013.
Hao, X., E. A. Silva, A. Mansson-Broberg, K. H. Grinnemo, A. J. Siddiqui, G. Dellgren, E. Wardell, L. A. Brodin, D. J. Mooney, and C. Sylven. Angiogenic effects of sequential release of VEGF-A165 and PDGF-BB with alginate hydrogels after myocardial infarction. Cardiovasc. Res. 75:178–185, 2007.
Hemadi, M., S. Shokri, F. Moramezi, R. Nikbakht, and A. Sobhani. Potential use of melatonin supplementation to protect vitrified testicular grafts from hypoxic-ischaemic damage. Andrologia 46:513–521, 2014.
Hemadi, M., M. Zargar, A. Sobhani, and A. Sobhani. Assessment of morphological and functional changes in neonate vitrified testis grafts after host treatment with melatonin. Folia Morphol. (Warsz) 70:95–102, 2011.
Henry, L., S. Labied, M. Fransolet, N. Kirschvink, S. Blacher, A. Noel, J. M. Foidart, M. Nisolle, and C. Munaut. Isoform 165 of vascular endothelial growth factor in collagen matrix improves ovine cryopreserved ovarian tissue revascularisation after xenotransplantation in mice. Reprod. Biol. Endocrinol. 13:12, 2015.
Hoare, T. R., and D. S. Kohane. Hydrogels in drug delivery: progress and challenges. Polymer 49:1993–2007, 2008.
Honaramooz, A., A. Snedaker, M. Boiani, H. Scholer, I. Dobrinski, and S. Schlatt. Sperm from neonatal mammalian testes grafted in mice. Nature 418:778–781, 2002.
House, M., C. C. Sanchez, W. L. Rice, S. Socrate, and D. L. Kaplan. Cervical tissue engineering using silk scaffolds and human cervical cells. Tissue Eng. Part A 16:2101–2112, 2010.
Howlader, N., A. M. Noone, M. Krapcho, J. Garshell, N. Neyman, S. F. Altekruse, C. L. Kosary, M. Yu, J. Ruhl, Z. Tatalovich, H. Cho, A. Mariotto, D. R. Lewis, H. S. Chen, E. J. Feuer, and K. A. Cronin. SEER Cancer Statistics Review, 1975–2010. Bethesda, MD: National Cancer Institute, 2014.
Huang, M., S. N. Vitharana, L. J. Peek, T. Coop, and C. Berkland. Polyelectrolyte complexes stabilize and controllably release vascular endothelial growth factor. Biomacromolecules 8:1607–1614, 2007.
Hussein, K. H., K. M. Park, K. S. Kang, and H. M. Woo. Biocompatibility evaluation of tissue-engineered decellularized scaffolds for biomedical application. Mater. Sci. Eng. C Mater. Biol. Appl. 67:766–778, 2016.
Hynes, R. O. The extracellular matrix: not just pretty fibrils. Science 326:1216–1219, 2009.
Jafari, M., Z. Paknejad, M. R. Rad, S. R. Motamedian, M. J. Eghbal, N. Nadjmi, and A. Khojasteh. Polymeric scaffolds in tissue engineering: a literature review. J Biomed Mater Res B Appl Biomater 104(11):2843–2853, 2015.
Kaneko, H., K. Kikuchi, M. Nakai, T. Somfai, J. Noguchi, F. Tanihara, J. Ito, and N. Kashiwazaki. Generation of live piglets for the first time using sperm retrieved from immature testicular tissue cryopreserved and grafted into nude mice. PLoS ONE 8:e70989, 2013.
Keros, V., K. Hultenby, B. Borgstrom, M. Fridstrom, K. Jahnukainen, and O. Hovatta. Methods of cryopreservation of testicular tissue with viable spermatogonia in pre-pubertal boys undergoing gonadotoxic cancer treatment. Hum. Reprod. 22:1384–1395, 2007.
Kim, T. H., H. K. Hargreaves, R. K. Brynes, H. K. Hawkins, V. K. Lui, J. Woodard, and A. H. Ragab. Pretreatment testicular biopsy in childhood acute lymphocytic leukaemia. Lancet 2:657–658, 1981.
Kim, T. H., H. K. Hargreaves, W. C. Chan, R. K. Brynes, C. Alvarado, J. Woodard, and A. H. Ragab. Sequential testicular biopsies in childhood acute lymphocytic leukemia. Cancer 57:1038–1041, 1986.
Labied, S., Y. Delforge, C. Munaut, S. Blacher, A. Colige, R. Delcombel, L. Henry, M. Fransolet, C. Jouan, S. Perrier d’Hauterive, A. Noel, M. Nisolle, and J. M. Foidart. Isoform 111 of vascular endothelial growth factor (VEGF111) improves angiogenesis of ovarian tissue xenotransplantation. Transplantation 95:426–433, 2013.
Laronda, M. M., A. E. Jakus, K. A. Whelan, J. A. Wertheim, R. N. Shah, and T. K. Woodruff. Initiation of puberty in mice following decellularized ovary transplant. Biomaterials 50:20–29, 2015.
Lee, D. R., M. T. Kaproth, and J. E. Parks. In vitro production of haploid germ cells from fresh or frozen-thawed testicular cells of neonatal bulls. Biol. Reprod. 65:873–878, 2001.
Lee, J., and K. Y. Lee. Local and sustained vascular endothelial growth factor delivery for angiogenesis using an injectable system. Pharm. Res. 26:1739–1744, 2009.
Lee, J. H., M. C. Gye, K. W. Choi, J. Y. Hong, Y. B. Lee, D. W. Park, S. J. Lee, and C. K. Min. In vitro differentiation of germ cells from nonobstructive azoospermic patients using three-dimensional culture in a collagen gel matrix. Fertil. Steril. 87:824–833, 2007.
Lee, J. H., H. J. Kim, H. Kim, S. J. Lee, and M. C. Gye. In vitro spermatogenesis by three-dimensional culture of rat testicular cells in collagen gel matrix. Biomaterials 27:2845–2853, 2006.
Lee, J. H., J. H. Oh, J. H. Lee, M. R. Kim, and C. K. Min. Evaluation of in vitro spermatogenesis using poly(d,l-lactic-co-glycolic acid) (PLGA)-based macroporous biodegradable scaffolds. J. Tissue Eng. Regen. Med. 5:130–137, 2011.
Liu, J., K. M. Cheng, and F. G. Silversides. Production of live offspring from testicular tissue cryopreserved by vitrification procedures in Japanese quail (Coturnix japonica). Biol. Reprod. 88:124, 2013.
Liu, Z., Y. H. Nie, C. C. Zhang, Y. J. Cai, Y. Wang, H. P. Lu, Y. Z. Li, C. Cheng, Z. L. Qiu, and Q. Sun. Generation of macaques with sperm derived from juvenile monkey testicular xenografts. Cell Res. 26:139–142, 2016.
Luyckx, V., M. M. Dolmans, J. Vanacker, C. Legat, C. Fortuno Moya, J. Donnez, and C. A. Amorim. A new step toward the artificial ovary: survival and proliferation of isolated murine follicles after autologous transplantation in a fibrin scaffold. Fertil. Steril. 101:1149–1156, 2014.
Luyckx, V., M. M. Dolmans, J. Vanacker, S. R. Scalercio, J. Donnez, and C. A. Amorim. First step in developing a 3D biodegradable fibrin scaffold for an artificial ovary. J. Ovarian Res. 6:83, 2013.
Milazzo, J. P., A. Travers, A. Bironneau, A. Safsaf, E. Gruel, C. Arnoult, B. Mace, O. Boyer, and N. Rives. Rapid screening of cryopreservation protocols for murine prepubertal testicular tissue by histology and PCNA immunostaining. J. Androl. 31:617–630, 2010.
Murphy, S. V., and A. Atala. 3D bioprinting of tissues and organs. Nat. Biotechnol. 32:773–785, 2014.
Nicodemus, G. D., and S. J. Bryant. Cell encapsulation in biodegradable hydrogels for tissue engineering applications. Tissue Eng. Part B Rev. 14:149–165, 2008.
Nomi, M., A. Atala, P. D. Coppi, and S. Soker. Principals of neovascularization for tissue engineering. Mol. Aspects Med. 23:463–483, 2002.
Ohta, H., and T. Wakayama. Generation of normal progeny by intracytoplasmic sperm injection following grafting of testicular tissue from cloned mice that died postnatally. Biol. Reprod. 73:390–395, 2005.
Patel, H., M. Bonde, and G. Srinivasan. Biodegradable polymer scaffold for tissue engineering. Trends Biomater. Artif. Organs 25:20–29, 2011.
Pati, F., J. Jang, D. H. Ha, S. Won Kim, J. W. Rhie, J. H. Shim, D. H. Kim, and D. W. Cho. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat Commun 5:3935, 2014.
Pelliniemi, L. J., J. Paranko, S. K. Grund, K. Frojdman, J. M. Foidart, and T. Lakkala-Paranko. Extracellular matrix in testicular differentiation. Ann. N. Y. Acad. Sci. 438:405–416, 1984.
Poels, J., G. Abou-Ghannam, S. Herman, A. Van Langendonckt, F. X. Wese, and C. Wyns. In search of better spermatogonial preservation by supplementation of cryopreserved human immature testicular tissue xenografts with N-acetylcysteine and testosterone. Front. Surg. 1:47, 2014.
Poels, J., A. Van Langendonckt, M. C. Many, F. X. Wese, and C. Wyns. Vitrification preserves proliferation capacity in human spermatogonia. Hum. Reprod. 28:578–589, 2013.
Pollanen, P. P., M. Kallajoki, L. Risteli, J. Risteli, and J. J. Suominen. Laminin and type IV collagen in the human testis. Int. J. Androl. 8:337–347, 1985.
Rao, A. V., and C. Shaha. N-acetylcysteine prevents MAA induced male germ cell apoptosis: role of glutathione and cytochrome c. FEBS Lett. 527:133–137, 2002.
Rathi, R., W. Zeng, S. Megee, A. Conley, S. Meyers, and I. Dobrinski. Maturation of testicular tissue from infant monkeys after xenografting into mice. Endocrinology 149:5288–5296, 2008.
Raya-Rivera, A. M., D. Esquiliano, R. Fierro-Pastrana, E. Lopez-Bayghen, P. Valencia, R. Ordorica-Flores, S. Soker, J. J. Yoo, and A. Atala. Tissue-engineered autologous vaginal organs in patients: a pilot cohort study. Lancet 384:329–336, 2014.
Reuter, K., J. Ehmcke, J. B. Stukenborg, M. Simoni, O. S. Damm, K. Redmann, S. Schlatt, and J. Wistuba. Reassembly of somatic cells and testicular organogenesis in vitro. Tissue Cell 46:86–96, 2014.
Richardson, T. P., M. C. Peters, A. B. Ennett, and D. J. Mooney. Polymeric system for dual growth factor delivery. Nat. Biotechnol. 19:1029–1034, 2001.
Rutz, A., M. M. Laronda, S. Xiao, K. A. Whelan, T. K. Woodruff, and R. N. Shah. Engineering a functional ovary with 3D biomaterial printing. Front. Bioeng. Biotechnol. 2016. doi:10.3389/conf.FBIOE.2016.01.02633.
Sadri-Ardekani, H., C. H. Homburg, T. M. van Capel, H. van den Berg, F. van der Veen, C. E. van der Schoot, A. M. van Pelt, and S. Repping. Eliminating acute lymphoblastic leukemia cells from human testicular cell cultures: a pilot study. Fertil Steril 101:1072–1078.e1, 2014.
Sasaki, T., R. Fassler, and E. Hohenester. Laminin: the crux of basement membrane assembly. J. Cell Biol. 164:959–963, 2004.
Schlatt, S., B. Westernstroer, K. Gassei, and J. Ehmcke. Donor-host involvement in immature rat testis xenografting into nude mouse hosts. Biol. Reprod. 82:888–895, 2010.
Schmidt, J. A., J. M. de Avila, and D. J. McLean. Effect of vascular endothelial growth factor and testis tissue culture on spermatogenesis in bovine ectopic testis tissue xenografts. Biol. Reprod. 75:167–175, 2006.
Schover, L. R., K. Brey, A. Lichtin, L. I. Lipshultz, and S. Jeha. Knowledge and experience regarding cancer, infertility, and sperm banking in younger male survivors. J. Clin. Oncol. 20:1880–1889, 2002.
Shetty, G., and M. L. Meistrich. The missing niche for spermatogonial stem cells: do blood vessels point the way? Cell Stem Cell 1:361–363, 2007.
Shikanov, A., Z. Zhang, M. Xu, R. M. Smith, A. Rajan, T. K. Woodruff, and L. D. Shea. Fibrin encapsulation and vascular endothelial growth factor delivery promotes ovarian graft survival in mice. Tissue Eng. Part A 17:3095–3104, 2011.
Shinohara, T., K. Inoue, N. Ogonuki, M. Kanatsu-Shinohara, H. Miki, K. Nakata, M. Kurome, H. Nagashima, S. Toyokuni, K. Kogishi, T. Honjo, and A. Ogura. Birth of offspring following transplantation of cryopreserved immature testicular pieces and in vitro microinsemination. Hum. Reprod. 17:3039–3045, 2002.
Silva, E. A., and D. J. Mooney. Effects of VEGF temporal and spatial presentation on angiogenesis. Biomaterials 31:1235–1241, 2010.
Simon-Yarza, T., F. R. Formiga, E. Tamayo, B. Pelacho, F. Prosper, and M. J. Blanco-Prieto. Vascular endothelial growth factor-delivery systems for cardiac repair: an overview. Theranostics 2:541–552, 2012.
Taylor, K. R., and R. L. Gallo. Glycosaminoglycans and their proteoglycans: host-associated molecular patterns for initiation and modulation of inflammation. FASEB J. 20:9–22, 2006.
Turkmen, S., A. Mentese, E. Karaguzel, Y. Karaca, A. Kucuk, A. Uzun, E. Yulug, and S. Turedi. A comparison of the effects of N-acetylcysteine and ethyl pyruvate on experimental testicular ischemia-reperfusion injury. Fertil. Steril. 98:626–631, 2012.
Van Eyck, A. S., C. Bouzin, O. Feron, L. Romeu, A. Van Langendonckt, J. Donnez, and M. M. Dolmans. Both host and graft vessels contribute to revascularization of xenografted human ovarian tissue in a murine model. Fertil. Steril. 93:1676–1685, 2010.
Van Saen, D., E. Goossens, C. Bourgain, A. Ferster, and H. Tournaye. Meiotic activity in orthotopic xenografts derived from human postpubertal testicular tissue. Hum. Reprod. 26:282–293, 2011.
Van Saen, D., E. Goossens, P. Haentjens, Y. Baert, and H. Tournaye. Exogenous administration of recombinant human FSH does not improve germ cell survival in human prepubertal xenografts. Reprod. Biomed. Online 26:286–298, 2013.
Vanacker, J., M. M. Dolmans, V. Luyckx, J. Donnez, and C. A. Amorim. First transplantation of isolated murine follicles in alginate. Regen. Med. 9:609–619, 2014.
Vanacker, J., V. Luyckx, M. M. Dolmans, A. Des Rieux, J. Jaeger, A. Van Langendonckt, J. Donnez, and C. A. Amorim. Transplantation of an alginate-matrigel matrix containing isolated ovarian cells: first step in developing a biodegradable scaffold to transplant isolated preantral follicles and ovarian cells. Biomaterials 33:6079–6085, 2012.
Wang, P., X. Jiang, Y. Jiang, X. Hu, H. Mou, M. Li, and H. Guan. In vitro antioxidative activities of three marine oligosaccharides. Nat. Prod. Res. 21:646–654, 2007.
Wyns, C., M. Curaba, B. Martinez-Madrid, A. Van Langendonckt, W. Francois-Xavier, and J. Donnez. Spermatogonial survival after cryopreservation and short-term orthotopic immature human cryptorchid testicular tissue grafting to immunodeficient mice. Hum. Reprod. 22:1603–1611, 2007.
Wyns, C., M. Curaba, S. Petit, B. Vanabelle, P. Laurent, J. F. Wese, and J. Donnez. Management of fertility preservation in prepubertal patients: 5 years’ experience at the Catholic University of Louvain. Hum. Reprod. 26:737–747, 2011.
Wyns, C., M. Curaba, B. Vanabelle, A. Van Langendonckt, and J. Donnez. Options for fertility preservation in prepubertal boys. Hum. Reprod. Update 16:312–328, 2010.
Wyns, C., A. Van Langendonckt, F. X. Wese, J. Donnez, and M. Curaba. Long-term spermatogonial survival in cryopreserved and xenografted immature human testicular tissue. Hum. Reprod. 23:2402–2414, 2008.
Yildiz, C., B. Mullen, K. Jarvi, C. McKerlie, and K. C. Lo. Effect of different cryoprotectant agents on spermatogenesis efficiency in cryopreserved and grafted neonatal mouse testicular tissue. Cryobiology 67:70–75, 2013.
Zhang, Y. S., K. Yue, J. Aleman, K. Mollazadeh-Moghaddam, S. M. Bakht, J. Yang, W. Jia, V. Dell’Erba, P. Assawes, S. R. Shin, M. R. Dokmeci, R. Oklu, and A. Khademhosseini. 3D bioprinting for tissue and organ fabrication. Ann. Biomed. Eng. 43(3):730–746, 2016.
Acknowledgments
The authors thank Mira Hryniuk, B.A., for reviewing the English language of the manuscript.
Funding
Studies conducted at the UCL were supported by grants from the Fonds National de la Recherche Scientifique de Belgique (Télévie Grants 7.4619.05; 7. 4.572.09.F; 7.4616.11.F; 7.4596.13; 7.4512.15) and the Fondation Salus Sanguinis. Anne des Rieux is a research associate from the FRS-FNRS (Fonds de la Recherche Scientifique, Belgique) and is a recipient of subsidies from the Fonds Spéciaux de Recherche Scientifique (FSR, UCL).
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Christiani Amorim oversaw the review of this article.
An erratum to this article is available at http://dx.doi.org/10.1007/s10439-017-1807-7.
Rights and permissions
About this article
Cite this article
Vermeulen, M., Poels, J., de Michele, F. et al. Restoring Fertility with Cryopreserved Prepubertal Testicular Tissue: Perspectives with Hydrogel Encapsulation, Nanotechnology, and Bioengineered Scaffolds. Ann Biomed Eng 45, 1770–1781 (2017). https://doi.org/10.1007/s10439-017-1789-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-017-1789-5