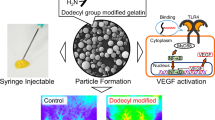
Abstract
Purpose
We hypothesize that a microsphere/hydrogel combination system could be useful for the local and sustained delivery of recombinant human vascular endothelial growth factor (rhVEGF) to enhance angiogenesis in vivo.
Methods
Poly(d,l-lactide-co-glycolide) (PLGA) microspheres containing rhVEGF were loaded into alginate gels by ionic cross-linking. The rhVEGF release from the system was monitored and bioactivity was tested in vitro. The combination system was subcutaneously injected into mice using a syringe, and new blood vessel formation was evaluated.
Results
Sustained rhVEGF release from the combination system was observed for 3 weeks, and the released rhVEGF remained bioactive. Endothelial cell proliferation was significantly enhanced when cells were cultured with the rhVEGF-releasing combination system in vitro. When the combination system was implanted, the granulation tissue layer was thicker with more newly formed blood vessels than that with a single dose VEGF injection.
Conclusion
The rhVEGF release was controlled by varying relative portions of microspheres and hydrogels in combination delivery systems, which efficiently promoted new blood vessel formation in vivo. This combination system could be a promising delivery vehicle for therapeutic angiogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Ischemic vascular diseases have recently become a leading cause of morbidity and mortality in the world (1). Tissues require oxygen and nutrients supplied through the surrounding capillaries. Insufficient supplies induce hypoxia, leading to cellular apoptosis. To date, surgical operations have been frequently performed to treat ischemic vascular diseases (2,3), however, limitations remain such as thrombosis, limited durability, and re-operation (4). Angiogenesis promotes capillary sprouting and extension from preexisting blood vessels, leading to new blood vessel formation (5). Various angiogenic molecules, stem cells or endothelial precursor cells, and genes have been utilized to treat ischemic vascular diseases (6–9). In addition, recent studies report that angiogenesis is useful for tissue engineering applications, especially for large tissue formation (10–12).
Vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), hepatocyte growth factor (HGF), insulin-like growth factor (IGF), angiopoietin-1, and angiopoietin-2 have been reported as potent angiogenic molecules (13–15). The VEGF family is one of the most potent angiogenic regulators, comprised of VEGF-A, VEGF-B, VEGF-C, and VEGF-D (16). Recombinant human vascular endothelial growth factor (rhVEGF) has 165 amino acids, and its bioactivity can be maintained by forming a dimer via disulfide linkages. Gene therapy can induce localized VEGF expression in ischemic myocardium for therapeutic angiogenesis (17). VEGF-B and its receptor VEGF-R1 promote angiogenesis in an in vivo ischemic hindlimb model by activating the Akt and eNOS-related pathway (18). Intracoronary rhVEGF administration was useful to treat patients with coronary artery disease (19).
VEGF is subject to enzymatic degradation and can be cleared from the body within comparatively short time periods, leading to repeated administration (20). For this reason, many drug delivery systems such as films, hydrogels, and nano- and microparticles have been used to control growth factor release and protect them from degradation (21). Hydrogels contain high water content and do not dissolve or dissociate, but maintain a three-dimensional structure in physiological conditions (22). Alginate gels have been widely used as an injectable tool to deliver various drug molecules (23). However, alginate gels rapidly release hydrophilic drug molecules during the initial stage. Polymeric micro- and nanoparticles have also been utilized to deliver bioactive molecules, including VEGF (24). These particles do not stay at the desired site without any supporting systems and are susceptible to phagocytosis by macrophages. In this study, we prepared a combination delivery system that consists of poly(d,l-lactide-co-glycolide) (PLGA) microspheres and alginate hydrogels containing rhVEGF. The rhVEGF release from the combination delivery system was investigated by varying mixing ratios between microspheres and hydrogels. Bioactivity of the released rhVEGF was tested using endothelial cells in vitro. We also tested if the rhVEGF-loaded combination system could enhance angiogenesis efficiently in vivo by subcutaneous injection into the back of mice, and the thickness of the granulation tissues and the number of newly formed blood vessels in the tissues were evaluated.
MATERIALS AND METHODS
Materials
Sodium alginate was purchased from FMC biopolymers (MW 200,000–300,000), and RESOMER® RG 502H (MW 10,000, 0.16–0.24 dl/g) was purchased from Boehringer Ingelheim. Recombinant human vascular endothelial growth factor (rhVEGF) was purchased from Peprotech Asia. Poly(vinyl alcohol) (PVA, MW 27,000–32,000), calcium sulfate (CaSO4), dimethyl sulfoxide (DMSO), and 3-(4,5-dime-thylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) were purchased from Sigma-Aldrich. Dulbecco’s phosphate buffered saline (DPBS) was purchased from Gibco. Ethyl acetate (EA) was purchased from Wako Pure Chemical Industries. Immunodeficient mice (BALB/c nude mice, female, 5 week-old) were supplied by Orient Bio Inc.
Preparation of Microsphere/Hydrogel Combination Systems Containing rhVEGF
PLGA microspheres containing rhVEGF were prepared using a water-in-oil-in-water (w/o/w) double emulsion method (25). Briefly, PLGA and rhVEGF were dissolved in EA and deionized water, respectively. The solutions were emulsified using a probe type sonicator (Branson Digital Sonifier®) for 10 s. The resulting emulsion (w/o) was poured into 4% aqueous PVA solution and emulsified again using a homogenizer (Ultra-Turrax® T25 basic, IKA®-Werke) for 5 min at 6,000 rpm (w/o/w). The obtained double emulsion was poured into 0.4% aqueous PVA solution (300 ml) and mixed thoroughly using a mechanical stirrer at 800 rpm for 3 h. The solidified microspheres were washed with de-ionized water five times and lyophilized. A microsphere/hydrogel combination system was prepared by ionically cross-linking a suspension of PLGA microspheres in an alginate solution using calcium sulfate (26). The mixing ratio of PLGA microspheres to alginate gel was varied from 0 to 1.0 (w/w).
In Vitro rhVEGF Release
Different amounts of PLGA microspheres were loaded into alginate hydrogels. The total rhVEGF in the system was kept constant by adding rhVEGF to the alginate solution (47.1 ng rhVEGF/disk). The gels were cut into disks (15 mm diameter and 2 mm thick) and washed with DPBS. The rhVEGF-loaded combination systems were placed in 12-well tissue culture plates (Corning), and DPBS was added (37°C, 5% CO2 atmosphere). At predetermined time intervals, the supernatant was removed and replaced with fresh DPBS. The amount of released rhVEGF was determined by an rhVEGF ELISA assay kit (R&D).
Bioactivity of Released rhVEGF
Human umbilical vein endothelial cells (HUVECs) were cultured in endothelial growth media (EGM-2) (Clonetics). For all experiments, cells from passage two to five were used. Cell viability was evaluated by MTT assay. Endothelial cells were seeded onto 96-well tissue culture plates and incubated for 12 h at 37°C under 5% CO2 atmosphere. Endothelial basal media (EBM-2) containing either intact or released rhVEGF was added to each well and incubated for 24 h. The cells were then washed with DPBS, and a solution of fresh media (180 μl) and MTT (20 μl, 0.5 mg/ml) was added to each well. The cells were incubated for 3 h at 37°C under 5% CO2 atmosphere. Formazan crystals were dissolved by adding DMSO, and absorbance was measured at 540 nm with a spectrophotometer (SpectraMax M2e).
In Vitro HUVEC Proliferation
Endothelial cells were seeded onto 12-well tissue culture plates, incubated for 12 h at 37°C under 5% CO2 atmosphere, and washed with DPBS twice. Combination systems containing 100 ng rhVEGF/disk were then placed in the upper insert of transwell (Corning Incorporated Costar®), and the cells were cultured for 7 days. At each time point, the number of cells was counted by MTT assay.
In Vivo Angiogenesis
The combination delivery system (100 μl) containing 10 μg/ml rhVEGF was subcutaneously injected into the dorsal site of immunodeficient mice using a syringe. In brief, animals were anesthetized by an intramuscular injection of ketamine (50.5 mg/ml) and xylazine hydrochloride (2.9 mg/ml). The combination delivery system was subcutaneously injected into the back of mice. The mice were sacrificed 3 weeks after implantation, and tissues surrounding the injection site were retrieved. All procedures were in compliance with Hanyang University Guidelines for the care and use of laboratory animals. The tissue samples were embedded into Tissue-Tek O.C.T. compound (Sakura) in aluminum foil molds and frozen (Revco). Cryostat tissue sections (10 μm thick) were prepared using a cryo-microtome (Leica Microsystems), and stained with hematoxylin and eosin (H & E). The microscopic images were obtained using an Axioskop 40 microscope (Zeiss), and analyzed by Image-Pro Plus Software (Media Cybernetics) for quantification.
Statistical Analysis
All data are presented as the mean ± standard deviation. Statistical analysis was performed with the Student’s t-test. Values of *P < 0.05, **P < 0.01, and ***P < 0.001 were considered statistically significant.
RESULTS
In Vitro rhVEGF Release
Combination delivery systems with various ratios of microspheres to hydrogels were prepared, and in vitro rhVEGF release profiles from the systems were investigated. The rhVEGF loading efficiency into the system was more than 90%, and the controlled release of rhVEGF from the combination system was achieved (Fig. 1). The release rates of rhVEGF from combination systems prepared at mixing ratios of 0, 0.5, and 1.0 (g/g, PLGA/alginate) were 6.6, 2.1, and 1.8 ng/day−1, respectively. While rhVEGF encapsulated in alginate hydrogels only (i.e., mixing ratio = 0) was almost completely released within 5 days of incubation, the sustained release was observed from combination systems. The release rate from the combination system decreased as the amount of rhVEGF-encapsulated microspheres increased in the system.
In vitro rhVEGF release from microsphere/hydrogel combination delivery systems prepared at different mixing ratios (closed squares 0; closed circles 0.5; closed triangles 1.0; PLGA/alginate = w/w). The VEGF release from a VEGF-loaded PLGA microsphere suspension in DPBS was also monitored (open triangles).
Bioactivity and Cell Proliferation
We next investigated whether the released rhVEGF remained bioactive. We tested the viability of endothelial cells cultured with EBM-2 (e.g., no growth factors in the media) after treating with either intact rhVEGF or released rhVEGF (Fig. 2). The released rhVEGF showed excellent bioactivity compared to intact rhVEGF.
In vitro proliferation of endothelial cells incubated with combination delivery systems was investigated to confirm that sustained rhVEGF release could maintain a constant therapeutic dose over time, leading to enhanced endothelial cell growth (Fig. 3). Cells cultured in EBM-2 media grew slowly due to the lack of growth factors in the media (no rhVEGF). Cell growth was not significantly affected by the presence of a combination system only (no rhVEGF). Cells treated with 100 ng rhVEGF/well showed enhanced growth, but it only lasted for a short time period and the growth rate (0.47 ±”0.07 day−1) returned to baseline (0.39 ± 0.11 day−1) (Table I). Interestingly, cell proliferation substantially increased when cells were cultured with the rhVEGF-releasing combination delivery system. The growth rate was remarkably high (0.90 ± 0.04 day−1), compared to cells cultured with the same amount of VEGF in the media.
In Vivo Angiogenesis
We next used an animal model to test whether combination delivery systems containing rhVEGF could be useful to improve angiogenesis in vivo. The microsphere/hydrogel combination system at a mixing ratio of 1.0 was subcutaneously injected into the back of immunodeficient mice using a syringe (1 μg rhVEGF/mouse). Tissue sections around the injection sites were retrieved after three weeks, and the newly formed granulation tissue thickness and blood vessel density were evaluated. Granulation tissues generated near the injection site are shown in Fig. 4. The granulation tissue thickness did not change significantly, even after single injection of rhVEGF or a suspension of rhVEGF-loaded microspheres at the implantation site. The granulation tissue thickness around the combination system containing rhVEGF increased significantly. The number of blood vessels in the granulation tissue layer also increased significantly (1,257 ± 348 vessels/mm2), compared with rhVEGF only (505 ± 161 vessels/mm2) or rhVEGF-containing microspheres (709 ± 246 vessels/mm2). No significant inflammatory response was observed.
Photomicrographs of representative tissue sections surrounding the implantation site with a DPBS (control), b combination system only (no VEGF), c single dose of VEGF, d VEGF-loaded microspheres in DPBS, and e VEGF-loaded combination system. The VEGF concentration remained constant (1 μg/animal). Original pictures were taken at 200× magnification. Arrows indicate the newly formed granulation tissue.
DISCUSSION
This study evaluated the ability of microsphere/hydrogel combination systems containing rhVEGF, prepared by embedding PLGA microspheres into alginate gels, to provide a localized delivery system for therapeutic angiogenesis. We previously reported that the relative proportions of microspheres and hydrogels are likely to be a key factor in controlling protein release from combination delivery systems, while neither the size nor the amount of protein loaded into the microspheres significantly influences the release behavior (26). We prepared rhVEGF-loaded PLGA microspheres (8 μg rhVEGF/g PLGA) with a mean diameter of 9.7 ± 2.1 μm, which were then used for a microsphere/hydrogel combination system. We found that the initial burst of rhVEGF released from the combination systems increased as the proportion of alginate gels increased, due to rapid release of the factor from the gel. As the amount of PLGA microspheres containing rhVEGF in the combination systems increased, sustained release behavior became prevalent (Fig. 1). Localized delivery of VEGF using various delivery vehicles, including alginate gels, was previously reported (27). However, the release rate of VEGF from alginate gels was fast and most angiogenic factors were depleted within 10 days. Interestingly our combination system improved the release behavior of VEGF in vitro in a controlled manner for more than three weeks. In addition, released rhVEGF was bioactive and substantially promoted HUVEC proliferation in vitro (Figs. 2 and 3). A single dose of rhVEGF did not efficiently enhance endothelial cell growth, compared with equivalent rhVEGF loaded into a combination system. The difference was attributed to the combination delivery system protecting the factor from enzymatic degradation. Additionally, the delivery system showed no significant cytotoxicity against endothelial cells, indicating a potential use as an injectable rhVEGF delivery system in vivo.
We tested whether combination delivery systems containing rhVEGF could effectively enhance angiogenesis in vivo. Although PLGA microspheres prolonged the release behavior of rhVEGF in vitro (Fig. 1), insufficient blood vessels were formed in vivo (Fig. 4). The lack of blood vessel formation was likely due to microspheres moving from the injection site and possible phagocytotic uptake by macrophages around the injection site. The newly formed granulation tissue was thicker with significantly more blood vessels when a combination system containing rhVEGF was injected into the mice (Figs. 5 and 6). This finding clearly indicates that the localized and sustained delivery of angiogenic factors is critical for angiogenesis in vivo.
Using rhVEGF as an angiogenic molecule has been frequently reported to treat ischemic disease (28). VEGF genes or proteins have been widely used for therapeutic angiogenesis at ischemic sites via systemic administration (29). These applications have been limited due to imprecise gene or protein localization into ischemic sites, which may lead to unnecessary side effects (e.g., hypotension, edema) caused by repeated administration (30). Thus, the localized delivery of angiogenic molecules using collagen (31) and PLGA (24) matrices was reported. These matrices required large doses to effectively enhance angiogenesis in vivo. Surprisingly, a small rhVEGF dose in our combination delivery system efficiently improved angiogenesis in vivo. Determining the optimum dose, systemically or locally administrated, is one critical factor in regulating angiogenesis. In addition, recent studies implicated that the spatial and temporal distribution of angiogenic molecules with a minimal dosage is critical in angiogenesis (32).
CONCLUSION
We fabricated an injectable microsphere/hydrogel combination system for localized rhVEGF delivery. The sustained rhVEGF release was achieved for three weeks using this combination system. Released rhVEGF remained bioactive and enhanced endothelial cell proliferation substantially for prolonged time periods in vitro. Subcutaneous injection into animals significantly enhanced new blood vessel formation at a small dose. This approach may have many useful applications in avoiding unnecessary multiple administrations in treating ischemic diseases, as well as tissue engineering.
References
Tierney EF, Gregg EW, Narayan KMV. Leading Causes of Death in the United States. JAMA 2006;295:383–4. doi:10.1001/jama.295.4.383-a.
Grossi EA, Galloway AC, LaPietra A, Ribakove GH, Ursomanno P, Delianides J, et al. Minimally invasive mitral valve surgery: a 6-year experience with 714 patients. Ann Thorac Surg 2002;74:660–4. doi:10.1016/S0003-4975(02)03754-2.
Varela E, Reavis KM, Hinojosa MW, Nguyen N. Laparoscopic gastric ischemic conditioning prior to esophagogastrectomy: Technique and review. Surgical Innovation 2008;15:132–5. doi:10.1177/1553350608317352.
Lange R, Weipert J, Homann M, Mendler N, Paek S-U, Holper K, et al. Performance of allografts and xenografts for right ventricular outflow tract reconstruction. Ann Thorac Surg 2001;71:S365–7. doi:10.1016/S0003-4975(01)02552-8.
Risau W. Mechanisms of angiogenesis. Nature 1997;386:671–4. doi:10.1038/386671a0.
Zwaginga JJ, Doevendans P. Stem cell-derived angiogenic/vasculogenic cells: Possible therapies for tissue repair and tissue engineering. Clinic Exp Pharmacol Physiol 2003;30:900–8. doi:10.1046/j.1440-1681.2003.03931.x.
Vincent KA, Jiang C, Boltje I, Kelly RA. Gene therapy progress and prospects: therapeutic angiogenesis for ischemic cardiovascular disease. Gene Therapy 2007;14:781–9. doi:10.1038/sj.gt.3302953.
Schmidt A, Brixius K, Bloch W. Endothelial precursor cell migration during vasculogenesis. Circulation Res 2007;101:125–36. doi:10.1161/CIRCRESAHA.107.148932.
Pattersonand C, Runge MS. Therapeutic myocardial angiogenesis via vascular endothelial growth factor gene therapy: Moving on down the road. Circulation 2000;102:940–2.
Wei G, Jin Q, Giannobile WV, Ma PX. Nano-fibrous scaffold for controlled delivery of recombinant human PDGF-BB. J Controlled Rel 2006;112:103–10. doi:10.1016/j.jconrel.2006.01.011.
Nomi M, Atala A, Coppi PD, Soker S. Principals of neovascularization for tissue engineering. Mol Aspects Med 2002;23:463–83.
Kannan RY, Salacinski HJ, Sales K, Butler P, Seifalian AM. The roles of tissue engineering and vascularisation in the development of micro-vascular networks: A review. Biomaterials 2005;26:1857–75. doi:10.1016/j.biomaterials.2004.07.006.
Nicosia R, Nicosia S, Smith M. Vascular endothelial growth factor, platelet-derived growth factor, and insulin-like growth factor-1 promote rat aortic angiogenesis in vitro. Am J Pathol 1994;145:1023–29.
Zakrzewska M, Marcinkowska E, Wiedlocha A. FGF-1: From Biology Through Engineering to Potential Medical Applications. Crit Rev Clin Lab Sci 2008;45:91–135. doi:10.1080/10408360701713120.
Tammela T, Enholm B, Alitalo K, Paavonen K. The biology of vascular endothelial growth factors. Cardiovasc Res 2005;65:550–63. doi:10.1016/j.cardiores.2004.12.002.
Crossand MJ, Claesson-Welsh L. FGF and VEGF function in angiogenesis: signalling pathways, biological responses and therapeutic inhibition. Trend Pharmacol Sci 2001;22:201–7. doi:10.1016/S0165-6147(00)01676-X.
Carmeliet P. VEGF gene therapy: stimulating angiogenesis or angioma-genesis? Nature Med 2000;6:1102–3. doi:10.1038/80430.
Silvestre J-S, Tamarat R, Ebrahimian TG, Le-Roux A, Clergue M, Emmanuel F, et al. Vascular endothelial growth factor-B promotes in vivo angiogenesis. Circulation Res 2003;93:114–23. doi:10.1161/01.RES.0000081594.21764.44.
Henry TD, Rocha-Singh K, Isner JM, Kereiakes DJ, Giordano FJ, Simons M, et al. Intracoronary administration of recombinant human vascular endothelial growth factor to patients with coronary artery disease. Am Heart J 2001;142:872–80. doi:10.1067/mhj.2001.118471.
Morishitaand M, Peppas NA. Is the oral route possible for peptide and protein drug delivery? Drug Discovery Today 2006;11:905–10. doi:10.1016/j.drudis.2006.08.005.
Langer R, Peppas NA. Advances in biomaterials, drug delivery, and bionanotechnology. AIChE J 2003;49:2990–3006. doi:10.1002/aic.690491202.
Lee KY, Yuk SH. Polymeric protein delivery systems. Progr Polym Sci 2007;32:669–97. doi:10.1016/j.progpolymsci.2007.04.001.
Hatefiand A, Amsden B. Biodegradable injectable in situ forming drug delivery systems. J Controlled Rel 2002;80:9–28. doi:10.1016/S0168-3659(02)00008-1.
Cleland JL, Duenas ET, Park A, Daugherty A, Kahn J, Kowalski J, et al. Development of poly(d,l-lactide-co-glycolide) microsphere formulations containing recombinant human vascular endothelial growth factor to promote local angiogenesis. J Controlled Rel 2001;72:13–24. doi:10.1016/S0168-3659(01)00258-9.
Yang Y-Y, Chung T-S, Bai X-L, Chan W-K. Effect of preparation conditions on morphology and release profiles of biodegradable polymeric microspheres containing protein fabricated by double-emulsion method. Chem Engin Sci 2000;55:2223–36. doi:10.1016/S0009-2509(99)00503-5.
Lee J, Lee KY. Injectable microsphere/hydrogel combination systems for localized protein delivery. Macromol. Biosci. in press 2009.
Lee KY, Peters MC, Mooney DJ. Comparison of vascular endothelial growth factor and basic fibroblast growth factor on angiogenesis in SCID mice. J Control Release 2003;87:49–56. doi:10.1016/S0168-3659(02)00349-8.
Zacchigna S, Papa G, Antonini A, Novati F, Moimas S, Carrer A, et al. Improved survival of ischemic cutaneous and musculocutaneous flaps after vascular endothelial growth factor gene transfer using adeno-associated virus vectors. Am J Pathol 2005;167:981–91.
Shimpo M, Ikeda U, Maeda Y, Takahashi M, Miyashita H, Mizukami H, et al. AAV-mediated VEGF gene transfer into skeletal muscle stimulates angiogenesis and improves blood flow in a rat hindlimb ischemia model. Cardiovascular Res 2002;53:993–1001. doi:10.1016/S0008-6363(01)00546-6.
Lee RJ, Springer ML, Blanco-Bose WE, Shaw R, Ursell PC, Blau HM. VEGF gene delivery to myocardium: Deleterious effects of unregulated expression. Circulation 2000;102:898–901.
Tabata Y, Miyao M, Ozeki M, Ikada Y. Controlled release of vascular endothelial growth factor by use of collagen hydrogels. J Biomater Sci Polym Edn 2000;11:915–30. doi:10.1163/156856200744101.
Caoand L, Mooney DJ. Spatiotemporal control over growth factor signaling for therapeutic neovascularization. Adv Drug Deliv Rev 2007;59:1340–50. doi:10.1016/j.addr.2007.08.012.
Acknowledgments
This work was supported by grant from World Class University Project, Ministry of Education, Science and Technology, Republic of Korea (grant no. 200900000000024).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, J., Lee, K.Y. Local and Sustained Vascular Endothelial Growth Factor Delivery for Angiogenesis Using an Injectable System. Pharm Res 26, 1739–1744 (2009). https://doi.org/10.1007/s11095-009-9884-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-009-9884-4