Abstract
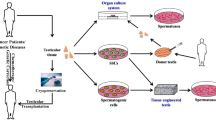
Destruction of spermatogonial stem cells in juvenile men survivors of pediatric cancers leads to infertility as a side effect of gonadotoxic therapies. Sperm freezing before cancer treatment is commonly used in the clinic for fertility preservation, but this method is not applicable for prepubertal boys due to the lack of mature sperm. In these cases, cryopreservation of testicular tissues is the only option for fertility preservation. Although controlled slow freezing (CSF) is the most common procedure for testicular tissue cryopreservation, vitrification can be used as an alternative method. Controlled vitrification has prevented cell damage and formation of ice crystals. Procedures were done easily and quickly with a brief exposure time to high concentration of cryoprotectants without expensive equipment. Different studies used vitrification of testicular tissues and they assessed the morphology of seminiferous tubules, apoptosis, and viability of spermatogonial cells. Transplantation of vitrified testicular tissue into infertile recipient mice as well as in vitro culture of vitrified tissues was done in previous studies and their findings showed complete spermatogenesis and production of mature sperm. Review articles usually have compared controlled slow freezing with vitrification. In this review, we focused only on the vitrification method and its results. Despite promising results, many studies have been done for finding an optimal cryopreservation protocol in order to successfully preserve fertility in prepubertal boys.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cryopreservation is a common method for long-term in vitro storage of organelles, cells, and tissues at an ultra-low temperature (− 196 °C), which preserves tissues and cells structurally and functionally. This method is widely used to preserve various cells (such as gametes and stem cells), embryos, and tissues by cooling the specimens to very low temperatures [1]. Cryopreservation is the best method for fertility preservation in patients suffering from cancer treatment [2]. Survival rates and longevity of childhood cancer have been increased up to higher than 80% by advancements in diagnosis and therapeutic approaches in recent decades [3, 4]. The gonadotoxic treatments of chemotherapy and radiotherapy revealed adverse side effects on testicular cells such as Sertoli, Leydig, and spermatogonial stem cells (SSCs). Therefore, fertility preservation of cancer survivors is taken into consideration around the world [5,6,7]. Sperm freezing is a well-established method to preserve fertility in adult patients [8]. This method cannot be used in prepubertal boys, because spermatogenesis does not begin until puberty. Testicular tissue cryopreservation before gonadotoxic treatments is an alternative process for fertility preservation in patients that could not able to produce mature sperm [5, 9, 10]. SSC isolation by enzymatic digestion of testicular tissues may affect cell viability, cell–cell interactions, and consequently the cryopreservation procedure. In addition, isolation and in vitro proliferation of SSCs are complicated processes prior to cryopreservation. Previous findings have suggested cryopreservation of testicular tissue maintains cellular viability and proliferation, cell to cell and cell to matrix interactions, tissue construction, and architectural integrity which play a key role in cellular signaling pathways. Therefore, researchers have indicated that the testicular tissues cryopreservation could be an appropriate approach for fertility preservation of cancer survivors [11,12,13].
Slow freezing and vitrification are various methods for testicular tissue cryopreservation. Controlled slow freezing (CSF or slow programmable freezing) supplemented by dimethyl sulfoxide (Me2SO) as a penetrating cryoprotective agent is the most common technique used for fertility preservation in humans [14,15,16]. Several studies have demonstrated that CSF can be used successfully for testicular tissue freezing and their results showed that the morphological characteristics of seminiferous tubules were well-preserved [17,18,19,20]. CSF is a complicated, time-consuming process and requires expensive equipment. Vitrification is a simple, novel, time-saving, and cost-effective method that has been used as an alternative to CSF [21]. Vitrification of mammalian tissues recently has gotten attention in human assisted reproduction technologies (ART), generation of domestic animals, and regenerative medicine [22, 23]. Most articles have compared the results of vitrification with slow freezing, and there is no review article that only has evaluated the effects of vitrification on testicular tissue after thawing. In this article, we focused on vitrification of human and animal testicular tissues, the effects of vitrification on apoptosis, in vitro propagation, viability, and spermatogenesis after thawing.
What Is Vitrification?
Vitrification is a physical mechanism in which a liquid solution solidifies to achieve a glass-like vitreous condition without ice formation [24]. Vitrification is a way for transforming cell suspensions directly from the liquid phase to a vitreous state by quick exposure to liquid nitrogen. Vitrification may provide an alternative approach to slow freezing. The ultra-fast cooling process of vitrification depends on the close interaction between the vitrification solution containing the cryoprotective agents and liquid nitrogen [25]. Exposure to high CPA concentrations at very low temperatures with immediate rapid cooling avoids ice nucleation in vitrification [34]. Equilibrium and nonequilibrium vitrification are two main vitrification approaches. The presence and composition of multimolar CPA mixtures into the cell suspensions are necessary for equilibrium vitrification. The nonequilibrium strategy categorized into two groups of carrier-based and carrier-free schemes uses high cooling rate with high concentrations of CPA. The low probability of chilling injuries resulted in comparatively high cell survival rate [1, 26]. Vitrification requires a high viscosity cryoprotectant (6–8 M) in order to avoid ice crystallization during freezing [27, 28]. The cryoprotectant prevents freezing damage caused by the cryopreservation process and preserves cells at a very low temperature. CPAs have low toxicity and should be able to penetrate cells [1]. Numerous CPAs have been used to reduce the amount of ice crystal formation according to cell types and cooling and warming rates [53]. On the other hand, sample size, cooling rate, warming rate, and CPA concentrations should be calibrated according to various cell types and tissue contents to gain the highest survival rate [34, 51]. It is possible to categorize CPAs into two groups: (1) permeating cryoprotectants and (2) non-permeating cryoprotectants [1, 29].
Permeating CPAs are small molecules such as Me2SO, glycerol, and 1,2-propanediol (PrOH); they can infiltrate plasma membrane and build hydrogen bonds with water molecules to reduce the freezing point, and prevent the occurrence of intracellular and extracellular ice crystals [30, 31]. Mixture of ethylene glycol (EG) and Me2SO is the most common permeating CPAs for vitrification of reproductive tissues [32]. Since high amount of one permeating CPA is considered much more toxic, mixture of two or more permeating CPAs is generally used and thereby reduces cytotoxicity [33,34,35]. The combination of several CPAs is successfully used in vitrification of embryo, ovary, testis, and articular cartilage [36,37,38,39].
Non-permeating CPAs are large molecules; they could not pass across the plasma membrane and remain in the extracellular matrix during freezing to support glass formation [32]. Sugars (e.g., sucrose, trehalose, and raffinose) and high molecular weight polymers (e.g., polyvinyl pyrrolidone and Ficoll) are non-permeating CPAs. They are relatively less toxic as compared to permeating CPAs. Their application leads to increased viscosity, allowing the use of small amounts of permeating CPAs without reducing the vitrification quality [40]. Sugars have several functions in the vitrification processes. They usually increase the viscosity of the vitrification solution and glass transition temperature needed for vitrification of extracellular solution. This process decreases the development of extracellular ice crystallization which causes cryoinjury in living cells [32, 40]. Sugars preserve cells from freezing damage through hydrogen bond formation and binding to cell membranes. For example, trehalose combines with the plasma membrane phospholipids, makes vitreous shells around the cells, and protects them from extracellular ice crystallization [41]. Difficult diffusion of CPA into multiple tissues is also a serious problem in cryopreservation of ovarian or testicular tissues that different cell types are tightly connected. Proper thickness of tissue sections and suitable frozen time in the range of 10–20 min are necessary for effective cryopreservation [42,43,44].
Vitrification History
During the last years, cryobiology and in vitro fertilization (IVF) have progressed in parallel to each other [44]. Kinetic vitrification was introduced by Father Basile J. Luyet, a Biology Professor. Luyet demonstrated that solutions could become solidified in ultra-high cooling rates and form amorphous glassy solid without any crystal formation, and then making a transparent glassy phase; this transparent stage was called “vitrification” [45]. Limited success in vitrification of chick hearts and neural tissue explants was revealed in 1950s [46]. In 1965, it was reported that the guinea pig uterine could be stored at − 79° C in a liquid state by using DMSO. The uterine would regain its contractile activity after warming but intracellular ice formation is reported at temperatures lower than − 79 °C [47, 48]. The first successful vitrification of living cells was demonstrated in 1968; glycerol as a cryoprotectant was introduced for vitrification of erythrocytes in this study. According to electron microscopy analysis, ice crystals formed neither outside nor inside the cells [49]. High concentration of cryoprotectant agents was applied. In 1978, ice formation kinetics in these cryoprotectants was a new achievement in vitrification of tissues and cells [50]. Based on this study, the combination of CPAs could facilitate vitrification and reduce the cryoprotectant toxicity.
Another study suggested that 55% v/v Me2SO was an optimal concentration for successful vitrification of the entire organs [34]. Preservation of complete tissue/organ was performed in another study and it was reported that ice crystal formation in the extracellular medium could be entirely suppressed by high concentration of CPAs. This experiment proposed a different method for vitrification and believed that both cooling and warming rates are essential factors for suppression of ice crystal formation and ensuring prosperous cryopreservation outcome [26]. In 1984, for the first time it was reported that the vitrification procedure could be used for cryopreservation of rabbits’ kidney [34]. In the last years, many ART laboratories have established successful oocyte and embryo vitrification. So far, in vivo organ function after vitrification was demonstrated in a few animals [51, 52].
From 1990 to 1998, researchers focused on high concentration of cryoprotectants and used a combination of permeable and nonpermeable CPAs for embryo vitrification to reduce cryoprotectant toxicity [33, 53,54,55]. The first vitrification procedure was performed using a conventional straw device and results displayed significant outcomes compared to slow freezing processes regarding live birth. Open pulled straw (OPS) device was invented in cattle in 1998 and results reported higher pregnancy rate by using a small amount of cryoprotectants [56]. The birth of a healthy baby from a vitrified oocyte was first recorded in 1999; researchers vitrified oocytes using ethylene glycol and sucrose in an OPS [57]. New devices and solutions were introduced by the commercial industry in the mid-2000s and they accelerated the use of vitrification in IVF laboratory clinics [58, 59].
Microdroplet vitrification was created in 2006; the microdroplet size was 1–2 μL and they were immediately plunged into liquid nitrogen containing a small amount of cryoprotectants [60]. The solid surface vitrification (SSV) procedure was introduced in 2010, and results of this experiment demonstrated high survival rate of oocyte and cryopreserved ovarian tissue [61]. Mazur et al. and Seki et al. are the pioneers of modern cryobiology; they have clearly showed that rapid to ultra-rapid warming is the primary determinant condition [62, 63]. A cryotech vitrification tool has been used since 2013 and resulted in 100% survival rate of bovine embryos following thawing [64]. Cryotech needs a small volume of cryoprotectants and enables vitrification of oocyte and embryo at any level of development. This tool is successful and findings revealed high survival rates [48, 65]. Similar to cryopreservation of human ovarian tissue, preservation of testicular tissue attracted significant attention in prepubertal boys who do not generate mature spermatozoa. Due to a lower success rate in post thaw cellular integrity, a successful cryopreservation method for testicular tissue is a cryobiological problem. Human reproductive tissue cryopreservation is used for three target groups: prepubertal persons (males and females), women without spouses, and patients that cannot detain cancer therapy for ovarian stimulation in IVF [66, 67].
Effective Factors in Vitrification Process
1. Cooling and Warming Rates
Vitrification efficacy is indeed determined by the two most relevant parameters for optimum performance of cryopreservation. High freezing rate is essential for cell survival and appropriate vitrification process. This can be done by direct interaction between the sample and liquid nitrogen or indirect contact when sample is enclosed by a closed container. Optimal cooling rate resulted in complete water migration out of the cells and vitrification of extracellular environment [68, 69]. Proper warming rate is another important factor in order to increase cell viability after vitrification. Fast warming has been used in most studies, by plunging cells immediately into the warming solution. In order to minimize osmotic shock, it is important to conduct this procedure using a set of media with gradual decrease of the osmotic pressure [26, 70].
2. Concentration of the Cryoprotectants
For attaining high cooling speeds, it is necessary to use high concentrations of cryoprotectants that limit crystallization. The critical concentration of cryoprotectants is needed for vitrification. Osmotic or chemical toxicity is one of the most detrimental consequences in some cryoprotectants [50]. Toxicity can be minimized by reducing either the temperature of the vitrification solution or the cryoprotectant exposure time. Moreover, replacement of penetrating cryoprotectants with non-permeating sugars and polymers can decrease cytotoxic effects [13, 30].
3. Sample Volume
The sample size should be reduced (i.e., < 1 μL) to decrease the vapor coat size, increase the cooling rate, and ensure that the sample has been enclosed by liquid instead of vapor. Special carriers are used for vitrification to minimize the vitrification solution and sample volume. Also, these carriers can increase cooling rate [50]. Recently, developing nano- and micro-scale techniques have enabled the handling of picoliter to nanoliter sample sizes. OPS, electron microscopy grids, Cryotop, and gel loading tip are examples of this method [28].
Testicular Tissue Vitrification
Several studies have recently used vitrification methods in cryopreservation of prepubertal testicular tissues in animal models (Table 1) [13, 39, 71,72,73,74] and humans [75,76,77,78]. These studies have shown that vitrification is a proper alternative technique to slow freezing [72, 75, 76, 78]. Ice crystal injury was eliminated in vitrification due to glassy solid state without intracellular crystal formation using high concentrations of cryoprotectants [79]. Some researchers prefer to use vitrification for testicular tissue cryopreservation due to lack of ice crystal formation, cost-effectiveness, and shorter procedure time (30 min versus 3 to 4 h) as compared to slow freezing [7]. Studies showed that complete spermatogenesis was seen in seminiferous tubules after warming, so vitrification is a suitable method for testicular tissue cryopreservation [80,81,82]. Despite the impressive results of vitrification in different studies, the application of this method in the cryopreservation of human samples is still in the experimental stage and further examinations are needed [7, 76]. Significant advancements have been made in vitrification agents and elimination of cross-contamination during the last 20 years. This method was utilized efficaciously for cryopreservation of stem cells, embryos, and cell–matrix systems [57, 83,84,85,86].
Can Apoptosis Take Place After Testicular Tissue Vitrification and Warming?
Under normal circumstances, apoptosis in testicular germ cells has been displayed to play a main role in controlling spermatogenesis and testicular tissue homeostasis. However, the high rate of apoptosis may induce harmful consequences in the male reproductive system [87]. Apoptosis is related to the expression of apoptotic factors such as caspase, Apaf-1, NF-KB, P53, death receptors, and anti-apoptotic factors such as BCL-2 [88,89,90].
Apoptosis is necessary for the maintenance of the SSC pool because it is involved in both mitotic and meiotic divisions. Any failure during consecutive divisions induced apoptosis to remove cells with genetic defects [91]. The mechanism of apoptosis mostly consists of two principal pathways, the extrinsic pathway or death receptor and the intrinsic mitochondrial pathway [92]. Apoptosis is distinguished by disorders in cell membrane integrity, cell contraction, cell to cell interactions, and degradation of chromatin as well as disintegrity in mitochondrial membrane, realization of cytochrome c into cytosol, cytoplasmic vacuolization, and cell decompression into membrane-bound residues called apoptotic bodies, which ultimately absorbed by phagocytic cells [93, 94]. Exposure of phosphatidylserine to the outer plasma membrane, caspase cascades activation, and the DNA cleavage are biochemical reactions during cell death [95]. In the extrinsic pathway, activation of death receptors (such as Fas, TNF) causes activation of the initiator caspase 8. Intrinsic or mitochondrial reactions are regulated by BCL2 family members, which enhanced mitochondrial membrane permeability and then release cytochrome C into the cytosol. Cytochrome C is involved in the formation of apoptosome complexes with Apaf-1, and induces caspase 9 activation. Activated caspase in intrinsic and extrinsic pathways upregulates caspase 3 and thus increases the occurrence of apoptosis [89, 91, 96]. P53 is a tumor suppressor and involved in apoptosis. Cell cycle arrest, DNA repair, and gene transcription associated with apoptosis are the central role of p53 [97]. Only a few studies have investigated the effect of vitrification on testicular cell apoptosis. Melatonin reduced the apoptotic index (TUNEL assay) in vitrified mice neonate testis [98]. It was also reported that an appropriate dose of antioxidants in vitrification medium of testicular grafts increased survival rate of spermatogenic cell lines; it also indicated that cytotoxic effects to SSCs in vitrified testes were decreased [98, 99]. Melatonin actually displayed a double role as a reactive oxygen (ROS) scavenger and a regulator of cell proliferation [100]. Vitrification of testicular tissue in the presence of melatonin did not really increase the expression of apoptotic genes (such as Bax and Fas) [100]. Recently, it has been shown that the use of DMSO during vitrification triggers early apoptotic pathways with a high expression of Fas-L and Fas in testicular tissue within the first 3 h of in vitro culture. Results stated an increase in the expression of BAX and reduction in the expression of BCL2 in vitrification groups. These findings suggested the role of p53-independent intrinsic pathway in apoptosis induction [101]. Expression of proteins involved in the autophagic process or apoptosis after vitrification of murine testicular tissue was revealed in another study [102]. In the current research, a small number of TUNEL-positive cells per seminiferous tubule were detected following the vitrification and in vitro culture of testicular tissues. In this study, the phagocytic activity of Sertoli cells directly promotes germ cell apoptosis via the extrinsic pathway involving Fas-L. These findings have proposed that the phagocytic function of the Sertoli cells was maintained even after vitrification. Similar to this study, in 2016 it was reported that 53p pathway signaling has not been influenced by in vitro spermatogenesis following vitrification [101, 102]. High doses of cryoprotectants are necessary for vitrification, but usually lead to the activation of genes involved in the apoptotic pathway [103]. Interestingly, experiments in 2019 observed that expression of BAX increased after a 24-h in vitro culture. According to these results, apoptosis pathways are more associated with suboptimal culture conditions rather than vitrification alone [104].
Testicular Tissue Culture After Vitrification and Warming
Cryopreserved testicular tissues can be cultured in vitro to produce sperm for fertility recovery in infertile cancer survivor patients. In vivo differentiation of SSCs can be obtained by autograft or xenograft transplantation [105]. Autotransplantation of cryopreserved testicular tissue is not recommended to prevent the potential reintroduction of malignant cells during autotransplantation, especially in leukemia patients [82, 106]. Xenografting also is associated with serious problems such as the transmission of DNA fragments or viruses of the gametes derived from animal donor [107].
In Vitro Culture
Previous experiments have revealed that three dimensional and organotypic culture systems provided successful formation of spermatozoa from prepubertal testes [16, 108, 109]. Unlike the culture of testicular cell suspension, the organotypic culture system provides higher preservation of tissue architecture and complex cellular interactions. Genetic and epigenetic abnormalities are both likely involved during in vitro spermatogenesis. Intact niche of spermatogonial cells and proper interactions between different testicular cells are significant advantages of the organotypic culture. According to previous experiments, addition of 10–6 mol/L retinol to the culture medium of fresh or cryopreserved testicular tissues enhances SSC differentiation and meiosis initiation [102, 105, 110, 111]. Functional spermatozoa are obtained recently from fresh [112], slow frozen, and vitrified prepubertal mice testicular tissue (Table 1) [16, 82]. Another study assessed vitrification of both human and mice testicular tissues. The histomorphometric evaluation of cryopreserved prepubertal testicular tissues and characteristics of seminiferous tubules were performed. Results indicated that organotypic culture of vitrified SSCs for 10 days maintained their proliferating capacity [39, 75]. The levels of testosterone and inhibin B were measured after the organotypic culture of vitrified human testicular tissues for 9 days. Results exhibited that production of testosterone decreased while the production of inhibin B was unchanged; they concluded inefficacy of culture conditions for Leydig cell growth [13]. Similarly, previous findings displayed reduced levels of hormones [17], but adequate testosterone generation was reported in 2008 [20]. In another study, a SSV technique has been used for mouse testicular tissue cryopreservation. Testicular tissues can be preserved after warming for 30 days. In addition, the presence of spermatozoa and functional Leydig cells was indicated. Also, the number of spermatozoa per milligram of tissue in the vitrification group was greater than in the CSF group. Results indicated that the morphological abnormalities and the proportion of pyknotic seminiferous tubules in the vitrification group were smaller than in the CSF group [16].
The longest period of testicular tissue culture following vitrification was reported in 2014 (Table 1). Vitrified testicular tissues were cultured on agarose gel for 52 days; their results confirmed the presence of spermatogenesis. Micro-insemination of round spermatids and sperm leads to production of offspring which consequently confirmed sperm functionality [82]. Expression of apoptotic genes was investigated after vitrification and short-term culture (for 20 h) of mouse testicular tissue. Findings proposed that the initiation of apoptosis following vitrification likely occurred by p53 transcription-independent pathway [101]. The expressions of the fundamental components of blood testis barrier (BTB) including CLDN11, CX43, and ZO-1 were evaluated for 4 weeks of organotypic culture after SSV, maturation of Sertoli cells, and progression of spermatogenesis investigated in this study [111]. Their results showed that in vitro spermatogenesis performed completely and the construction of the BTB could not interfere with SSV protocols [111]. The effect of fast warming (50 c for 5 s) on cat testicular tissues after vitrification was investigated. After 5 days of organotypic culture, results showed increased survival and reanimation of vitrified prepubertal testicular tissue. Also, viability and differentiation of germ cells after vitrification and appropriate warming were reported [113]. The effects of vitrification on cell membrane integrity and expression of genes which participated in cell proliferation and stress response, as well as somatic and germ cell specific markers, were assessed 2 and 24 h after warming and in vitro organ culture [104].
In Vivo Culture
Some experts claim that implantation is the most possible way to assess the reproductive capability of a frozen-thawed testis organ [98]. Small fragments of testicular tissues were autografted or xenografted into immunodeficient mice [114]. In this respect, several studies evaluated progression of spermatogenesis in vitrified testicular tissue after xenografting to nude mice (Table 1) [72, 77, 80, 81, 115]. Similar to results of slow freezing, normal cellular organization and tubular consistency of prepubertal mouse testicular tissue were observed after xenotransplantation of vitrified testicular fragments for 4 months [72].
The viability and functionality of vitrified-warmed neonatal mouse testicular tissues 3 months after transplantation were investigated in 2018. Findings revealed that high levels of cryoprotectants improved vitrification but resulted in lower androgen concentrations, which may be related to Leydig cell damage [115]. Piglet testicular tissues were vitrified in an experiment in 2013, and results reported the longest duration of transplantation (almost 1 year). They have produced porcine offspring using sperm obtained from immature testicular fragments after cryopreservation and transplantation into recipient immunodeficient mice [81].
Spermatogenesis After Vitrification
The main task after vitrification is to establish optimal conditions for the resumption of spermatogenesis by testicular tissues. Isolation of late-stage male germ cells (i.e., elongated spermatid, spermatozoa) from frozen-thawed testicular tissues has been beneficial for fertility preservation [114, 116]. Restoration of spermatogenesis can typically be achieved by in vivo or in vitro culture of vitrified tissues summarized in Table 1.
In Vivo Spermatogenesis
Some researchers believed that transplantation is the only practical method to determine the reproductive capacity of frozen-thawed testicular tissues [98] and much more beneficial results were obtained via an association between cryopreservation procedures and in vivo culture of transplanted testis[114, 117].
Abrishami et al. was one of the pioneers for xenografting testicular tissues after vitrification into nude mice and porcine. Vitrified testicular fragments revealed normal spermatogenesis which led to production of round and elongated spermatids. Integrity in germinal epithelium of seminiferous tubules without histological disruption was reported under light microscopy evaluation after vitrification [80].
Several studies evaluated spermatogenesis after vitrification and transplantation of testicular tissues, including Hemadi et al. (2011) [98] in mice, Baert et al. (2012) [72] in mice, Kaneko et al. (2013, 2017) [81, 118] in pig, Pukazhenthi et al. (2015) [119] in lamb, Yamini et al. (2016) [120] in mice, and Yildiz et al. (2018) [115] in mice (Table 1). These studies displayed that recovery of spermatogenesis after transplantation was observed in seminiferous tubules. Spermatozoa were successfully obtained in studies of Kaneko et al. [81] and Yildiz et al. [115].
Investigation of spermatogenesis after xenotransplantation of human vitrified testicular tissues was only performed by an experiment in 2013. They compared the vitrification with slow freezing and their results showed the proliferation of SSCs after vitrification and successful orthotopic xenograft into nude mice for 6 months. Moreover, they believed that SSCs were able to initiate spermatogenesis, but germ cells arrested at the pachytene spermatocytes have been reported [77]. Unfortunately, SSC numbers during slow freezing, vitrification, and fresh graft were significantly reduced as compared to non-grafted tissues. These results indicated that the xenotransplantation and cryopreservation protocol could be involved in the reduction of SSCs after transplantation [77]. The mean number of seminiferous tubules decreased and only type A spermatogonia are seen in the treated grafts after transplantation [77, 98]. The successful connection between recipient blood circulatory system and transplanted tissue as well as proper nutrient, oxygen, and hormone supply confirmed the achievement of the xenotransplantation [121]. Another study discovered that the number of SSCs in melatonin-treated grafts was higher than in other groups after transplantation of testicular tissue [99].
A variety of theories have been proposed to describe SSC loss, delayed maturation, and limited development of cryopreserved testicular tissue after implantation. They included the following:
-
1.
Proper graft size is important for the vitrification method. Tissue degradation increases with the size of fragments due to decrement in penetration rate, which results in overexposure of surface cells to cryoprotectants [40].
-
2.
Residual cryoprotectant in the vitrified-warmed tissues after washing exhibited cytotoxic effects; some studies believed that cryopreservation decreased the spermatogenic potential of implanted testicular tissues and leads to SSC dysfunction [122, 123]. However, another study showed that cryoinjury caused by cryopreservation did not compromise the in vivo developmental potential of testicular tissues [72].
-
3.
Ischemic injury of grafted testicular tissue prior to revascularization caused tissue necrosis or induction of apoptosis [77, 124]. Some researchers considered higher level of apoptosis after transplantation on the first 3 days which decreased within 2–3 weeks. Ischemia–reperfusion injury is associated with severe damage to SSC niche and the interstitial vascular system; both of them are essential for preserving functional SSCs and tissue stability [19, 125, 126].
-
4.
Another factor is the host environmental efficiency. The hypothalamic-pituitary–gonadal axis of the recipient mouse regulated the endocrine function of implanted testicular tissue [127]. This theory was confirmed by findings in pigs and monkeys, where exogenous gonadotropins increased the testicular tissue graft maturation and differentiation in recipient mice [126, 128].
In Vitro Spermatogenesis
Many studies have examined in vitro culture of vitrified testicular tissues, but only a few focused on the spermatogenesis ability of vitrified testicular samples in vitro (Table 1). As previously mentioned, mice vitrified testicular tissues cultured on agarose gel and production of haploid spermatozoa was reported [82]. In 2015, the formation of flagellate spermatozoa and functional Leydig cells after organotypic culture of vitrified mice testicular tissue using the SSV process for 30 days was demonstrated [16]. Findings of another study using the SSV technique showed the formation of round and elongated spermatids after organotypic culture of vitrified samples for 4 weeks [111]. Finally, DNA methylation and histone modifications were assessed in vitrified mouse prepubertal testicular tissues. They also cultured mouse vitrified testicular tissues at a gas–liquid interface system for 30 days and their results confirmed formation of spermatozoa (Table 1) [105].
Human Testicular Tissue Vitrification
Cryopreservation of testicular cells and tissues has become a common strategy in the field of infertility [129]. Vitrification has developed from experimental studies to a standard cryopreservation procedure for human reproductive cells and tissues, specifically for oocytes, zygotes, and blastocysts. But this method is still considered experimental in the context of human testicular tissue. More experiments are needed for the optimization of cryopreservation process and the development of appropriate strategies in order to produce sperm from cryopreserved tissue or cells [130, 131]. No studies have reported the production of sperm after transplantation or in vitro culture of prepubertal human testis tissue or SSCs [130]. Only a few studies have been conducted on the vitrification of human testicular tissues (Table 2).
So far, various researches focused on the vitrification of human testicular tissues [75,76,77, 132]. The mixture of permeable (ET and Me2SO) and nonpermeable (sucrose) cryoprotectants was used for vitrification of testicular tissues. Curaba et al. [75] only used two permeable cryoprotectants (ET and Me2SO). We recently know that the use of non-permeating and permeating CPAs in vitrification of mammalian tissue reduces the cytotoxicity of permeating CPAs and increases vitrification clinical outcomes, especially in vitrification of oocyte and embryo [133]. However, the results of this study were promising; they demonstrated this technique could protect the integrity of human STs, and also support proliferation and viability of SSCs in organotypic culture system [75].
In another study, cryopreservation of adult human testicular tissues using four different methods of controlled slow freezing (CSF), uncontrolled slow freezing (USF), solid surface vitrification (SSV), and direct cover vitrification (DCV) was investigated. According to their results, SSV reduced spermatogonia numbers in testicular tissues. They believed that it is created by the mechanical forces and cellular stress produced by extracellular ice formation. They recommended the use of USF instead of CSF for human testicular tissue banking, due to the effective results [76]. In 2014, cryoprotectant formulation similar to a previous study in 2013 was applied [76]. They investigated the effects of slow freezing and vitrification on ROS production. They discovered that slow freezing was more successful than vitrification because vitrification increased significantly reactive oxygen levels than slow freezing [132]. Poels and colleagues [77] were the only authors that xenografted the immature human testicular tissue after vitrification and they investigated the spermatogenesis resumption. Their results reported that spermatogenesis arrests at the pachytene stage [77]. Because of limited studies in vitrification of human testicular tissues, cryopreservation of human testicular tissue in the clinic generally requires additional research.
Offspring Generation After Vitrification
Few researches have been conducted on offspring generation after vitrification. Only two studies evaluated the generation of live offspring after testicular tissue vitrification and warming [81, 134]. Fertile sperm was obtained from the organotypic culture of vitrified testicular tissues in the previous research. Micro-insemination of round spermatids and sperm resulted in offspring production. This study concluded that slow freezing and vitrification were both useful for cryopreservation of mouse testicular tissue. Produced offspring grew normally and progeny was generated upon natural mating [82]. Interestingly, they believed that the cryopreservation process is less important than the culture system factors for spermatogenesis outcomes. In other words, proper culture system is necessary for progression of spermatogenesis and sperm production from cryopreserved testicular tissues [82].
Yokonishi et al. were able to achieve sperm and live birth after in vitro culture of vitrified testicular fragments [82]. In another study, vitrified immature pig testicular fragments were transplanted to nude mice. Sperm obtained from recipient mice on days 230 to 250 generated sperm injected into the porcine oocyte by micro-insemination and embryos transferred to recipient gilts. Results of testicular graft histomorphometric analysis and levels of inhibin and testosterone after vitrification and xenotransplantation exhibited no significant differences between groups [81].
Comparison Between Vitrification and Slow Freezing
CSF is the most common method for human fertility preservation [14, 15]. This procedure is also effective in animals [20, 135]. Vitrification is a revolutionary method for prepubertal testicular tissue preservation that supports the ability of tissues to trigger or complete spermatogenesis after warming [77, 81, 82, 115]. Since vitrification is simpler than CSF and does not require expensive instruments or a long procedure time, it is a better method than CSF [7]. Several experiments have shown that this method is preferable to CSF in regard to the post-thaw cell viability, tubal integrity, morphological alterations, and number of flagellated spermatozoa [7, 13, 16].
The analysis compared only slow freezing and vitrification of testicular tissues in terms of spermatogonial viability, but their functionality was not assessed [75, 77]. Some scientists believed that vitrification is a safer method because it prevents ice crystal formation and chilling injury [72, 75].
According to experiments of Beart et al. (2013) [76], Tang et al. (2014) [132], Pukazhenthi et al. (2015) [119], and Yildiz et al. (2018) [115], slow freezing is superior to vitrification. The amounts of ROS and heme oxygenase-1 gene expression in both the vitrification and slow freezing groups were measured in previous experiments. Results stated slow freezing induced HO-1 expression and reduced ROS significantly than vitrification. This study concluded that the slow freezing procedure was more efficient than the vitrification [132]. After that, it is reported that slow freezing of lamb testicular tissues was more efficient than vitrification in cellular integrity, functionality, and progression of spermatogenesis after xenotransplantation [119]. Similar to previous studies, results of another experiment in 2018 showed that controlled-rate freezing reduced cryoinjury of tissue constituents than vitrification [115, 119, 132]. Findings of another study concluded that spermatogenic ability was maintained by high concentrations of vitrification solution although caused severe damage to Leydig cells; consequently, lower androgenic activity was determined. Low to intermediate concentration of cryoprotectants could not support post-thaw spermatogenesis probably due to tissue permeation failure [115].
Conclusion
Spermatogenesis is a sensitive and complicated process that requires specific niche. This microenvironment may be damaged after vitrification and warming. The principal purpose of testicular tissue cryopreservation is to maintain immature germ cells obtained from pubertal boys for the generation of future offspring. Vitrification appears to be a promising technology for fertility preservation of young boys as an alternative to CSF. This approach does not require expensive devices and can be performed even outside the laboratory environment. Despite the promising results of vitrification, numerous problems related to this procedure remained unsolved. One problem is to establish an effective nontoxic concentration of permeating and non-permeating CPAs for mammalian tissues. Another problem is a controversy that testicular tissue fragments or germ cell suspensions after enzymatic digestion of testicular tissues were used for SSC cryopreservation. Twenty years have been passed since the first report of human immature testicular tissue cryopreservation in prepubertal boys, but fertility restoration has not been achieved yet. This is mainly related to the absence of an optimal freezing–thawing protocol that supports isolation, propagation, and transplantation of SSCs after cryopreservation. Although testicular tissue cryopreservation is in the experimental stage, it is now highly considered and ethically accepted. Various factors such as type of cryoprotectant agents and exposure time, size of testicular tissue fragments, and freezing and warming rates play important roles in successful vitrification; further studies are suggested to be done for exact evaluation of these factors.
Code Availability
Not applicable.
References
Jang TH, et al. Cryopreservation and its clinical applications. Integr Med Res. 2017;6(1):12–8.
Liu X, et al. Male cancer patient sperm cryopreservation for fertility preservation: 10-year monocentric experience. Basic Clin Androl. 2021;31(1):1–9.
Aslam I, et al. Fertility preservation of boys undergoing anti-cancer therapy: A review of the existing situation and prospects for the future. Hum Reprod. 2000;15(10):2154–9.
Brenner H, Steliarova-Foucher E, Arndt V. Up-to-date monitoring of childhood cancer long-term survival in Europe: methodology and application to all forms of cancer combined. Ann Oncol. 2007;18(9):1561–8.
Benvenutti L, et al. Wistar rats immature testicular tissue vitrification and heterotopic grafting. JBRA Assist Reprod. 2018;22(3):167–73.
Trottmann M, et al. Semen quality in men with malignant diseases before and after therapy and the role of cryopreservation. Eur Urol. 2007;52(2):355–67.
Radaelli MRM, et al. A comparison between a new vitrification protocol and the slow freezing method in the cryopreservation of prepubertal testicular tissue. JBRA Assist Reprod. 2017;21(3):188–95.
Di Santo M, et al. Human sperm cryopreservation: update on techniques, effect on DNA integrity, and implications for ART. Adv Urol. 2012.
Brinster RL. Male germline stem cells: from mice to men. Science. 2007;316(5823):404–5.
Yango P, et al. Optimizing cryopreservation of human spermatogonial stem cells: comparing the effectiveness of testicular tissue and single cell suspension cryopreservation. Fertil Steril. 2014;102(5):1491-1498.e1.
Ogawa T, Ohmura M, Ohbo K. The niche for spermatogonial stem cells in the mammalian testis. Int J Hematol. 2005;82(5):381–8.
Spradling A, Drummond-Barbosa D, Kai T. Stem cells find their niche. Nature. 2001;414(6859):98–104.
Gouk SS, et al. Cryopreservation of mouse testicular tissue: prospect for harvesting spermatogonial stem cells for fertility preservation. Fertil Steril. 2011;95(7):2399–403.
Keros V, et al. Methods of cryopreservation of testicular tissue with viable spermatogonia in pre-pubertal boys undergoing gonadotoxic cancer treatment. Hum Reprod. 2007;22(5):1384–95.
Wyns C, et al. Management of fertility preservation in prepubertal patients: 5 years’ experience at the Catholic University of Louvain. Hum Reprod. 2011;26(4):737–47.
Dumont L, et al. Assessment of the optimal vitrification protocol for pre-pubertal mice testes leading to successful in vitro production of flagellated spermatozoa. Andrology. 2015;3(3):611–25.
Keros V, et al. Optimizing cryopreservation of human testicular tissue: comparison of protocols with glycerol, propanediol and dimethylsulphoxide as cryoprotectants. Hum Reprod. 2005;20(6):1676–87.
Kvist K, et al. Cryopreservation of intact testicular tissue from boys with cryptorchidism. Hum Reprod. 2006;21(2):484–91.
Wyns C, et al. Spermatogonial survival after cryopreservation and short-term orthotopic immature human cryptorchid testicular tissue grafting to immunodeficient mice. Hum Reprod. 2007;22(6):1603–11.
Milazzo JP, et al. Comparison of conditions for cryopreservation of testicular tissue from immature mice. Hum Reprod. 2008;23(1):17–28.
Slabbert M. Investigating alternative sperm preservation methods for assisted reproductive technologies. 2013, University of Pretoria.
Pomeroy KO, et al. The ART of cryopreservation and its changing landscape. Fertil Steril. 2022;117(3):469–76.
Yokota Y, et al. Successful pregnancy following blastocyst vitrification: case report. Hum Reprod. 2000;15(8):1802–3.
Rall WF, Fahy GM. Ice-free cryopreservation of mouse embryos at -196 degrees C by vitrification. Nature. 1985;313(6003):573–5.
Heo YS, et al. “Universal” vitrification of cells by ultra-fast cooling. Technology. 2015;3(01):64–71.
Yavin S, Arav A. Measurement of essential physical properties of vitrification solutions. Theriogenology. 2007;67(1):81–9.
Karlsson JO, Toner M. Long-term storage of tissues by cryopreservation: critical issues. Biomaterials. 1996;17(3):243–56.
Zhang X, et al. Emerging technologies in medical applications of minimum volume vitrification. Nanomedicine (Lond). 2011;6(6):1115–29.
Sambu S. A Bayesian approach to optimizing cryopreservation protocols. PeerJ. 2015;3:e1039–e1039.
Shaw JM, Jones GM. Terminology associated with vitrification and other cryopreservation procedures for oocytes and embryos. Hum Reprod Update. 2003;9(6):583–605.
Karlsson JO. Cryopreservation: freezing and vitrification. Science. 2002;296(5568):655–6.
Yong KW, et al. Review of non-permeating cryoprotectants as supplements for vitrification of mammalian tissues. Cryobiology. 2020;96:1–11.
Ali J, Shelton JN. Design of vitrification solutions for the cryopreservation of embryos. J Reprod Fertil. 1993;99(2):471–7.
Fahy GM, et al. Vitrification as an approach to cryopreservation. Cryobiology. 1984;21(4):407–26.
Wusteman M, Robinson M, Pegg D. Vitrification of large tissues with dielectric warming: biological problems and some approaches to their solution. Cryobiology. 2004;48(2):179–89.
Baril G, et al. Successful direct transfer of vitrified sheep embryos. Theriogenology. 2001;56(2):299–305.
Song YC, et al. Vitreous preservation of articular cartilage grafts. J Invest Surg. 2004;17(2):65–70.
Jafarabadi M, Abdollahi M, Salehnia M. Assessment of vitrification outcome by xenotransplantation of ovarian cortex pieces in γ-irradiated mice: morphological and molecular analyses of apoptosis. J Assist Reprod Genet. 2015;32(2):195–205.
Curaba M, et al. Cryopreservation of prepubertal mouse testicular tissue by vitrification. Fertil Steril. 2011;95(4):1229-34 e1.
Amorim CA, et al. Vitrification as an alternative means of cryopreserving ovarian tissue. Reprod Biomed Online. 2011;23(2):160–86.
Quan GB, et al. Comparison of the effect of various disaccharides on frozen goat spermatozoa. Biopreserv Biobank. 2012;10(5):439–45.
Gosden RG. Gonadal tissue cryopreservation and transplantation. Reprod Biomed Online. 2002;4:64–7.
Hovatta O. Cryopreservation and culture of human ovarian cortical tissue containing early follicles. Eur J Obstet Gynecol Reprod Biol. 2004;113(Suppl 1):S50–4.
Fuller B, Paynter S. Fundamentals of cryobiology in reproductive medicine. Reprod Biomed Online. 2004;9(6):680–91.
Luyet BJ. The vitrification of organic colloids and of protoplasm. Biodynamica. 1937;29:1–14.
Gonzales F, Luyet B. Resumption of heart-beat in chick embryo frozen in liquid nitrogen. Biodynamica. 1950;7(126–128):1–5.
Farrant J. Mechanism of cell damage during freezing and thawing and its prevention. Nature. 1965;205(4978):1284–7.
Schiewe M, Anderson R. Vitrification: the pioneering past to current trends and perspectives of cryopreserving human embryos, gametes and reproductive tissue. J Biorepository Sci Appl Med. 2017;5:57–68.
Rapatz G, Luyet B. Electron microscope study of erythrocytes in rapidly cooled suspensions containing various concentrations of glycerol. Biodynamica. 1968;10(210):193–210.
Boutron P, Kaufmann A. Stability of the amorphous state in the system water-glycerol-dimethylsulfoxide. Cryobiology. 1978;15(1):93–108.
Fahy GM, et al. Physical and biological aspects of renal vitrification. Organogenesis. 2009;5(3):167–75.
Finger EB, Bischof JC. Cryopreservation by vitrification: a promising approach for transplant organ banking. Curr Opin Organ Transplant. 2018;23(3):353–60.
Shaw JM, Diotallevi L, Trounson AO. A simple rapid 4–5 m dimethyl-sulfoxide freezing technique for the cryopreservation of one-cell to blastocyst stage preimplantation mouse embryos. Reprod Fertil Dev. 1991;3(5):621–6.
Shaw JM, et al. Vitrification properties of solutions of ethylene glycol in saline containing PVP, Ficoll, or dextran. Cryobiology. 1997;35(3):219–29.
Mukaida T, et al. Vitrification of human embryos based on the assessment of suitable conditions for 8-cell mouse embryos. Hum Reprod. 1998;13(10):2874–9.
Vajta G, et al. Open pulled straw (OPS) vitrification: a new way to reduce cryoinjuries of bovine ova and embryos. Mol Reprod Dev:Inc Gamete Res. 1998;51(1):53–8.
Kuleshova L, et al. Birth following vitrification of a small number of human oocytes. Hum Reprod. 1999;14(12):3077–9.
Cobo A, et al. Use of cryo-banked oocytes in an ovum donation programme: a prospective, randomized, controlled, clinical trial. Hum Reprod. 2010;25(9):2239–46.
Zhu D, et al. Vitrified-warmed blastocyst transfer cycles yield higher pregnancy and implantation rates compared with fresh blastocyst transfer cycles - time for a new embryo transfer strategy? Fertil Steril. 2011;95(5):1691–5.
Chang C-C, Huang J-C, Shen P-C. Method for microdrop vitrification of cells. 2006, Google Patents.
Xing W, et al. Solid-surface vitrification is an appropriate and convenient method for cryopreservation of isolated rat follicles. Reprod Biol Endocrinol. 2010;8(1):1–9.
Seki S, Mazur P. Ultra-rapid warming yields high survival of mouse oocytes cooled to -196°c in dilutions of a standard vitrification solution. PLoS One. 2012;7(4).
Mazur P, Seki S. Survival of mouse oocytes after being cooled in a vitrification solution to -196°C at 95° to 70,000°C/min and warmed at 610° to 118,000°C/min: a new paradigm for cryopreservation by vitrification. Cryobiology. 2011;62(1):1–7.
Gutnisky C, et al. Evaluation of the Cryotech Vitrification Kit for bovine embryos. Cryobiology. 2013;67(3):391–3.
Allahbadia GN, Gandhi G. Vitrification in assisted reproduction. 2016: Springer.
Fahy GM, et al. Cryopreservation of organs by vitrification: perspectives and recent advances. Cryobiology. 2004;48(2):157–78.
Armstrong AG, et al. Ovarian tissue cryopreservation in young females through the Oncofertility Consortium’s National Physicians Cooperative. Future Oncol. 2018;14(4):363–78.
Liebermann J, et al. Potential importance of vitrification in reproductive medicine. Biol Reprod. 2002;67(6):1671–80.
Kader AA, et al. Factors affecting the outcome of human blastocyst vitrification. Reprod Biol Endocrinol. 2009;7:99.
Cho HJ, et al. An improved protocol for dilution of cryoprotectants from vitrified human blastocysts. Hum Reprod. 2002;17(9):2419–22.
Poels J, et al. Vitrification of non-human primate immature testicular tissue allows maintenance of proliferating spermatogonial cells after xenografting to recipient mice. Theriogenology. 2012;77(5):1008–13.
Baert Y, et al. Orthotopic grafting of cryopreserved prepubertal testicular tissue: in search of a simple yet effective cryopreservation protocol. Fertil Steril. 2012;97(5):1152-1157.e2.
Liu I, Cheng KM, Silversides FG. Production of live offspring from testicular tissue cryopreserved by vitrification procedures in Japanese quail (Coturnix japonica). Biol Reprod. 2013;88(5).
Chatdarong K, Thuwanut P, Morrell JM. The development of cat testicular sperm cryopreservation protocols: effects of tissue fragments or sperm cell suspension. Theriogenology. 2016;85(2):200–6.
Curaba M, et al. Can prepubertal human testicular tissue be cryopreserved by vitrification? Fertil Steril. 2011;95(6):2123 e9–12.
Baert Y, et al. What is the best cryopreservation protocol for human testicular tissue banking? Hum Reprod. 2013;28(7):1816–26.
Poels J, et al. Vitrification preserves proliferation capacity in human spermatogonia. Hum Reprod. 2013;28(3):578–89.
Sa R, et al. Cryopreservation of human testicular diploid germ cell suspensions. Andrologia. 2012;44(6):366–72.
Pegg DE. The role of vitrification techniques of cryopreservation in reproductive medicine. Hum Fertil. 2005;8(4):231–9.
Abrishami M, et al. Cryopreservation of immature porcine testis tissue to maintain its developmental potential after xenografting into recipient mice. Theriogenology. 2010;73(1):86–96.
Kaneko H, et al. Generation of live piglets for the first time using sperm retrieved from immature testicular tissue cryopreserved and grafted into nude mice. PLoS One. 2013;8(7):e70989.
Yokonishi T, et al. Offspring production with sperm grown in vitro from cryopreserved testis tissues. Nat Commun. 2014;5:4320.
Bhakta G, et al. Cryoreservation of alginate-fibrin beads involving bone marrow derived mesenchymal stromal cells by vitrification. Biomaterials. 2009;30(3):336–43.
Kuleshova LL, et al. Vitrification of encapsulated hepatocytes with reduced cooling/warming rates. Cryo-Letters. 2004;25(4):241–54.
Tan FCK, et al. Optimization of cryopreservation of stem cells cultured as neurospheres: comparison between vitrification, slow-cooling and raped cooling “freezing” protocols. Cryo-Letters. 2007;28(6):445–60.
Wen F, et al. Vitreous cryopreservation of nanofibrous tissue-engineered constructs generated using mesenchymal stromal cells. Tissue Eng-Part C: Methods. 2009;15(1):105–14.
Martincic DS, et al. Germ cell apoptosis in the human testis. Pflugers Arch. 2001;442(6 Suppl 1):R159–60.
Aggarwal A, et al. Adverse effects associated with persistent stimulation of Leydig cells with hCG in vitro. Mol Reprod Dev. 2009;76(11):1076–83.
Nagata S, Golstein P. The Fas death factor. Science. 1995;267(5203):1449–56.
Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2(9):647–56.
Shaha C, Tripathi R, Mishra DP. Male germ cell apoptosis: regulation and biology. Philos Trans R Soc Lond B Biol Sci. 2010;365(1546):1501–15.
Igney FH, Krammer PH. Death and anti-death: tumour resistance to apoptosis. Nat Rev Cancer. 2002;2(4):277–88.
Majno G, Joris I. Apoptosis, oncosis, and necrosis an overview of cell death. Am J Pathol. 1995;146(1):3–15.
Papaliagkas V, et al. The proteins and the mechanisms of apoptosis: a mini-review of the fundamentals. Hippokratia. 2007;11(3):108–13.
Fadok VA, et al. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148(7):2207–16.
Aitken RJ, et al. Apoptosis in the germ line. Reproduction. 2011;141(2):139–50.
Soussi T, Caron de Fromentel C, May P. Structural aspects of the p53 protein in relation to gene evolution. Oncogene, 1990;5(7):945–52.
Hemadi M, et al. Assessment of morphological and functional changes in neonate vitrified testis grafts after host treatment with melatonin. Folia Morphol (Warsz). 2011;70(2):95–102.
Hemadi M, et al. Potential use of melatonin supplementation to protect vitrified testicular grafts from hypoxic-ischaemic damage. Andrologia. 2014;46(5):513–21.
Gholami M, et al. Does prepubertal testicular tissue vitrification influence spermatogonial stem cells (SSCs) viability? J Assist Reprod Genet. 2013;30(10):1271–7.
Hajiaghalou S, et al. Comparison of apoptosis pathway following the use of two protocols for vitrification of immature mouse testicular tissue. Theriogenology. 2016;86(8):2073–82.
Dumont L, et al. Evaluation of apoptotic- and autophagic-related protein expressions before and after IVM of fresh, slow-frozen and vitrified pre-pubertal mouse testicular tissue. Mol Hum Reprod. 2017;23(11):738–54.
Orrenius S, Nicotera P, Zhivotovsky B. Cell death mechanisms and their implications in toxicology. Toxicol Sci. 2011;119(1):3–19.
Bebbere D, et al. Gene expression analysis of ovine prepubertal testicular tissue vitrified with a novel cryodevice (E.Vit). J Assist Reprod Genet. 2019;36(10):2145–54.
Oblette A, et al. DNA methylation and histone post-translational modifications in the mouse germline following in-vitro maturation of fresh or cryopreserved prepubertal testicular tissue. Reprod Biomed Online. 2019;39(3):383–401.
Jahnukainen K, Mitchell RT, Stukenborg JB. Testicular function and fertility preservation after treatment for haematological cancer. Curr Opin Endocrinol Diabetes Obes. 2015;22(3):217–23.
Patience C, Takeuchi Y, Weiss RA. Zoonosis in xenotransplantation. Curr Opin Immunol. 1998;10(5):539–42.
Abu Elhija M, et al. Differentiation of murine male germ cells to spermatozoa in a soft agar culture system. Asian J Androl. 2012;14(2):285–93.
Arkoun B, et al. Does soaking temperature during controlled slow freezing of pre-pubertal mouse testes influence course of in vitro spermatogenesis? Cell Tissue Res. 2016;364(3):661–74.
Dumont L, et al. Vitamin A prevents round spermatid nuclear damage and promotes the production of motile sperm during in vitro maturation of vitrified pre-pubertal mouse testicular tissue. Mol Hum Reprod. 2016;22(12):819–32.
Rondanino C, et al. Establishment, maintenance and functional integrity of the blood-testis barrier in organotypic cultures of fresh and frozen/thawed prepubertal mouse testes. Mol Hum Reprod. 2017;23(5):304–20.
Sato T, et al. In vitro production of functional sperm in cultured neonatal mouse testes. Nature. 2011;471(7339):504–7.
Lima DBC, Silva L, Comizzoli P. Influence of warming and reanimation conditions on seminiferous tubule morphology, mitochondrial activity, and cell composition of vitrified testicular tissues in the domestic cat model. PLoS One. 2018;13(11):e0207317.
Woods EJ, et al. Fundamental cryobiology of reproductive cells and tissues. Cryobiology. 2004;48(2):146–56.
Yildiz C, et al. Comparison of cryosurvival and spermatogenesis efficiency of cryopreserved neonatal mouse testicular tissue between three vitrification protocols and controlled-rate freezing. Cryobiology. 2018;84:4–9.
Kimura Y, Yanagimachi R. Mouse oocytes injected with testicular spermatozoa or round spermatids can develop into normal offspring. Development. 1995;121(8):2397–405.
Gilmore JA, et al. Determination of optimal cryoprotectants and procedures for their addition and removal from human spermatozoa. Hum Reprod. 1997;12(1):112–8.
Kaneko H, et al. Production of sperm from porcine fetal testicular tissue after cryopreservation and grafting into nude mice. Theriogenology. 2017;91:154–62.
Pukazhenthi BS, et al. Slow freezing, but not vitrification supports complete spermatogenesis in cryopreserved, neonatal sheep testicular xenografts. PLoS One. 2015;10(4):e0123957.
Yamini N, et al. Developmental potential of vitrified mouse testicular tissue after ectopic transplantation. Cell J. 2016;18(1):74–82.
Poels J, et al. In search of better spermatogonial preservation by supplementation of cryopreserved human immature testicular tissue xenografts with N-acetylcysteine and testosterone. Front Surg. 2014;1:47.
Wu B, et al. iNOS enhances rat intestinal apoptosis after ischemia-reperfusion. Free Radic Biol Med. 2002;33(5):649–58.
Frederickx V, et al. Recovery, survival and functional evaluation by transplantation of frozen–thawed mouse germ cells. Hum Reprod. 2004;19(4):948–53.
Israely T, et al. Reducing ischaemic damage in rodent ovarian xenografts transplanted into granulation tissue. Hum Reprod. 2006;21(6):1368–79.
Wyns C, et al. Long-term spermatogonial survival in cryopreserved and xenografted immature human testicular tissue. Hum Reprod. 2008;23(11):2402–14.
Rathi R, et al. Maturation of testicular tissue from infant monkeys after xenografting into mice. Endocrinology. 2008;149(10):5288–96.
Schlatt S, et al. Modulating testicular mass in xenografting: a model to explore testis development and endocrine function. Endocrinology. 2010;151(8):4018–23.
Zeng W, et al. The length of the spermatogenic cycle is conserved in porcine and ovine testis xenografts. J Androl. 2006;27(4):527–33.
Pietzak EJ 3rd, et al. Histology of testicular biopsies obtained for experimental fertility preservation protocol in boys with cancer. J Urol. 2015;194(5):1420–4.
Kilcoyne KR, Mitchell RT. Fertility preservation: testicular transplantation for fertility preservation: clinical potential and current challenges. Reproduction. 2019;158(5):F1-f14.
Onofre J, et al. Cryopreservation of testicular tissue or testicular cell suspensions: a pivotal step in fertility preservation. Hum Reprod Update. 2016;22(6):744–61.
Tang W, et al. Up-regulation of heme oxygenase-1 expression modulates reactive oxygen species level during the cryopreservation of human seminiferous tubules. Fertil Steril. 2014;102(4):974-980 e4.
Nagy ZP, Shapiro D, Chang CC. Vitrification of the human embryo: a more efficient and safer in vitro fertilization treatment. Fertil Steril. 2020;113(2):241–7.
Yokonishi T, et al. Offspring production with sperm grown in vitro from cryopreserved testis tissues. Nat Commun. 2014;5(1):1–6.
Shinohara T, et al. Birth of offspring following transplantation of cryopreserved immature testicular pieces and in-vitro microinsemination. Hum Reprod. 2002;17(12):3039–45.
Acknowledgements
We would like to thank the Anatomical Department of Tehran University of Medical Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
This was a review article on existing literature and did not need review by the institutional ethics committee.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nikmahzar, A., Khadivi, F., Abbasi, M. et al. Testicular Tissue Vitrification: a Promising Strategy for Male Fertility Preservation. Reprod. Sci. 30, 1687–1700 (2023). https://doi.org/10.1007/s43032-022-01113-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-022-01113-8