Abstract
This study presents the development of three new chiral stationary phases. Three new peptide chiral stationary phases with a main chain of l-proline, l-phenylalanine, l-valine and l-leucine and different terminals of l-citrulline, l-lysine and l-tryptophan immobilized on 3-aminopropyltrimethoxysilane modified silica gel were prepared; furthermore, successful analyses and characterizations were conducted using Fourier transform infrared spectra, elemental analysis, and thermogravimetric analysis. After this, the enantioselective performance of the three peptide stationary phases columns was evaluated. The evaluation used nine racemic compounds under normal-phase high-performance liquid chromatography mode. Optimized enantiomeric separation conditions were established. Under these conditions, the resolutions of flurbiprofen, naproxen, benzoin, 1,1ʹ-bi-2-naphthol and ketoprofen on the CSP-1 column were 1.61, 2.0, 0.62, 0.52 and 1.20, respectively. In addition, the reproducibility of the CSP-1 column was also investigated. The results of the investigation illustrated that the stationary phases have good reproducibility (RSD = 0.12%, n = 5).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chirality is a common natural phenomenon that is also one of the basic properties of natural materials. Most substances in organisms such as proteins, polysaccharides and nucleic acids are chiral, and the metabolic activities of organisms are often associated with chirality [1]. Enantiomers have similar physicochemical properties in an achiral environment and differ only through an optical one (rotation of polarized light); however, when introduced in a chiral environment such as the human body, they can exhibit totally different pharmacokinetic (absorption, distribution, metabolism and excretion) and pharmacodynamic (quantitative or qualitative differences in pharmacologic or toxicologic effects) profiles generating different therapeutic responses [2,3,4]. The separation of chiral compounds into single enantiomers is therefore of great importance in many fields including pharmaceutical, food additive, agrochemical, biological environments and medicine [5].
Since 1980s, high-performance liquid chromatography (HPLC) based on chiral stationary phases (CSPs) has become the most effective and widely used method for enantiomeric separation [6,7,8,9]. A CSP is very essential for chiral HPLC which enables enantioseparation. Therefore, the studies upon CSPs and chiral recognition mechanism were always attractive in past decades [10,11,12]. Till now, there have been numerous studies on natural CSPs including polysaccharides [13, 14], macrocyclic glycopeptides [15, 16], proteins [17], cyclodextrins [18] and artificial CSPs such as crown ethers [19, 20], chiral porous materials [21, 22] and Pirkle-type materials [23]. Hence, the development of more versatile CSPs with high enantioselectivity and low cost continues to be a research focus.
Amino acids are a class of naturally occurring chiral small molecules with special structures containing amino and carboxyl groups, and some of them also have aromatic structures, which enable them to form hydrogen bonds, electrostatic interactions and π–π interactions with chiral compounds. For this reason, amino acids are often used as chiral monomers in a wide range of chiral research. For example, seven CSPs with different numbers of proline units or different linkage arms were prepared by Huang et al. It was found that the separation capacity of the 53 analytes increased with the number of prolines and that stationary phases containing shorter arm lengths such as 3-methylaminopropyl silica had better separation capacity [24]. New proline oligopeptide CSPs containing two and four proline units, respectively, were prepared by Li et al., and the separation performance of 53 enantiomers was evaluated. It was found that the chiral stationary phase containing four proline units was effective in separating 31 of the enantiomers and was comparable to the Whelk O2 column compared to the commercial columns, although less so than the Chiralcel AD-H and OD-H columns, indicating that this is a promising stationary phase [25]. A series of ionic CSP of dipeptides and tripeptides using a variety of amino acids (Phe, Leu) were prepared by Buszewski et al., and their chromatographic performance was evaluated using hydrophilic interaction chromatography and reversed-phase liquid chromatography. It was found that hydrophobic, electrostatic and hydrogen bonding interactions play an important role in the enantiomeric recognition of these stationary phases [26].
Herein, based on previous studies in our laboratory [27], three new peptide CSPs were prepared with a main chain of l-proline (l-Pro), l-phenylalanine (l-Phe), l-valine (l-Val) and l-leucine (l-Leu). Compared to the CSPs previously studied, valine and leucine with spatial resistance were introduced in the main chain to increase the chiral recognition site of the stationary phase. At the same time, the introduction of different terminals including l-citrulline (l-Cit), which forms a hydrogen-bonded urea group structure, l-lysine (l-Lys), which forms a space-blocked aliphatic chain structure, and l-tryptophan (l-Trp), which forms a space-blocked aromatic ring structure, and all three terminal amino acids have a free amino group, all of which structures enhance the enantiomeric separation of the stationary phase. Therefore, in this paper, three new peptide CSPs with a main chain of l-Pro, l-Phe, l-Val and l-Leu and different terminals of l-Cit (CSP-1), l-Lys (CSP-2) and (l-Trp (CSP-3) immobilized on 3-aminopropyltrimethoxysilane modified silica gel (APS) were prepared. The chromatographic separation performance of the three new chiral peptide columns was evaluated by nine racemic compounds.
Materials and Methods
Chemicals and Reagents
All reagents and solvents were obtained from commercial suppliers and were used without further purification. Amino acid derivatives were purchased from LeYan reagent company (Shanghai, China), and 3-aminopropyltrimethoxysilane was obtained from Aladdin Chemicals (Shanghai, China). Spherical silica gel (particle size, 5 µm; pore size, 120 Å and surface area 300 m2 g−1) was purchased from Daisogel Chemicals (Osaka, Japan). And all of racemic compounds used were purchased from Aladdin Chemicals (Shanghai, China).
Instruments and Methods
The infrared absorption spectrum was determined with FTIR-8400 (Shimadzu, Japan), and the thermal stability of the product was analyzed with STA 449 C simultaneous thermal analyzer (Netzsch, Germany). The element analysis was carried out by Vario III elemental analyzer (Elementar, Germany). The stationary phase materials were loaded into an empty column tube (150 mm × 4.6 mm) using a GLK100 column loader (Wuxi Galeck Chromatography Technology Co., Ltd.). The HPLC experiment was performed by P230II system (Dalian Elite Analytical Instruments Co.).
Preparation of Chiral Stationary Phases
Preparation of 3-Aminopropyltrimethoxysilane Silica Gel
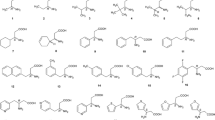
The synthetic route of the stationary phase is shown in Fig. 1. A 6.6 mL of 3-aminopropyltriethoxysilane sample was slowly added to a solution of activated silica gel (2 g) in anhydrous toluene. The reaction solution was heated to reflux under nitrogen protection for 12 h. Modified silica (APS) was isolated by filtration and washed with toluene, methanol and acetone and dried under reduced pressure.
Synthetic scheme for the preparation of CSP-1. a l-Pro methyl ester, DCM, TBTU, DIEA, 0 ℃ naturally warms to room temperature; b hydrogen chloride gas in methanol, 4 mol L−1; Boc-l-Val, DCM, TBTU, DIEA, 0 ℃ naturally warms to room temperature; c hydrogen chloride gas in methanol, 4 mol L−1; Boc-l-Leu, DCM, TBTU, DIEA, 0 ℃ naturally warms to room temperature; d hydrogen chloride gas in methanol, 4 mol L−1; Boc-l-Cit, DCM, TBTU, DIEA, 0 ℃ naturally warms to room temperature; e NaOH, MeOH; f APS, DCM, TBTU, DIEA, 0 ℃ naturally warms to room temperature; g hydrogen chloride gas in methanol, 4 mol L−1; h activated silica gel, 3-aminopropyltriethoxysilane, anhydrous toluene, 110 ℃
Preparation of CSP-1
The synthetic route of the stationary phase is shown in Fig. 1. N, N-diisopropylethylamine (DIEA; 3.0 g, 23.2 mmol) was added to a solution of l-Pro methyl ester (2 g, 15.5 mmol) and Boc-l-Phe (4.5 g, 17.1 mmol) in dry dichloromethane (30 mL) at 0 ℃. After 10 min, O-benzotriazole-N, N, Nʹ, Nʹ-tetramethyluronium tetrafluoroborate (TBTU; 7.4 g, 23.2 mmol) was slowly added to the solution. The reaction mixture was allowed to warm to room temperature overnight with stirring and monitored by thin layer chromatography (TLC). The reaction mixture was washed with 5% citric acid (15 mL × 2), aqueous 5% sodium bicarbonate (15 mL × 2) and saturated brine (15 mL × 2) in turn. After this, the organic layer was dried over anhydrous sodium sulfate and evaporated. The crude product was purified by column chromatography [petroleum ether/ethyl acetate (EtOAc) = 4:1] to afford compound 1. Next, compound 1 was added to a solution of hydrogen chloride gas in methanol (4 mol L−1). The resulting mixture was stirred for 6 h at room temperature. After evaporation of the solvent in vacuum, the crude intermediate was obtained. It was used without further purification for the synthesis of compound 2. The synthesis of compound 2, 3 and 4 refers to the synthesis method of compound 1. Boc-l-Val, Boc-l-Leu and Boc-l-Cit were used instead of Boc-l-Phe. Then, aqueous sodium hydroxide (1.7 g of sodium hydroxide in 15 mL water) was slowly added to a solution of compound 4 in methanol (30 mL) at 0 ℃. The reaction mixture was left to warm to room temperature overnight with stirring. The organic solvent was removed in vacuum, and the residual aqueous solution was partitioned with EtOAc. Then, the organic phase was extracted with water (15 mL × 2). The combined aqueous extract was acidified to pH = 5–6 with aqueous hydrochloric acid (HCl, 1 mol L−1). The aqueous phase was extracted with dichloromethane (20 mL × 3). The combined organic extract was dried over anhydrous sodium sulfate and concentrated to produce the desired compound 5.
TBTU was slowly added to a solution of APS (2 g) and compound 5 (1.6 g, 2.2 mmol, HPLC purity: 98.6%) in dry dichloromethane at 0 ℃. The reaction mixture was left to warm to room temperature overnight with stirring. Compound 6 was isolated by filtration and washed with dichloromethane, methanol and acetone and dried under reduced pressure. According to the literature [28], compound 6 was added to a solution of hydrogen chloride gas in methanol (4 mol L−1). The resulting mixture was stirred for 12 h at room temperature. CSP-1 was isolated by filtration and washed with dichloromethane, methanol and acetone and dried under reduced pressure.
Preparation of CSP-2 and CSP-3
CSP-2 and CSP-3 were prepared according to the above CSP-1 preparation procedure. Replacement of l-Cit by (S)-2,6-Di-Boc-aminohexanoic acid and Boc-l-Trp was done to prepare the corresponding intermediates and CSP-2 and CSP-3. (The specific synthesis route is shown in Fig. 1).
Sample Preparation
The chiral sample to be tested was dissolved in methanol at a concentration of 1 mg mL−1 and stored at − 20 ℃.
Packing the Column
The empty column tube was first connected to the column loading machine. The stationary phase material was dispersed with 20 mL of cyclohexanol/isopropanol (v/v, 1:1) and poured into a stainless-steel column (4.6 × 150 mm). The machine was run at 35 MPa pressure for 30 min using methanol and isopropyl alcohol as the driving agent; then the pressure was adjusted to zero and maintained in that condition for 30 min.
Results and Discussion
Chiral Stationary Phase Design
Amino acids can form hydrogen bond, dipole–dipole and π–π interaction with chiral reagents, and their derivatives have been widely used as chiral building block in CSPs. In this experiment, four amino acids were selected as the main chain for the chiral stationary phase of the peptide. All four amino acids are hydrophobic, and Pro contains a secondary amine and has a dihedral angle structure, Phe has a phenyl ring, and Val and Leu contain isopropyl and isobutyl, respectively. These structures create a spatial barrier for the peptides and affect the recognition of chiral separation. Therefore, good enantiomeric separation may be achieved by the combination of the advantages of Pro, Phe, Val and Leu. In addition, the introduction of aliphatic branched chains with spatial barriers and urea structures can form hydrogen bonds and aromatic ring structures with large spatial site barriers at the capping terminal. Therefore, several chiral selectors with main chains of l-Pro-l-Phe-l-Val-l-Leu and different terminals of l-Cit, l-Lys and l-Trp as shown in Fig. 1 were designed.
Characterizations of the Modified Silica Gel
Infrared Spectra
The functional groups of the synthesized stationary phase materials were assayed by Fourier transform infrared spectra (FT-IR). The overlaid FT-IR spectra of CSP-1, CSP-2 and CSP-3 were illustrated in Figure S1 (see support information for details). A strong absorption peak at 1105 cm−1 was assigned to the Si–O–Si stretching vibration in APS, CSP-1, CSP-2 and CSP-3. An amide I absorption band at 1666 cm−1 (CSP-1), 1641 cm−1 (CSP-2) and 1645 cm−1 (CSP-3) and an amide II absorption band at 1538 cm−1 (CSP-1), 1560 cm−1 (CSP-2) and 1562 cm−1 (CSP-3) have been recorded in the CSPs IR spectrum. Moreover, out-of-plane bending vibrations of the benzene at about 686 cm−1 and framing vibrations of the benzene at 1454 cm−1 (CSP-1), 1450 cm−1 (CSP-2) and 1446 cm−1 (CSP-3) were also observed. The absorption bands were characteristic of amide, benzene rings, and their presence confirmed that the stationary phase materials have been successfully synthesized.
Elemental Analysis
In addition, the successful preparation of CSPs was verified by the results of the elemental analysis (EA) shown in Table 1. Firstly, the carbon content of silica gel increased to 5.15% and the nitrogen content increased to 1.84% after grafting APS on bare silica. This showed that APS was bonded on bare silica. Compared with APS, the carbon, nitrogen and hydrogen contents of CSPs were increased to different degrees. This indicated that the peptides were successfully received on silica gel. According to the formula, the surface bonding amounts of the chiral stationary phases CSP-1, CSP-2 and CSP-3 were 0.41 mmol g−1, 0.38 mmol g−1 and 0.32 mmol g−1, respectively.
The calculation formula of the surface bonding is as follows:
Bonding amount µ (mmol g−1) = 10 × (C1 − C0)/(M × n), C1 and C0 represent the percentage of nitrogen in the stationary phase and pure silica gel, respectively, C0 = 0, M is the molar mass of nitrogen, and n refers to the number of nitrogen atoms in the chiral selector.
Thermogravimetric Analysis
Thermogravimetric analysis (TGA) was applied to evaluate the thermal stability of these prepared CSPs between 50 and 800 ℃. The results are given in in Figure S2 (see support information for details). The results showed that silica gel had good thermal stability with a weight loss of <1.6% until 800 ℃. The weight loss of silica gel to be about 100 ℃ was attributable to the loss of physically adsorbed water on the silica surface. Compared with silica gel, APS, CSP-1, CSP-2 and CSP-3 exhibited the characteristic that their weight of samples was decreased with an increase of temperature. The weight loss rate of APS was about 8.7% with increasing temperature, due to the loss of 3-methoxyaminopropylsilane bound to silica gel under the influence of the temperature. In the same way, the weight loss ratio of peptide on the CSP-1 reached approximately 20.3%. The weight loss ratio of peptide on CSP-2 and CSP-3 was up to 21% and 19.6%. In the temperature range of 300–500 ℃, the substance on the surface of the silica gel decomposed rapidly and the material quality of CSPs rapidly decreases. In the range of 500–700 ℃, the decomposition rate of the CSPs became slow. This shows that the CSPs material stability was good below 300 ℃. Compared with the thermogravimetric curve of APS, it was shown that the CSPs materials were successfully prepared on silica gel.
Chromatographic Separations
In our case, enantioseparation performance of nine racemic compounds (Fig. 2) including flurbiprofen, ketoprofen, naproxen, loxoprofen, ibuprofen, benzoin, 1,1ʹ-bi-2-naphthol, naringenin and lansoprazole on both CSP-1, CSP-2 and CSP-3 was determined by HPLC in normal phase mode and reversed phase mode, respectively. It was found that the enantiomeric separation in the reversed-phase mode was not as effective as that in the normal-phase mode. Therefore, the following discussion focuses on the normal phase mode and investigated in detail the chromatographic conditions, including the isopropanol content and formic acid content of the mobile phase and the effect of flow rate on retention and resolution.
Separation of Enantiomers on Three Chiral Stationary Phases
The chromatograms of these racemic compounds on the CSPs are presented in Figures S3, S4 and S5 (see support information for details), and chromatographic parameters including k1, α and Rs of these chiral compounds are listed in Table 2. Some typical chromatograms are shown in Fig. 3. As shown in Table 2, on the CSP-1 column, enantiomeric separation of flurbiprofen (Rs = 1.61) and naproxen (Rs = 2.0) was achieved and partial enantioseparations of benzoin (Rs = 0.62), 1,1ʹ-bi-2-naphthol (Rs = 0 0.52) and ketoprofen (Rs = 1.2) were achieved. Baseline separation of flurbiprofen (Rs = 1.79) and ketoprofen (Rs = 1.98) was performed on the CSP-2 column, despite partial enantioseparations for benzoin (Rs = 0.46) and 1,1ʹ-bi-2-naphthol (Rs = 0.54). On the CSP-3 column, partial enantioseparations of the enantiomers were achieved for 1,1ʹ-bi-2-naphthol (Rs = 0.71) and flurbiprofen (Rs = 0.72). The results showed that the CSP-1, CSP-2 and CSP-3 exhibited different separation capabilities for these nine chiral compounds. The difference in separation performance may be caused by the structure of the end of the capping terminal affecting the chiral recognition ability of the CSPs.
Representative chromatograms of naproxenon (a) CSP-1 and ketoprofen (b) on CSP-2. Separation conditions as shown in Table 2
When the mobile phase conditions are n-hexane/isopropanol/0.1% formic acid, isopropanol was chosen as the organic phase to investigate the effect of the isopropanol content on the enantiomeric separation with the same amount of formic acid was explored. Figure 4a shows the chromatogram of flurbiprofen at CSP-1 with increasing isopropanol content. As exhibited in Fig. 4a, the retention time of flurbiprofen was decreased with the increase of isopropanol concentration but the resolution of flurbiprofen showed an increase first and decrease then with increasing isopropanol content. The upward trend may be due to increased separation of flurbiprofen enantiomers by CSP-1 with increased isopropanol content, and the downward trend may be due to isopropanol occupying the hydrogen bond site on the CSP-1, resulting in weaker recognition of flurbiprofen enantiomers by CSP-1. Similarly, Fig. 4b shows the chromatogram of ketoprofen at CSP-2 with increasing isopropanol content. As shown in Fig. 4b, the retention time and resolution of ketoprofen showed the same trend as that of flurbiprofen.
Effect of isopropanol content on the chromatographic separation of flurbiprofen (a), ketoprofen (b). Chromatographic conditions: injection volume, 5 μL; column temperature, 30 ℃; detection wavelength, 254 nm. Mobile phases conditions as shown in Table 2
Effect of Flow Rate on Separation Performance
The kinetic factors cannot be ignored during the HPLC separation process. Therefore, the effect of mobile phase flow rate on the chiral separation of CSP-1 and CSP-2 was investigated on the basis of the optimum separation conditions for each analyte. As shown in Figure S6 (see support information for details), on CSP-1 and CSP-2 column, the k1 values of each analyte presented a decreased trend as the flow rate increased. That is, the retention time for each analyte decreased again with increasing flow rate. The effect of flow rate on Rs of these chiral compounds is shown in Fig. 5. On the CSP-1 column, the Rs values of benzoin, 1,1-bi-2-naphthol and naproxen showed an increase first and decrease then with increasing flow rate, but ketoprofen showed the opposite trend. And the Rs values of flurbiprofen showed an increasing trend with increasing flow rate. On the CSP-2 column, the Rs values of flurbiprofen and naproxen tended to decreased first and increased then with increasing flow rate, but the Rs values of 1,1-bi-2-naphthol presented increased first and decreased then. And the Rs values of ketoprofen tended to decrease with increasing flow rate.
Effect of flow rate on Rs of chiral compounds on a CSP-1 and b CSP-2. Chromatographic conditions: injection volume, 5 μL; column temperature, 30 ℃; detection wavelength, 254 nm. Mobile phases conditions as shown in Table 2
Effect of Acid Additives (Formic Acid) on Separation Performance
For most racemic compounds, it is usually necessary to add additives to the mobile phase to increase resolution. Due to the presence of large number of ionizable groups on the surface of CSPs, the structure may change at different formic acid contents. Therefore, the formic acid content is also a factor affecting the retention and separation of chiral enantiomers.
Under the same chromatographic conditions, 0.1% and 0.2% formic acid were added to n-hexane to investigate the changes in k1 and Rs of the chiral compounds on CSP-1 and CSP-2 columns, respectively. As shown in Fig. 6a and c, on the CSP-1 and CSP-2 columns, the k1 values of each analyte presented a decreased with increasing formic acid content. It was speculated that the degree of protonation of the chiral compounds increased with the increase of formic acid content, which weakens the interaction force between the chiral compounds and the CSPs. Therefore, the formic acid content has an important effect on the retention of these enantiomers. Similarly, the formic acid content of mobile phase affected the Rs of chiral compounds in HPLC. As shown in Fig. 6b, on the CSP-1 column, the Rs values of benzoin, flurbiprofen, ketoprofen and naproxen showed a decreasing trend with the increase of formic acid content, but the Rs values of 1,1-bi-2-naphthol showed slight increase. As shown in Fig. 6d, on the CSP-2 column, the Rs values of flurbiprofen, ketoprofen and 1,1-bi-2-naphthol showed a decrease with increasing formic acid content, except for the Rs values of naproxen which showed gradual increase. Compared with Fig. 6b and d, the Rs values of benzoin, 1,1-bi-2-naphthol, flurbiprofen and naproxen were higher on CSP-1 than on CSP-2. The cause of this may be the effect of the capping terminal structure of CSPs. Compared to CSP-2, the terminal urea group of CSP-1 enhanced the spatial site resistance, π–π interaction, hydrophobic interaction and hydrogen bonding interaction between the analyte and CSP-1, thereby improving the chiral recognition of the chiral enantiomer by CSP-1.
Effect of formic acid content on k1 (a, c) and Rs (b, d) of chiral compounds on a, b CSP-1 and c, d CSP-2. Chromatographic conditions: injection volume, 5 μL; column temperature, 30 ℃; detection wavelength, 254 nm. Mobile phases conditions as shown in Table 2
Reproducibility of the Chromatographic Column
In order to prove that the CSP-1 column has good reproducibility, naproxen was chosen as testing sample. The reproducibility was investigated on the CSP-1 column with 5 µL per injection and five manual injections. As shown in Figure S7 (see support information for details), the RSD value of the retention factor for the first peaking isomer of the CSP-1 was calculated with 0.12% (n = 5).
Effect of the n-hexane Content in the Mobile Phase on the Retention
When the mobile phase conditions are n-hexane/isopropanol/0.1% formic acid, the retention curves of the nine stereoisomers (Fig. 2) on the three peptide CSPs were investigated. First, plots of the retention times (tR) of five pairs of enantiomers with carboxyl groups against the n-hexane content are presented in Fig. 7. The retention of the five pairs of enantiomers with carboxyl groups c analytes exhibits obviously “U-shape” curve. In the normal phase chromatography, the retention increases with the increase of n-hexane content. Presently, such weird phenomenon is unclear if considering their highly hydrophilicity property. But it suggests the special selectivity of such column.
In addition, plots of the tR of four pairs of other enantiomers against the n-hexane content are presented in Fig. 8. As shown in Fig. 8, for the other compounds, the retention time of racemic compounds increases with the increase of n-hexane content. Therefore, according to the analyte characteristics and chromatographic conditions, the U-shaped curves of the retention against n-hexane can be regarded as the balance function between the hydrophilic (hydrogen bond) and hydrophobic interactions between prepared CSP-1/2/3 and the analytes.
Increased retention of five pairs of enantiomers with carboxyl groups was observed at lower and at higher n-hexane content in the mobile phase, indicating that different mechanisms are most likely employed. The “three-point rule” is considered to be the most suitable mechanism to explain enantiomeric separation on amino acid-derived CSPs, because the amino acids are small molecules. Therefore, at a lower n-hexane content (<20%), it may be that isopropanol impairs hydrogen bonding as it competes for the H-bonding site with the analyte molecule. In higher n-hexane content mobile phases (increased retention of stereoisomers starting at 50% n-hexane content in the CSPs; Fig. 7), it may be that hydrophobic interaction played a dominant role in CSPs.
Conclusions
Three new peptide CSPs with a main chain of l-Pro-l-Phe-l-Val-l-Leu and different terminals of l-Cit, l-Lys and l-Trp immobilized on APS were prepared, and the enantiomeric separation ability of these columns was evaluated by nine analytes under the normal-phase HPLC condition. Baseline separation of flurbiprofen (Rs = 1.61) and naproxen (Rs = 2.0) was achieved, and partial enantioseparations of benzoin (Rs = 0.62), 1,1ʹ-bi-2-naphthol (Rs = 0.52) and ketoprofen (Rs = 1.2) were achieved. The effect of chromatographic conditions on retention and separation was investigated in detail, and it was found that the enantiomers were best separated when the mobile phase was low in isopropanol (<10%) and high in hexane (>90%) and low in formic acid (0.1%). From the comparison of CSP-1, CSP-2 and CSP-3, it was observed that they exhibited notable difference in enantioseparation. Further, it was indicated that enhanced enantioselectivity is affected by the structure of the capping terminal. For example, the capped terminal urea group structure of CSP-1 increased the hydrogen bonding force between the analyte and the CSP-1. Therefore, peptides as CSP are worthy of further studying.
Data availability
The data underlying this article are available in the article and in its supplementary material.
References
Qi L, Qiao J (2022) Progress of chiral ligand-exchange capillary electrophoresis for enantioseparation. J Chromatogr A 1679:63381. https://doi.org/10.1016/j.chroma.2022.463381
Cheng QS, Ma Q, Pei HB, Liang H, Zhang XJ, Jin XN, Liu NJ, Guo RB, Mo ZL (2023) Chiral metal-organic frameworks materials for racemate resolution. Coordin Chem Rev 484: 215120. https://doi.org/10.1016/j.ccr.2023.215120
Brooks W, Guida W, Daniel K (2011) The significance of chirality in drug design and development. Curr Top Med Chem 760: 11. https://doi.org/10.2174/156802611795165098
Blaser HU (2013) Chirality and its implications for the pharmaceutical industry. Rend Lincei 24:213–216. https://doi.org/10.1007/s12210-012-0220-2
Zhu QJ, Cai ZW, Zhou PL, Sun XX, Xu J (2023) Recent progress of membrane technology for chiral separation: a comprehensive review. Sep Purif Technol 309: 123077. https://doi.org/10.1016/j.seppur.2022.123077
Mane S (2016) Racemic drug resolution: a comprehensive guide. Anal Methods 8:567–7586. https://doi.org/10.1039/c6ay02015a
Gao H, Fu LL, Cai ML, Chen W, Bai ZW (2021) Preparation of 6-amino-6-deoxy cellulose and its derivatives used as chiral separation materials. Carbohydr Polym 259: 117756. https://doi.org/10.1016/j.carbpol.2021.117756
Peluso P, Mamane V, Dallocchio R, DessìA CS (2020) Noncovalent interactions in high-performance liquid chromatography enantioseparations on polysaccharide-based chiral selectors. J Chromatogr A 1623:46120. https://doi.org/10.1016/j.chroma.2020.461202
Chankvetadze B (2020) Recent trends in preparation, investigation and application of polysaccharide-based chiral stationary phases for separation of enantiomers in high-performance liquid chromatograph. Trends Anal Chem 122: 115709. https://doi.org/10.1016/j.trac.2019.115709
Chankvetadze B (2012) Recent developments on polysaccharide-based chiral stationary phases for liquid-phase separation of enantiomers. J Chromatogr A 1269:26–51. https://doi.org/10.1016/j.chroma.2012.10.033
Tsui HW, Ye PW, Huang X (2020) Effect of solvents on the chiral recognition mechanisms of immobilized cellulose-based chiral stationary phase. J chromatogr A 1637: 461796. https://doi.org/10.1016/j.chroma.2020.461796
Shen J, Okamoto Y (2016) Efficient separation of enantiomers using stereoregular chiral polymers. Chem Rev 116:1094–1138. https://doi.org/10.1021/acs.chemrev.5b00317
Ferraro JM, Umstead WJ (2023) Chiral separation of cannabichromene, cannabicyclol and their acidic analogs on polysaccharide chiral stationary phases. Molecules 28:1164. https://doi.org/10.3390/molecules28031164
Zeng QL, Wen Q, Xiang Y, Zhang L (2018) Chiral stationary phases. J Chromatogr A 1571:240–244. https://doi.org/10.1016/j.chroma.2018.08.010
Kažoka H, Turovska B, Upmanis T, Veinberg G (2022) Enantioseparation of 4C-substituted pyrrolidin-2-one derivatives on polysaccharide and macrocyclic glycopeptide chiral stationary phases. Chromatographia 85:489–495. https://doi.org/10.1007/s10337-022-04145-z
Syame KR, Caroline W (2019) Characterization of three macrocyclic glycopeptide stationary phases in supercritical fluid chromatography. J Chromatogr A 160:4460–4485. https://doi.org/10.1016/j.chroma.2019.460485
Xu SJ, Wang YY, Tang YX, Ji YB (2018) A protein-based mixed selector chiral monolithic stationary phase in capillary electrochromatography. New J Chem 42:13520–13528. https://doi.org/10.1039/c8nj02309c
Tang S, Wang W, Hou HP, Liu YQ, Liu K, Geng N, Sun LQ, Luo AQ (2022) A β-cyclodextrin covalent organic framework used as a chiral stationary phase for chiral separation in gas chromatography. Chin Chem Let 33:898–902. https://doi.org/10.1016/j.cclet.2021.06.089
Ohnishi A, Shibata T, Imase T, Shinkura S, Nagai K (2021) Achiral molecular recognition of substituted aniline position isomers by crown ether type chiral stationary phase. Molecules 493:26. https://doi.org/10.3390/molecules26020493
Sung JY, Jin M, Lee SM, An SY, Jin JS (2021) Unusual enantiomeric separation due to residual amines in chiral crown ether stationary phase linked by long alkyl chain. Talanta 235: 122739. https://doi.org/10.1016/j.talanta.2021.122739
Chen H, Gu ZG, Zhang J (2012) Surface chiroselective assembly of enantiopure crystalline porous films containing bichiral building blocks. Chem Sci 12: 12346–12352. https://doi.org/10.1039/d1sc03089b
Slater B, Hill M, Ladewig B (2021) Solvent-induced enantioselectivity reversal in a chiral metal organic framework. J Sep Sc 44:3319–3323. https://doi.org/10.1002/jssc.202100322
Ismail O, Pasti L, Ciogli A, Villani C, Kocergin J, Anderson S, Gasparrini FA, Cavazzini A, Catani M (2016) Pirkle-type chiral stationary phase on core-shell and fully porous particles: are superficially porous particles always the better choice toward ultrafast high-performance enantioseparations. J Chromatogr A 1466:96–104. https://doi.org/10.1016/j.chroma.2016.09.001
Huang M, Chen H, Li TY (2006) Improvement of proline chiral stationary phases by varying peptide length and linker. J Chromatogr A 1113:109–115. https://doi.org/10.1016/j.chroma.2006.01.128
Huang JM, Zhang P, Chen H, Li TY (2005) Preparation and evaluation of proline-based chiral columns. Anal Chem 77:3301–3308. https://doi.org/10.1021/ac050050s
Buszewski B, Skoczylas M (2019) Multi-parametric characterization of amino acid-and peptide-silica stationary phases. Chromatographia 82:153–166. https://doi.org/10.1007/s10337-018-3569-2
Ma ZY, Shang PP, Liu DL, Nie YY, Liu YL, Guo XY, Wei BB, Bai LG, Qiao XQ (2023) Preparation and chromatographic performance of chiral peptide-based stationary phases for enantiomeric separation. Chirality 1–9. https://doi.org/10.1002/chir.23564
Li Y, Jiang DJ, Huang DY, Huang MX, Lia LJ (2015) Preparation and characterization of tripeptide chiral stationary phases with varying amino acid sequences and terminal groups. Anal Methods 7:3772–3778. https://doi.org/10.1039/c5ay00691k
Acknowledgements
This work was supported by grants from Hebei Natural Science Foundation (nos. H2017201075, B2020201056).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Xinyuan Guo and Panpan Shang. The first draft of the manuscript was written by Xinyuan Guo. Research program planning and writing—review and editing were performed by Benben Wei and Wenrong Du. Conceptualization, writing—review and editing, supervision, project administration and funding acquisition were performed by Wenrong Du and Yong Lan. Zhengyue Ma revised the whole manuscript and finished the final draft. Xiaoqiang Qiao and Ligai Bai put forward some useful suggestions for the revision and improvement of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guo, X., Shang, P., Wei, B. et al. Preparation and Enantiomeric Separation of l-Pro-l-Phe-l-Val-l-Leu Peptide Stationary Phases. Chromatographia 86, 541–551 (2023). https://doi.org/10.1007/s10337-023-04267-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-023-04267-y