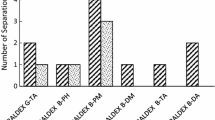
Abstract
Liquid chromatographic resolution of racemic compounds containing a primary amino group has been known to be most successful when chiral crown ether-based chiral stationary phases (CSPs) are used. Among various crown ether-based CSPs, the stationary phase based on (+)-(18-crown-6)-2,3,11,12-tetracarboxylic acid covalently bonded to silica gel has been successfully applied in the resolution of various racemic compounds containing primary amino groups. In this chapter, the preparation of the CSP based on (+)-(18-crown-6)-2,3,11,12-tetracarboxylic acid covalently bonded to silica gel and examples for the application to the enantioseparation of racemic compounds including α-amino acids, cyclic amines, amino alcohols, and chiral drugs are described.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Hyun MH (2003) Characterization of liquid chromatographic chiral separation on chiral crown ether stationary phases. J Sep Sci 26:242–250
Hyun MH (2005) Development and application of crown ether-based HPLC chiral stationary phases. Bull Kor Chem Soc 26:1153–1163
Hyun MH (2006) Preparation and application of HPLC chiral stationary phases based on (+)-(18-crown-6)-2,3,11,12-tetracarboxylic acid. J Sep Sci 29:750–761
Choi HJ, Hyun MH (2007) Liquid chromatographic chiral separations by crown ether-based chiral stationary phases. J Liq Chromatogr Rel Technol 30:853–875
Sousa LR, Sogah GDY, Hoffman DH, Cram DJ (1978) Host-guest complexation. 12. Total optical resolution of amine and amino ester salts by chromatography. J Am Chem Soc 100:4569–4576
Sogah GDY, Cram DJ (1979) Host-guest complexation. 14. Host covalently bound to polystyrene resin for chromatographic resolution of enantiomers of amino acid and ester salts. J Am Chem Soc 101:3035–3042
Shinbo T, Yamaguchi T, Nishimura K, Sugiura M (1987) Chromatographic separation of racemic amino acids by use of chiral crown ether-coated reversed-phase packings. J Chromatogr 405:145–153
Shinbo T, Yamaguchi T, Yanagishita H, Kitamoto D, Sakaki K, Sugiura M (1992) Improved crown ether-based chiral stationary phase. J Chromatogr 625:101–108
Hyun MH, Han SC, Lipshutz BH, Shin Y-J, Welch CJ (2001) New chiral crown ether stationary phase for the liquid chromatographic resolution of α-amino acid enantiomers. J Chromatogr A 910:359–365
Hyun MH, Han SC, Choi HJ, Kang BS, Ha HJ (2007) Effect of the residual silanol group protection on the liquid chromatographic resolution of racemic primary amino compounds on a chiral stationary phase based on optically active (3,3’-diphenyl-1,1’-binaphthyl)-20-crown-6. J Chromatogr A 1138:169–174
Choi HJ, Ha HJ, Han SC, Hyun MH (2008) Liquid chromatographic resolution of β-amino acids on CSPs based on optically active (3,3’-diphenyl-1,1’-binaphthyl)-20-crown-6. Anal Chim Acta 619:122–128
Choi HJ, Cho HS, Han SC, Hyun MH (2009) HPLC of fluoroquinolone antibacterials using chiral stationary phase based on enantiomeric (3,3’-diphenyl-1,1’-binaphthyl)-20-crown-6. J Sep Sci 32:536–541
Choi HJ, Jin JS, Hyun MH (2008) Liquid chromatographic direct resolution of aryl α-amino ketones on a residual silanol group-protecting chiral stationary phase based on optically active (3,3’-diphenyl-1,1’-binaphthyl)-20-crown-6. J Chromatogr B 875:102–107
Choi HJ, Jin JS, Hyun MH (2009) Liquid chromatographic direct resolution of tocainide and its analogs on a (3,3’-diphenyl-1,10-binaphthyl)-20-crown-6-based chiral stationary phase containing residual silanol protecting n-octyl groups. Chirality 21:11–15
Behr J-P, Girodeau J-M, Hayward RC, Lehn J-M, Sauvage J-P (1980) Molecular receptors. Functionalized and chiral macrocyclic polyethers derived from tartaric acid. Helv Chim Acta 63:2096–2111
Hyun MH, Jin JS, Lee W (1998) Liquid chromatographic resolution of racemic amino acids and their derivatives on a new chiral stationary phase based on crown ether. J Chromatogr A 822:155–161
Berkecz R, Ilisz I, Misicka A, Tymecka D, Fulop F, Choi HJ, Hyun MH, Peter A (2009) HPLC enantioseparation of β 2-homoamino acids using crown ether-based chiral stationary phase. J Sep Sci 32:981–987
Hyun MH, Jin JS, Koo HJ, Lee W (1999) Liquid chromatographic resolution of racemic amines and amino alcohols on a chiral stationary phase derived from crown ether. J Chromatogr A 837:75–82
Hyun MH, Han SC, Jin JS, Lee W (2000) Separation of the stereoisomers of fluoroquinolone antibacterial agents on a crown-ether-based chiral HPLC stationary phase. Chromatographia 52:473–476
Hyun MH, Tan G, Cho YJ (2004) Liquid chromatographic resolution of aryl α-amino ketones on chiral stationary phases based on (+)-(18-crown-6)-2,3,11,12-tetracarboxylic acid. J Liq Chromatogr Rel Technol 27:1671–1680
Hyun MH, Min HJ, Cho YJ (2003) Resolution of tocainide and its analogues on liquid chromatographic chiral stationary phases based on (+)-(18-crown-6)-2,3,11,12-tetracarboxylic acid. Bull Kor Chem Soc 24:911–915
Lee A, Choi HJ, Hyun MH (2010) Liquid chromatographic direct resolution of flecainide and its analogs on a chiral stationary phase based on (+)-(18-Crown-6)-2,3,11,12-tetracarboxylic acid. Chirality 22:693–698
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media, LLC
About this protocol
Cite this protocol
Hyun, M.H. (2013). Enantioseparations of Primary Amino Compounds by High-Performance Liquid Chromatography Using Chiral Crown Ether-Based Chiral Stationary Phase. In: Scriba, G. (eds) Chiral Separations. Methods in Molecular Biology, vol 970. Humana Press, Totowa, NJ. https://doi.org/10.1007/978-1-62703-263-6_9
Download citation
DOI: https://doi.org/10.1007/978-1-62703-263-6_9
Published:
Publisher Name: Humana Press, Totowa, NJ
Print ISBN: 978-1-62703-262-9
Online ISBN: 978-1-62703-263-6
eBook Packages: Springer Protocols