Abstract
Polar-ionic and hydro-organic mobile phase mode of high-performance liquid chromatographic separations of 23 sterically constrained primary β3-amino acid enantiomers containing, alkyl, aryl or heteroaryl side-chains were carried out by using newly developed Cinchona alkaloid-based zwitterionic chiral selectors and the stationary phases Chiralpak ZWIX(+)™ and ZWIX(−)™. In the polar-ionic mode, the effects of the composition of the bulk solvent and the natures of the co- and counter-ions, while in the hydro-organic mode, the effects of the pH, the counter-ion concentration and the structures of the analytes were investigated. The separations of the enantiomers of these 23 primary β3-amino acids, which can be classified as a series of quasi- (pseudo-) homologs, were optimized in both chromatographic modes. The elution sequence was determined in most cases and a reversal of elution order on ZWIX(+)™ and ZWIX(−)™ column was observed. On the basis of this intermolecular recognition model between the selectors and the given enantiomers an indirect assignment of the resolved enantiomer via chromatography is proposed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although of less importance than their α-analogs, β-amino acids are at the focus of current interest by virtue of their valuable pharmacological properties (Ma 2003). They serve as essential structural units of a number of biologically active compounds found in natural products (Juaristi and Soloshonok 2005). In recent years, extensive investigations have been carried out on the chemistry and pharmacology of β-amino acids, in particular because of their importance in pharmaceutical research (Bandala and Juaristi 2009). β-Amino acids are applied in the synthesis of heterocyclic derivatives in a wide range of structural diversity, and a rapidly-growing class of oligomeric peptides with significant biological and catalytic properties (Sleebs et al. 2009; Fülöp and Martinek 2012). It is clear that, following the synthesis of any chiral compound, a check on the stereochemistry of the final product demands a sensitive and informative analytical method. Among the possible methods, enantioselective high-performance liquid chromatography (HPLC) is routinely used for the discrimination of enantiomers. Chromatographic enantioseparations of β-amino acids have been surveyed in several review articles (Hyun 2005; Ilisz et al. 2008, 2009, 2012; Lämmerhofer 2010). The direct enantioresolution of β-amino acids has been successfully achieved with both low molecular weight (Gecse et al. 2013; Ilisz et al. 2014a, b; Pataj et al. 2014a, b; Péter 2002; Sipos et al. 2012a) and macromolecular selectors (SOs) (D’Aquarica et al. 2000; Berkecz et al. 2009; Pataj et al. 2014a, b; Sipos et al. 2010, 2012b; Sardella et al. 2014a, b).
Cinchona alkaloid-based zwitterionic ion-exchange-type chiral SOs generally operate in slightly acidic polar-ionic mobile phases allowing three modes of ion exchange: anion-exchange, cation-exchange and zwitterion-exchange modes on amphoteric compounds such as amino acids and small peptides (Hoffmann et al. 2008; 2009a, b; Pell et al. 2012; Wernisch et al. 2012; Wernisch and Lindner 2012. To date, relatively few papers have been published for selectands (SAs) operating under reversed-phase conditions (Hoffmann et al. 2009a, b; Zhang et al. 2014a, b).
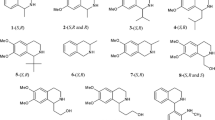
The focus of this paper is the evaluation of the effect of mobile phase compositions and separation conditions on the enantioseparation of 23 primary β3-amino acid enantiomers belonging in a series of quasi- (pseudo-) homologs possessing alkyl, aryl and heteroaryl side-chains in β-position (Fig. 1). The zwitterionic Cinchona alkaloid-based SOs and chiral stationary phases (CSPs) (Fig. 2) were operated in polar-ionic mode (PIM) and hydro-organic mobile phase mode. The effects of the mobile phase composition, the natures and concentrations of the mobile phase additives, the pH of the mobile phase, the temperature and the structural features of the SAs on the retention and enantioselectivity are discussed. On the basis of stereochemically well-defined reference compounds in these β3-amino acid series, the sequence of elution of the enantiomers was determined in most cases.
Chemicals and reagents
Racemic 3-aminobutanoic acid (1) and 3-aminopentanoic acid (2) (Fig. 1) were prepared from the corresponding α,β-unsaturated acids by benzylamine addition and subsequent debenzylation of the products with 20 % metallic palladium on charcoal in a hydrogen atmosphere (Zilkha and Rivlin 1958; Gedey et al. 2001). (R)-3-Aminobutanoic acid (1) was prepared by the same method, but (R)-(+)-α-methylbenzylamine was used in the addition step instead of benzylamine (Furukawa et al. 1977). The other aliphatic racemic β3-amino acids, 3-amino-4-methylpentanoic acid (3), 3-amino-4,4-dimethylpentanoic acid (4), 3-amino-4-methylhexanoic acid (5), 3-amino-4-ethylhexanoic acid (6), 3-amino-3-cyclohexylpropanoic acid (7), 3-amino-3-(3-cyclohexen-1-yl)propanoic acid (8), and β3-amino acids possessing aromatic side-chains, 3-amino-3-phenylpropanoic acid (9), 3-amino-3-phenylbutanoic acid (10), 3-amino-4-(3,4,6-trifluorophenyl)butanoic acid (16), 3-amino-3-(3-pyridyl)propanoic acid (19), 3-amino-3-(2-thienyl)propanoic acid (20) 3-amino-3-(3-thienyl)propanoic acid (21), 3-amino-3-(2-furyl)propanoic acid (22) and 3-amino-3-(3-furyl)propanoic acid (23) were synthetized from the corresponding aldehydes by a modification of the procedure of (Rodionov and Malivinskaya 1926; Shih et al. 1978; Lázár et al. 1998; Solymár et al. 2002).
The hydrochlorides of (S)-3-amino-4-methylpentanoic acid (3), (S)-3-amino-3-cyclohexylpropanoic acid (7), (S)-3-amino-3-phenylpropanoic acid (9), (S)-3-amino-3-phenylbutanoic acid (10) and (S)-3-amino-4-(3,4,6-trifluorophenyl)butanoic acid (16) were prepared by the method of Gedey et al. (2001). The hydrochlorides of (S)-3-amino-3-heteroarylpropanoic acids (20-23) were prepared by acidic hydrolysis of the butyramides of the corresponding ethyl (S)-3-amino-3-heteroarylpropanoates.
Enantiomerically pure (R)- and (S)-3-amino-5-phenylpentanoic acid (11), (R)- and (S)-3-amino-4-(2-naphthyl)butanoic acid (12), (R)- and (S)-3-amino-4-(3-methylphenyl)butanoic acid (13), (R)- and (S)-3-amino-4-(4-methylphenyl)butanoic acid (14), (R)- and (S)-3-amino-4-(4-chlorophenyl)butanoic acid (15), (R)- and (S)-3-amino-4-(3-pyridyl)butanoic acid (17) and (R)- and (S)-3-amino-4-(4-pyridyl)butanoic acid (18) were from Solvay-Peptisyntha (Brussels, Belgium).
Methanol (MeOH) and acetonitrile (MeCN) of HPLC grade, and ammonia (NH3), ethylamine (EA), diethylamine (DEA), triethylamine (TEA), propylamine (PA) tripropylamine (TPA), butylamine (BA), tributylamine (TBA), formic acid (FA) and glacial acetic acid (AcOH) of analytical reagent grade were purchased from VWR International (Radnor, PA, USA). Milli-Q water was further purified by filtration on a 0.45-μm filter, type HV, Millipore (Molsheim, France). Aqueous triethylammonium acetate (TEAA) buffer was prepared from TEA with the appropriate molarity, and the specified pH was adjusted with AcOH.
Instrumentation
Chromatographic measurements were carried out on a 1100 Series HPLC system from Agilent Technologies (Waldbronn, Germany), consisting of a solvent degasser, a pump, an autosampler, a column thermostat, a multiwavelength UV–Vis detector and a corona-charged aerosol detector from ESA Biosciences, Inc. (Chelmsford, MA, USA). Data acquisition and analysis were carried out with ChemStation chromatographic data software from Agilent Technologies. The alternative Waters Breeze system consisted of a 1525 binary pump, a 487 dual-channel absorbance detector, a 717 plus autosampler and Empower 2 data manager software (Waters Chromatography, Milford, MA, USA). The columns were thermostated in a Spark Mistral column thermostat (Spark Holland, Emmen, The Netherlands).
The Chiralpak ZWIX(+)™ and ZWIX(−)™ columns (150 × 3.0 mm I.D., 3-μm particle size for both columns) were from Chiral Technologies Europe (Illkirch, France).
Chromatography was performed in isocratic mode at a flow rate of 0.6 ml min−1 and a column temperature of 25 °C (if not otherwise stated). UV detection was accomplished at selected wavelengths of 215, 230 and 265 nm. The void volume of the columns (t 0) was determined by injecting acetone dissolved in MeOH, with detection at 280 nm. All the SAs were dissolved in MeOH in the concentration range 0.5–1.0 mg ml−1 and further diluted with mobile phase.
pK a values were calculated by using ACD/pK a DB 7.0 software from ACDLabs (Advanced Chemistry Development, Ontario, Canada). pH measurements were carried out on an Orion model 420A pH Meter (Boston, USA).
Results and discussion
For the investigated set of analytes (SAs), the carboxy and primary amino groups provide the chargeable sites for the most pronounced electrostatic interactions. The various aliphatic and aromatic side-chains and other structural features influence the overall hydrophobicity, π-character, bulkiness, rigidity, steric effects, etc., of the molecules, resulting in particular effects on their interactions with the SOs following a stereochemically demanding SO–SAs molecular recognition model.
PIM-based enantioseparations on ZWIX(+)™ and ZWIX(−)™ columns
In the PIM, a mixture of MeOH as protic solvent and MeCN as aprotic solvent ensured the best enantioseparation on the Cinchona alkaloid-based zwitterionic chiral ion-exchangers (Hoffmann et al. 2009a, b; Pell et al. 2012). On ZWIX(−)™, the effects of variation of the mobile phase compositions for analytes possessing alkyl (1) or aryl side-chains (9) were investigated in mixtures of MeOH/MeCN (75/25, 50/50 and 60/40 v/v) containing 25 mM TEA and 50 mM AcOH as bulk solvents; the ratio of acid and base was kept constant at 2:1, ensuring weakly acidic conditions. The retention of the enantiomers of the β3-amino acids was substantially greater in the presence of MeCN in the mobile phase. For 1, k 1 increased from 2.27 to 6.71, and for 9 from 2.73 to 7.93 (Supplementary Fig. 1, Online Resource). The increase in k 1 in the presence of MeCN is probably due to the decreasing solvation effect in the mobile phase, triggering stronger electrostatic interactions between the SAs and the SOs. The slight increase in α (approximately 20 %) with increasing MeCN content was attributed to more pronounced electrostatic and H-bonding interactions. R S changed in parallel with the k and α values, i.e. it increased with increasing MeCN content. MeCN contents above 60 v % resulted in extremely high retention. These results indicate that the ratio MeOH/MeCN sensitively influences the performance of the zwitterionic CSPs and the chiral separation. In view of these results, the subsequent experiments were carried out with MeOH/MeCN (50/50 v/v), as the most broadly applicable mixture.
In the PIM, the natures of the various acid and base additives in the mobile phase greatly influence the chromatographic parameters and play an important role in the optimization of the enantioseparation on Cinchona alkaloid-based CSPs. For a more detailed investigation of the effects of the natures of the base and acid additives, 1 and 9 were separated with the same bulk solvent composition, MeOH/MeCN (50/50 v/v) containing 25 mM base and 50 mM AcOH (or FA), on the ZWIX(−)™ column. As bases, NH3, EA, DEA, TEA, PA, TPA, BA and TBA were selected, which differ in the degree and nature of their alkyl substitution on the N atom, and either AcOH or FA (data for FA are not shown) was used as acid. The acid-to-base concentration ratio was kept at ~2, ensuring that all the bases within the system were present in their protonated, “ammonium ion” form. The experimental results in Fig. 3 reveal that, in general, k 1 increased as the degree of alkyl substitution on the N atom increased (EA < DEA < TEA; PA < TPA; BA < TBA). The presence of ethyl-, propyl- and t-butyl-substituted bases generally resulted in slightly higher k 1 values than that for the n-butyl-substituted amines. As compared with to the monosubstituted bases the presence of more apolar trisubstituted bases probably influenced the solvation of the ionic analytes disadvantageously, resulting in increased retention.
Effects of the natures of acid and base additives on the chromatographic parameters of analytes 1 and 9. Chromatographic conditions: column, ZWIX(−)™; mobile phase, MeOH/MeCN (50/50 v/v) containing 50.0 mM AcOH and 25.0 mM NH3; 50.0 mM AcOH and 25.0 mM EA; 50.0 mM AcOH and 25.0 mM DEA; 50.0 mM AcOH and 25.0 mM TEA; 50.0 mM AcOH and 25.0 mM PA; 50.0 mM AcOH and 25.0 mM TPA; 50.0 mM AcOH and 25.0 mM BA; 50.0 mM AcOH and 25.0 mM TBA; flow rate, 0.6 ml min−1; detection, 230 nm; symbols, a
k
1
:  , α
, α
 , R
S
, R
S
 for 1; b
k
1
for 1; b
k
1
 , α
, α
 , R
S
, R
S
 for 9
for 9
The nature of the base exerted a slight effect on the selectivity, but α varied in only a narrow range 1.18–1.22 for 1, and 1.56–1.65 for 9 on ZWIX(−)™. The natures of the applied bases influenced the resolution, but no general trend could be observed. The findings were similar when FA was used as acid additive instead of AcOH, with slightly higher k values. However, the enantioselectivity remained in the same range as in the case of AcOH (data not shown).
Hydro-organic mobile phase-based enantioseparations on ZWIX(+)™ and ZWIX(−)™ columns
Most of the chiral separations with the Cinchona alkaloid-based zwitterionic CSPs were carried out with the application of water-free polar-ionic or polar-organic mobile phases consisting of MeOH or MeOH/MeCN bulk solvents with acid and base additives (Hoffmann et al. 2008, 2009a, b; Ilisz et al. 2014a, b; Pataj et al. 2014a, b; Pell et al. 2012; Wernisch et al. 2012; Wernisch and Lindner 2012). Relatively few papers have been published on chromatographic enantioseparation by zwitterionic phases with water-containing eluents. A small number of examples for the enantioseparation of free α-amino acids (Ala, Asp, Asn, Phe, Pro, α-Me-mTyr and Trp analogs, secondary amino acids) have been described with low or moderate water contents in the mobile phase (Hoffmann et al. 2009a, b; Ilisz et al. 2014c; Zhang et al. 2014a, b). The mobile phases were MeOH/MeCN (49/49 v/v), MeOH/THF (49/49 v/v) or pure MeOH, all containing 2 v % water, or MeOH/H2O (90/10 or 80/20 v/v) or MeCN/H2O (90/10 or 80/20 v/v), all containing acid and base in a ratio of 2:1.
To illustrate the possibilities in hydro-organic mobile phase separations, analytes containing aliphatic (4) or aromatic (16) side-chains were employed, when the application of MeOH/MeCN or MeOH as bulk solvent containing more than 2 v % H2O resulted in only partial separation with poor peak shapes. In the presence of water in the mobile phase, the best separation in the enantioseparation of primary β3-amino acid enantiomers was achieved with MeCN as bulk solvent containing not more than 10 % water.
It is expected that ion-pairing processes will also dominate in the retention of zwitterionic analytes in the hydro-organic mobile phase. Under slightly acidic hydro-organic mobile phase conditions, with MeCN/H2O containing a TEAA buffer, the tertiary amino group within the quinuclidine ring (pK a ≈ 9, Lindner and Lämmerhofer 1996) is protonated, while the negatively charged strong sulfonic acid (calculated pK a 1.78) in diluted aqueous solution is expected to be dissociated. ACD calculations for the acidic site in 4 and 16 gave pK a values of 3.8 and 3.7, respectively, while the corresponding values for the basic sites were 10.6 and 9.5, respectively. Decrease of the pH from 6.00 to 4.00 in the 25 mM aqueous TEAA of the TEAA/MeCN (10/90 v/v) eluent system slightly increased the retention and separation factors of 4 and 16 on both ZWIX(+)™ and ZWIX(−)™ (Supplementary Fig. 5, Online Resource). The k 1 values for 4 and 16 on ZWIX(+)™ increased by about 20 %, and on ZWIX(−)™ by 10 %. α for 4 varied in the interval 1.34–1.39 and for 16 in the interval 1.25–1.33 on both columns. A pH that produced higher k 1 and α values also yielded better resolution. The observed effects may probably be attributed to the changes in the protonation, that either directly affects the ionic or dipolar interactions between the analyte and the CSP, or indirectly influences the separation by changing the conformation of the SO. The retention is therefore probably governed by the simultaneous double ion-pairing of the chargeable sites of the SA and SO.
Under slightly acidic conditions, where ion-pairing takes place between the SA and SO, long-range ionic interaction occurred between the cationic site of the SA and the strong anionic site of SO as the primary interaction, followed and supported by the second ion-pairing process, leading to the formation of sterically defined intermediate SO–SA complexes. In this case, the counter-ions present in the mobile phase will compete for the interaction sites with the SA, i.e. the application of a higher counter-ion concentration should result in lower retention. According to the stoichiometric displacement model (Kopaciewicz et al. 1983; Stahlberg 1999), a linear relationship should be obtained for the plot of the logarithm of the retention factor of the first-eluted enantiomer (log k 1) vs. the logarithm of the counter-ion concentration (log c).
In order to investigate of the validity of the simple displacement model in our case, the concentration of TEA was varied between 5.0 and 50 mM (the acid-to-base ratio was kept constant at 2:1 by the addition of AcOH). The obtained results, presented as log k 1 vs. log c curves in Fig. 4, clearly confirm the stoichiometric displacement model, which can serve as a proof of the occurrence of an ion-exchange process. Figure 4 depicts the slopes and intercepts for 4 and 16 under isocratic conditions as functions of the counter-ion concentration. The slopes relate to the effective charge number and are determined by the ratio of the effective charges of the SA and the counter-ion. A slope close to 1.0 means that both the counter-ion and the SA are protonated and have comparable charges (Hoffmann et al. 2007). The slopes of the log k 1 vs. log c plots for 4 were 0.29 on both ZWIX(+)™ and ZWIX(−)™ columns, while those for 16 were slightly lower, 0.21 (Fig. 4). The results are in good accordance with the literature data (Hoffmann et al. 2009a), where slopes in the interval 0.1–0.2 were obtained for zwitterionic SAs on zwitterionic CSPs. This result draws attention to the marked difference between zwitterionic CSPs and ionic CSPs that contain only cationic or anionic ion-exchange sites; through variation of the counter-ion concentration, the retention on zwitterionic CSPs applied in the hydro-organic mobile phase can be adjusted in only a limited range. Under hydro-organic mobile phase conditions for both analytes (4 and 16) on both CSPs, practically identical slopes were obtained for each enantiomer, i.e. the enantioselectivity remained almost constant when the counter-ion concentration was increased.
Influence of the counter-ion concentration of 4 and 16 on the retention of the first-eluting enantiomer (k 1). Chromatographic conditions: column, ZWIX(+)™ or ZWIX(−)™; mobile phase, aqueous TEAA (pH 4.0)/MeCN (10/90 v/v) containing 5, 12.5, 25 or 50 mM TEA (the pH of the aqueous phase was adjusted by the addition of AcOH); flow rate, 0.6 ml min−1; detection, 215, 230 and 260 nm; symbols, k 1 filled square for 4, and filled circle for 16 on ZWIX(−)™, and open square for 4, and open circle for 16 on ZWIX(+)™
In the PIM (mobile phase, MeOH/MeCN (50/50 v/v) containing 50 mM AcOH and 25 mM DEA), higher k 1 values were generally obtained than in the hydro-organic mobile phase (mobile phase, MeCN/H2O (90/10 v/v) containing 50 mM AcOH and 25 mM TEA) on both the ZWIX(+)™ and the ZWIX(−)™ column (Tables 1, 2). The α values were also higher in the PIM, but in some cases α was higher in the hydro-organic mobile phase, mainly for SAs containing an aromatic ring on ZWIX(+)™. However, the elution sequence remained identical in all cases, which strongly supports the concept of a consistent molecular recognition model between the SOs and SAs, modulated only slightly by the mobile phase components (bulk solvent, amine and acid additives).
In both the PIM and the hydro-organic mobile phase, higher k 1 values were usually observed on ZWIX(+)™ than on ZWIX(−)™ (exceptions were 5, 12 and 16 in the PIM), but the α values were generally higher on ZWIX(−)™ (exceptions were 7, 12 and 16 in the hydro-organic mobile phase). Opposite elution sequence were observed in all cases on ZWIX(+) and ZWIX(−).
Structure–retention (selectivity) relationships
The sterically demanding structures of the constrained but quasi- (pseudo-) homologoes SAs (Fig. 1) influence the retention and the chiral recognition. Tables 1 and 2 report the k and α values, including the elution sequence, observed with the most frequently applied mobile phases on the ZWIX(+)™ and ZWIX(−)™ columns in this study. At the same mobile phase composition in both the PIM and the hydro-organic mobile phase, the k 1 and α values for analytes with alkyl side-chains depend strongly on the chain length and the bulk of the side-chain.
In order to determine the specific structural effects of the alkyl substituents in the investigated primary β3-amino acids on chromatographic data such as k 1 and α, the volume of the side-chain of the substituents (V a) was considered. The steric effect of a substituent on the reaction rate has been reported to be characterized by the size-descriptor of the molecule, V a [the volume of the side-chain (V a, Meyer parameter) of the substituents were calculated in a dimension nm3/molecule; Meyer 1986]. For both columns, at a constant mobile phase composition of MeOH/MeCN (50/50 v/v) containing 50 mM AcOH and 25 mM DEA, or of MeCN/H2O (90/10 v/v) containing 50 mM AcOH and 25 mM TEA, the chromatographic parameters k 1 and α correlated closely with V a. The data in Fig. 5 reveal that the retention factors depended strongly on the volume of the alkyl group: via steric effects, a bulkier substituent evidently inhibited the overall interaction with the SO, and the retention decreased. However, the molecular structure has a significant effect on the degree of enantiorecognition too; a longer chain length and a bulkier molecular structure resulted in improved chiral recognition. Our results demonstrated that the steric effects of alkyl side-chains exerted a considerable influence on the retention (and chiral discrimination) of primary β3-amino acids. Nonetheless, it should be emphasized that the stereochemically driven elution sequence remained, which is a strong sign of a consistent multiple site intermolecular interaction model dominated by the simultaneously occurring double ion-pairing in concert with space describing sectors.
Dependence of retention factors and separation factors of 1, 2, 4 and 6 on the Meyer substituent parameter (V a). Chromatographic conditions: column, ZWIX(+)™ or ZWIX(−)™; mobile phase, MeOH/MeCN (50/50 v/v) containing 25 mM TEA and 50 mM AcOH (PIM) or H2O/MeCN (10/90 v/v) containing 25 mM TEA and 50 mM AcOH (hydro-organic mobile phase); flow rate, 0.6 ml min−1; detection 215 and 230 nm or corona detector; temperature, 25°C; symbols, k 1 and α filled square in PIM, in open triangle hydro-organic mobile phase on ZWIX(+)™; and k 1 and α filled circle in PIM, filled diamond in hydro-organic mobile phase on ZWIX(−)™
For 9–12 (containing an aromatic ring), the k 1 values proved to be considerably higher, whereas the enantioselectivity generally remained at the same level as seen for 1–6. The π character of the SAs may contribute to the retention through interaction with the quinoline moieties of the SO; an especially enhanced interaction was observed for the naphthalene ring-containing 12 as compared with 10, but this was not manifested in markedly improved enantioselectivity. A methyl group on the benzyl ring, independently of its position (13 and 14 vs. 10) gave rise to small effects on k 1 and α. The presence of the –Cl or –F group in 15 and 16 may improve the interaction with the SO through H-bonding to some extent relative to 10, this being manifested in most cases in higher k 1 and α values in both chromatographic modes and on both columns. However, the presence and position of the –N–, –S– and –O– groups (SAs 17–19, 20–21 and 22–23) are associated with only moderate effects on the retention and enantioselectivity. The larger k 1 values of 17–19 as compared with 9 and 10 may be attributed to the difference in the effects of the pyridinium and the benzyl ring. The ortho or meta position of the –S– and –O– groups in 20 vs. 21 and 22 vs. 23 exerts a steric influence on the interaction, the meta position of the heteroatoms resulting in higher k 1 and α values, supporting a favored SA-SO interaction. Again, the stereochemically motivated elution sequence remained consistent.
Sequence of elution of primary β3-amino acid enantiomers
The chiral SOs of the Chiralpak ZWIX(+)™ and ZWIX(−)™ CSPs are actually mutually diastereomeric (Fig. 2), but in most cases they behave as pseudo-enantiomers (Hoffmann et al. 2009b, Pell et al. 2012). On switching from ZWIX(+)™ to ZWIX(−)™, therefore, the sequence of elution of the enantiomers of SAs should in principle be reversed, assuming very similar intermolecular interactions as it was observed for the series of β3-amino acids (Tables 1, 2). Enantiomer elution order specified in Tables 1 and 2 presented some reversals of elution orders dependent on the type of substituents (alkyl, alkene and aryl) in the β3-amino acids within this series of analytes both on ZWIX(+) and ZWIX(−) columns. However, it turns out that these supposed reversals of elution orders are due to the changes of Cahn-Ingold-Prelog (CIP; Cross and Klyne 1976) priorities of the substituents but not a change in the molecular recognition mechanism. If relative configurations are considered the elution order is persistent throughout the series. Absolute configuration assignments based on elution orders seem therefore be possible.
A careful inspection of the results in Tables 1 and 2 reveals that the sterically demanding molecular recognition mechanism is consistent. It relies on the double ion-pair formation between the SO and SAs and the steric parameters associated with the more or less bulky substituents around the chiral centers describing the chiral space and the binding grooves of the SO and SAs. Accordingly, it is confirmed that the sequence of elution of the 1, 10–18 with (R)-configuration corresponds to the sequence of elution of 3, 7, 9, 20–23 with (S)-configuration, also being eluted first on the ZWIX(+) column.
For the ZWIX(−) column, we see exactly the reverse situation. From these observations, it may be stated that the sequence of elution for this group of ampholytic analytes follows a sterically well-defined binding model. As a consequence, it could be used to assign the absolute configuration of the first-eluted peak of 2, 4, 5, 6, 8 and 19 chromatographically. It is an indirect method of course, but the relatively large number of proved examples for the series of quasi- (pseudo-) homologs β3-amino acids indicate that the concept is very reasonable. Some typical chromatograms of the enantiomeric separations are presented in Fig. 6.
Selected chromatograms of β3-amino acids. Chromatographic conditions: column, ZWIX(+)™ or ZWIX(−)™; mobile phase, for analytes 3, 7, 11 and 17 MeOH/MeCN (50/50 v/v) containing 25 mM TEA and 50 mM AcOH (PIM) and for analytes 10, 12, 15 and 16 H2O/MeCN (10/90 v/v) containing 25 mM TEA and 50 mM AcOH (hydro-organic mobile phase); flow rate, 0.6 ml min−1; detection 215 and 230 nm or corona detector; temperature, 25 °C
Temperature dependence and thermodynamic parameters
Temperature generally influences enantioselective retention and separation (Castells and Carr 2000; Götmar et al. 2000; Fornstedt et al. 1998; Peyrin et al. 1997). In order to determine the enthalpic and entropic contributions to the retention, van’t Hoff plots are generally applied (Chester and Coym 2003), based on the equation
where k is the retention factor, ΔH° is the standard enthalpy of transfer of the solute from the mobile phase to the stationary phase, ΔS° is the standard entropy of transfer of the solute from the mobile phase to the stationary phase, R is the gas constant, T is temperature in Kelvin and ϕ is the phase ratio ϕ = V S /V M [the ratio of the volumes of the stationary phase (V S) and the mobile phase (V M)]. If the stationary phase volumes for the two enantiomers are the same, the Δ(ΔH°) and Δ(ΔS°) values for the separated enantiomers can be determined through a modification of Eq. 1, from the relationship
where α is the selectivity factor (α = k 2/k 1), Δ(ΔH°) expressing the difference in standard enthalpy change, and Δ(ΔS°) expressing the difference in standard entropy change for the two enantiomers moving from the mobile to the stationary phase.
In order to investigate the effects of temperature on the chromatographic parameters for SAs possessing alkyl, aryl or a substituted-aryl side-chains (4, 10 and 16), a variable-temperature study was carried out in the hydro-organic mobile phase on ZWIX(+)™ and ZWIX(−)™ columns over the temperature range 10–50 °C (in 10 °C increments). Experimental data for the mobile phase of aqueous 25 mM TEAA (pH 4.0)/MeCN (10/90 v/v) are presented in Supplementary Tables 1 and 2 (Online Resource). Comparison of the retention and separation factors on ZWIX(+)™ reveals that all of the recorded k 1 and α values decreased with increasing temperature, indicating that an increase in separation temperature lowers the separation factor, α. Transfer of the SA from the mobile phase to the stationary phase is generally an exothermic process and consequently k (and α) decreases with increasing temperature. However, on the ZWIX(−)™ column the behavior of 4, 10 and 16 was unique; k increased but α decreased, whereas increasing temperature. Increasing k and α values with increasing temperature were earlier observed for nonchiral separations (Adlof and List 2004; Wu et al. 2004; and Yogo et al. 2011) and recently also for separation of the enantiomers of neutral compounds (Matarashvili et al. 2013) and zwitterionic compounds (Ilisz et al. 2014b, c). It should be noted that this behavior was seen only on ZWIX(−)™ [not on ZWIX(+)™], indicating that the changes in the three-dimensional structure and solvation of ZWIX(−)™ with temperature probably contribute to this unusual effect. Further studies are required for a better understanding of the mechanism of this behavior.
As concerns the resolution, on ZWIX(+)™ this also decreased with increasing temperature, while on ZWIX(−)™ for 4 and 10 it first increased and then decreased, but for 16 it increased continuously with increasing temperature. Increasing temperature may improve the peak symmetry (improving the kinetics of separation) and efficiency, and the resolution may therefore also improve. However, at higher temperatures the decrease in α compensates the improvement in the kinetics of separation and R S decreases again.
The changes in retention factors, selectivity and resolution with temperature were not consistent, and an extensive study relating to the thermodynamics of these systems was therefore carried out. The initial step involved the accumulation of accurate chromatographic data, from which van’t Hoff plots were constructed Eqs. (1) and (2). As a general trend, the selectivity (ln α vs. 1/T) gave clearly linear van’t Hoff plots, as indicated by the correlation coefficients in Table 3.
The differences in the changes in standard enthalpy, Δ(ΔH°), reflect the relative ease of transfer of the analytes from the mobile to the stationary phase. A negative Δ(ΔH°) value indicates a favorable exothermic process of transfer of the enantiomers from the mobile to the stationary phase. On both ZWIX(+)™ and ZWIX(−)™, negative Δ(ΔH°) values were found (Table 3). The Δ(ΔH°) values ranged from −1.3 to −0.6 kJ mol−1 and were lower on ZWIX(+)™. The more negative Δ(ΔH°) values point to the more efficient transfer of the enantiomers between the mobile and the stationary phase and/or the stronger interaction of the more retained enantiomers with the SO.
The trend in the change in Δ(ΔS°) is similar to that in Δ(ΔH°). Under these conditions, Δ(ΔS°) also has negative values parallel with the Δ(ΔH°) values. Δ(ΔS°) ranged from −2.3 to −0.03 J mol−1 K−1 and was lower on ZWIX(+)™. In the case of negative Δ(ΔS°), the adsorbed enantiomers exhibited an increased order and/or a loss in the degrees of freedom on the SO. The strong interaction and formation of a highly ordered SO–SA complex resulted in a significant loss of freedom, indicating a thermodynamically unfavorable process.
The relative contributions of the enthalpic and entropic terms to the free energy of adsorption can be visualized through the enthalpy/entropy ratio Q [Q = Δ(ΔH°)/[298 × Δ(ΔS°)] calculated at 298 K (Table 3). Comparison of the Q values for the individual analytes revealed that the enantioselective discrimination was enthalpically driven (Q > 1.0), and especially high Q values were obtained for 10 and 16 on ZWIX(−)™.
A comparison of the −Δ(ΔG°) values calculated for the two columns (ranging from −0.5 to −0.8 kJ mol−1) demonstrated that ZWIX(+)™ and ZWIX(−)™ exhibited similar binding with the SAs. Under the conditions applied, the intermolecular interactions leading to the SO–SA complex formation were exothermic, with a corresponding negative entropic contribution.
Conclusions
HPLC methods were developed for separation of the enantiomers of 23 β3-amino acids by using Cinchona-based CSPs, i.e. Chiralpak ZWIX(+)™ and ZWIX(−)™, in PIM and RPM. By variation of the chromatographic modes, the separations of the stereoisomers were optimized; as a result, baseline resolution was achieved for all the investigated SAs in at least one chromatographic system. The composition of the bulk solvent, the natures and concentrations of the co- and counter-ions, and the structures of the SAs were found to be as important factors in the enantioselective separation process. The elution sequence was determined in most cases and was found to be opposite on the ZWIX(+)™ and ZWIX(−)™ columns. Reversal of elution order dependent on the type of substituent side-chains of β3-amino acids were due to the change of CIP priorities of the substituents but not a change in the molecular recognition mechanism. As this was based on the experiments on a relatively large series of homologs it could be used as a tool for an indirect assignment of the absolute configurations of similarly structured β3-amino acids chromatographically.
References
Adlof R, List G (2004) Analysis of triglyceride isomers by silver-ion high-performance liquid chromatography: effect of column temperature on retention time. J Chromatogr A 46:109–113
Bandala Y, Juaristi E (2009) Recent developments in the synthesis of β-amino acids. In: Hughes AB (ed) Amino acids, peptides and proteins in organic chemistry. origins and synthesis of amino acids, vol 1. Wiley-VCH, Weinheim, pp 291–365
Berkecz R, Ilisz I, Benedek G, Fülöp F, Armstrong DW, Péter A (2009) High-performance liquid chromatographic enantioseparation of 2-aminomono- and dihydroxycyclopentanecarboxylic and 2-aminodihydroxycyclohexanecarboxylic acids on macrocyclic glycopeptide-based phases. J Chromatogr A 1216:927–932
Castells CB, Carr RW (2000) A study of the thermodynamics and influence of temperature on chiral high-performance liquid chromatographic separations using cellulosetris (3,5-dimethylphenylcarbamate) coated zirconia stationary phases. Chromatographia 52:535–542
Chester TL, Coym JW (2003) Effect of phase ratio on van’t Hoff analysis in reversed-phase liquid chromatography, and phase-ratio-independent estimation of transfer enthalpy. J Chromatogr A 1003:101–111
Cross LC, Klyne W (1976) IUPAC Nomenclature of organic chemistry, section F, stereochemistry. Pure Appl Chem 45:11–30
D’Aquarica I, Gasparrini F, Misiti D, Zappia G, Cimarelli C, Palmieri G, Carotti A, Cellamare S, Villani C (2000) Application of a new chiral stationary phase containing the glycopeptide antibiotic A-40,926 in the direct chromatographic resolution of β-amino acids. Tetrahedron Asymmetry 11:2375–2385
Fornstedt T, Sajonz P, Guichon G (1998) A closer study of chiral retention mechanisms. Chirality 10:375–381
Fülöp F, Martinek TA (2012) Peptidic foldamers: ramping up diversity. Chem Soc Rev 41:687–702
Furukawa M, Okawara T, Terawaki Y (1977) Asymmetric syntheses of β-amino acids by the addition of chiral amines to C=C double bonds. Chem Pharm Bull 25:1319–1325
Gecse Z, Ilisz I, Nonn M, Grecsó N, Fülöp F, Agneeswari R, Hyun MH, Péter A (2013) High-performance liquid chromatographic enantioseparation of isoxazoline-fused 2-aminocyclopentanecarboxylic acids on a chiral ligand-exchange stationary phase. J Sep Sci 36:1335–1342
Gedey S, Liljeblad A, Lázár L, Fülöp F, Kanerva LT (2001) Preparation of highly enantiopure beta-amino esters by Candida antarctica lipase A. Tetrahedron Asymmetry 12:105–110
Götmar G, Fornstedt T, Guiochon G (2000) Retention mechanism of β-blockers on an immobilized cellulase. Relative importance of the hydrophobic and ionic contributions to their enantioselective and nonselective interactions. Anal Chem 72:3908–3915
Hoffmann CV, Lämmerhofer M, Lindner W (2007) Novel strong cation-exchange type chiral stationary phase for the enantiomer separation of chiral amines by high-performance liquid chromatography. J Chromatogr A 1161:242–251
Hoffmann CV, Pell R, Lämmerhofer M, Lindner W (2008) Synergistic effects on enantioselectivity of zwitterionic chiral stationary phases for separations of chiral acids, bases, and amino acids by HPLC. Anal Chem 80:8780–8789
Hoffmann CV, Reischl R, Maier NM, Lämmerhofer M, Lindner W (2009a) Stationary phase-related investigations of quinine-based zwitterionic chiral stationary phases operated in anion-, cation-, and zwitterion-exchange modes. J Chromatogr A 1216:1147–1156
Hoffmann CV, Reischl R, Maier NM, Lämmerhofer M, Lindner W (2009b) Investigations of mobile phase contributions to enantioselective anion- and zwitterion-exchange modes on quinine-based zwitterionic chiral stationary phases. J Chromatogr A 1216:1157–1166
Hyun MH (2005) Development and application of crown ether-based HPLC chiral stationary phases. Bull Korean Chem Soc 26:1153–1163
Ilisz I, Berkecz R, Péter A (2008) Application of chiral derivatizing agents in the high-performance liquid chromatographic separation of amino acid enantiomers: a review. J Pharm Biomed Anal 47:1–15
Ilisz I, Berkecz R, Péter A (2009) Retention mechanism of high-performance liquid chromatographic enantioseparation on macrocyclic glycopeptide-based chiral stationary phases. J Chromatogr A 1216:1854–1860
Ilisz I, Aranyi A, Pataj Z, Péter A (2012) Recent advances in the direct and indirect liquid chromatographic enantioseparation of amino acids and related compounds: a review. J Pharm Biomed Anal 69:28–41
Ilisz I, Grecsó N, Palkó M, Fülöp F, Lindner W, Péter A (2014a) Structural and temperature effects on enantiomer separations of bicyclo[2.2.2]octane-based 3-amino-2-carboxylic acids on cinchona alkaloid-based zwitterionic chiral stationary phases. J Pharm Biomed Anal 98:130–139
Ilisz I, Pataj Z, Gecse Z, Szakonyi Z, Fülöp F, Lindner W, Péter A (2014b) Unusual temperature-induced retention behavior of constrained β-amino acid enantiomers on the zwitterionic chiral stationary phases ZWIX(+) and ZWIX(−). Chirality 26:385–393
Ilisz I, Gecse Z, Pataj Z, Fülöp F, Tóth G, Lindner W, Péter A (2014c) Direct high-performance liquid chromatographic enantioseparation of secondary amino acids on Cinchona alkaloid-based chiral zwitterionic stationary phases. Unusual temperature behavior. J Chromatogr A. doi:10.1016/j.chroma.2014.06.087
Juaristi E, Soloshonok VA (2005) Enantioselective synthesis of β-amino acids, 2nd edn. Wiley-VCH, New York
Kopaciewicz W, Rounds MA, Fausnaugh F, Regnier FE (1983) Retention model for high-performance ion-exchange chromatography. J Chromatogr A 266:3–21
Lämmerhofer M (2010) Chiral recognition by enantioselective liquid chromatography: mechanisms and modern chiral stationary phases. J Chromatogr A 1217:814–856
Lázár L, Martinek T, Bernáth G, Fülöp F (1998) A simple synthesis of β-alkyl-substituted β-amino acids. Synth Commun 28:219–224
Lindner W, Lämmerhofer M (1996) Quinine and quinidine derivatives as chiral selectors I. Brush type chiral stationary phases for high-performance liquid chromatography based on cinchonan carbamates and their application as chiral anion exchangers. J Chromatogr A 741:33–48
Ma JS (2003) Unnatural amino acids in drug discovery. Chim Oggi Chem Today 21:61–68
Matarashvili I, Chankvetadze L, Fanali S, Farkas T, Chankvetadze B (2013) HPLC separation of enantiomers of chiral arylpropionic acid derivatives using polysaccharide-based chiral columns and normal-phase eluents with emphasis on elution order. J Sep Sci 36:140–147
Meyer AZ (1986) Molecular mechanics and molecular shape. Part 4. Shape and steric parameters. J Chem Soc Perkin Trans 2:1567–1986
Pataj Z, Ilisz I, Gecse Z, Szakonyi Z, Fülöp F, Lindner W, Péter A (2014a) Effect of mobile phase composition on the liquid chromatographic enantioseparation of bulky monoterpene-based β-amino acids by applying chiral stationary phases based on Cinchona alkaloid. J Sep Sci 37:1075–1082
Pataj Z, Ilisz I, Grecsó N, Palkó M, Fülöp F, Armstrong DW, Péter A (2014b) Enantiomeric separation of bicyclo[2.2.2]octane-based 2-amino-3-carboxylic acids on macrocyclic glycopeptide chiral stationary phases. Chirality 26:200–208
Pell R, Sic S, Lindner W (2012) Mechanistic investigations of cinchona alkaloid-based zwitterionic chiral stationary phases. J Chromatogr A 1269:287–296
Péter A (2002) Direct high-performance liquid-chromatographic enantioseparation of apolar β-amino acids on a quinine derived chiral anion exchanger stationary phase. J Chromatogr A 955:141–150
Peyrin E, Guillaume YC, Guinchard C (1997) Interactions between dansyl amino acids and human serum albumin using high-performance liquid chromatography: mobile-phase pH and temperature considerations. Anal Chem 69:4979–4984
Rodionov WM, Malivinskaia EF (1926) Zur darstellung von aryl-β-amino-fettsäuren Berichte 59:2952–2958
Sardella R, Ianni F, Lisanti A, Marinozzi M, Scorzoni S, Natalini B (2014a) The effect of mobile phase composition in the enentioseparation of pharmaceutically relevant compounds with polysaccharide-based stationary phases. Biomed Chromatogr 28:159–167
Sardella R, Ianni F, Lisanti A, Scorzoni S, Marini F, Sternativo S, Natalini B (2014b) Direct chromatographic enantioresolution of fully constrained β-amino acids: exploring the use of high-molecular weight chiral selectors. Amino Acids 46:1235–1242
Shih Y-E, Wang J-S, Chen C-T (1978) Studies on potential anti-tumor agents. 3. synthesis of 4-arylcyclophosphamides. Heterocycles 9:1277–1285
Sipos L, Ilisz I, Pataj Z, Szakonyi Z, Fülöp F, Armstrong DW, Péter A (2010) High-performance liquid chromatographic enantioseparation of monoterpene-based 2-amino carboxylic acids on macrocyclic glycopeptide-based phases. J Chromatogr A 1217:6956–6963
Sipos L, Ilisz I, Aranyi A, Gecse Z, Nonn M, Fülöp F, Hyun MH, Péter A (2012a) High-performance liquid chromatographic enantioseparation of unusual isoxazoline-fused 2-aminocyclopentanecarboxylic acids on (+)-(18-crown-6)-2,3,11,12-tetracarboxylic acid-based chiral stationary phases. Chirality 24:817–824
Sipos L, Ilisz I, Nonn M, Fülöp F, Pataj Z, Armstrong DW, Péter A (2012b) High-performance liquid chromatographic enantioseparation of unusual isoxazoline-fused 2-aminocyclopentanecarboxylic acids on macrocyclic glycopeptide-based chiral stationary phases. J Chromatogr A 1232:142–151
Sleebs BE, Van Nguyen TT, Hughes AB (2009) Recent advances in stereoselective synthesis and application of β-amino acids. Org Prep Proc Int 41:429–478
Solymár M, Fülöp F, Kanerva LT (2002) Candida antarctica lipase A—a powerful catalyst for the resolution of heteroaromatic β-amino esters. Tetrahedron Asymmetry 13:2383–2388
Stahlberg J (1999) Retention models for ions in chromatography. J Chromatogr A 855:3–55
Wernisch S, Lindner W (2012) Versatility of cinchona-based zwitterionic chiral stationary phases: enantiomer and diastereomer separations of non-protected ologopeptides utilizing a multi-modal chiral recognition mechanism. J Chromatogr A 1269:297–307
Wernisch S, Pell R, Lindner W (2012) Increments to chiral recognition facilitating enantiomer separations of chiral acids, bases, and ampholytes using Cinchona-based zwitterion exchanger chiral stationary phases. J Sep Sci 35:1560–1572
Wu N, Yehl PM, Gauthier D, Dovletoglu A (2004) Retention and thermodynamic studies of piperazine diastereomers in reversed-phase liquid chromatography. Chromatographia 59:189–195
Yogo K, Takemura C, Saito Y, Jinno K (2011) An abnormal temperature dependence of alkylpyrazines’ retention in reversed-phase liquid chromatography. Anal Sci 27:1257–1260
Zhang T, Holder E, Franco P, Lindner W (2014a) Method development and optimization on cinchona and chiral sulfonic acid-based zwitterionic stationary phases for enantiomer separations of free amino acids by high-performance liquid chromatography. J Chromatogr A 1363:191–199
Zhang T, Holder E, Franco P, Lindner W (2014b) Zwittewrionic chiral stationary phases based on cinchona and sulfonic acids fort the direct stereoselectice separation of amino acids and other amphoteric compounds. J Sep Sci 37:1237–1247
Zilkha A, Rivlin J (1958) Syntheses of DL-β-aminobutyric acid and its N-alkyl derivatives. J Org Chem 23:94–96
Acknowledgments
This work was supported by Hungarian National Science Foundation grant OTKA K 108847. AP gratefully acknowledges the support of Pilar Franco (Chiral Technologies Europe) for the Chiralpak columns. RP, PZ and PB gratefully acknowledge the support of the Ministry of Education, Youth and Sports of the Czech Republic (NPU LO 1305), Operational Program “Education for Competitiveness” (CZ.1.07/2.3.00/30.0041), and Palacký University in Olomouc (IGA_PrF_2014031).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: D. Tsikas.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ilisz, I., Grecsó, N., Papoušek, R. et al. High-performance liquid chromatographic separation of unusual β3-amino acid enantiomers in different chromatographic modes on Cinchona alkaloid-based zwitterionic chiral stationary phases. Amino Acids 47, 2279–2291 (2015). https://doi.org/10.1007/s00726-015-2006-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-015-2006-1