Abstract
This work describes the separation performance of the amphiphilic star-shaped calix[4]resorcinarene (C4A-CL) as the stationary phase for capillary gas chromatography (GC). The statically coated C4A-CL capillary column exhibited medium polarity and high column efficiency of 3877 plates m−1 determined by naphthalene at 120 °C. Importantly, the C4A-CL column exhibited extremely high resolving capability for aliphatic analytes with varying polarity, including n-alkanes, esters, ketones, alcohols, and bromoalkanes. In addition, the C4A-CL column exhibited high selectivity and resolving capability for positional, structural and cis-/trans-isomers. Among them, the C4A-CL column displayed advantageous resolving capability over the commercial DB-17 column for aromatic amine isomers. Moreover, it was applied for the determination of isomer impurities in real samples, showing good potential in GC applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Calixarenes (CAs), which are known as the third generation of host supramolecules after crown ethers and cyclodextrins, are macrocyclic compounds consisting of phenolic units linked by methylene bridges [1]. A number of selective factors should be considered in CAs configuration; these factors include conformation, cavity size, and substituents [2]. Their high selectivity, and good chemical and thermal stability endow them good candidates as stationary phases for gas chromatography (GC). Mangia et al. first used p-tert-butylcalix[8]arene as stationary phase in packed GC and studied its retention behavior for alkanols, chlorinated hydrocarbons, and aromatic compounds [3]. Mŭnk et al. studied the inclusion properties of p-tert-butylcalix[4]arene by GC [4]. However, the unsubstituted calixarenes are difficult to coat onto the capillary column walls because of their high melting point and poor solubility, thereby leading to poor column efficiency and separation ability. Subsequently, calixarenes are usually used together with polysiloxanes either in physical mixtures or chemically grafted polymers to improve the column efficiency and separation performance [5,6,7,8].
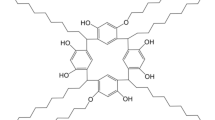
ε-Caprolactone (CL) is a monomer used to synthesize poly ε-caprolactone and is widely used in pharmaceutical industry [9]. In synthetic compounds, CL can provide many excellent physicochemical properties to the synthesized compounds. The amphipathic straight chain, which is gained from the open loop of CL, has five nonpolar methylenes and one polar ester group on a structural unit. The straight-chain ε-caprolactone is introduced into the subject molecule of CAs to form a star-shaped compound structure [10, 11]. This structure is beneficial in breaking the intramolecular H bonds in CAs to increase the dissolving ability and decrease the glass transition temperature, thereby improving the film formation of CAs significantly. Calix[4]resorcinarene is a type of CAs that contains eight phenolic hydroxyl groups. Calix[4]resorcinarene becomes a core molecule due to its excellent star-shaped compounds, which are attributed to multiple functional groups. Therefore, the star-shaped calix[4]resorcinarene can be used as the stationary phase of GC.
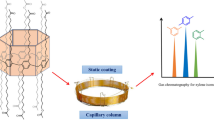
As illustrated in Scheme 1, we report the investigation of utilizing the amphiphilic star-shaped calix[4]resorcinarene (C4A-CL) as the stationary phase for GC separations. First, the C4A-CL capillary column was fabricated by static coating method and characterized for its column efficiency and polarity. Then, it was investigated for its separation performance and retention behaviors by employing more than a dozen mixtures covering a wide range of analytes and isomers. Afterwards, the C4A-CL column was applied for the determination of isomer impurities in real samples. To our knowledge, this is the first report on employing the CL-modified CAs with a star-shaped conformation in GC separations.
Experimental
Materials and Equipments
All the reagents and solvents employed were commercially available and were used as received without further purification. All the analytes were of analytical grade and dissolved in dichloromethane. Untreated fused-silica capillary tubing (0.25 mm, i.d.) was purchased from Yongnian Ruifeng Chromatogram Apparatus Co., Ltd. (Hebei, China). The commercial capillary column DB-17 (30 m × 0.25 mm, i.d., 0.25 μm film thickness, and 50% phenyl 50% dimethyl polysiloxane) was purchased from Agilent Technologies.
An Agilent 7890A gas chromatograph equipped with a split/splitless injector, a flame ionization detector (FID), and an autosampler was used for GC separations. All the separations were performed under the following GC conditions: nitrogen of high purity (99.999%) as carrier gas, injection port at 250 °C, split ratio at 60:1, FID detector at 250 °C. Oven temperature programs for the GC separations were individually provided in their figure captions. 1H NMR spectra were recorded on a Bruker Biospin 400 MHz instrument using TMS as the internal standard. IR spectra were recorded on a Bruker Platinum ART Tensor II FT-IR spectrometer. MALDI-TOF-MS was recorded on a Bruker BIFLEX III mass spectrometer. Thermogravimetric analysis (TGA) was used on a DTG-60AH instrument (Shimadzu, Japan). Scanning electron microscopy (SEM) images were recorded on a Zeiss Sigma 500 microscope (Zeiss, Germany).
Synthesis of the C4A-CL Stationary Phase
The C4A-CL was synthesized by the procedures described in Fig. 1. First, 1,3-bis(2-hydroxyethoxy)benzene (2 g, 10.09 mmol), hendecanal (1.71 g, 10.05 mmol), and 37% HCl (4 mL) were added in ethanol (5 mL). The mixture was stirred for 16 h at 90 °C under nitrogen, cooled to room temperature, and filtered and washed with methanol:water = 1:1 (V:V), getting white sticky solid. Afterwards, the white sticky solid (0.2 g, 0.14 mmol), caprolactone (0.128 g, 1.12 mmol), and Sn(Oct)2(20 mg) were added in toluene (5 mL) and refluxed at 100 °C for 24 h under nitrogen, cooled to room temperature, dissolved in tetrahydrofuran, and precipitated in diethyl ether to obtain the white solid product (C4A-CL, 87% yield). m.p. 37–39 °C. 1H NMR (400 MHz, DMSO-d6) δ: 7.75 ~ 5.87 (m, 8H), 4.51 (d, J = 24.4 Hz, 8H), 4.35 ~ 4.26 (m, 4H), 3.96 (dt, J = 13.2, 6.8 Hz, 16H), 3.85 ~ 3.65 (m, 8H), 3.62 ~ 3.32 (m, 16H), 2.29 ~ 2.22 (m, 8H), 1.79 ~ 1.58 (m, 8H), 1.52 (dd, J = 8.8, 7.6 Hz, 16H), 1.41 ~ 1.13 (m, 72H), 0.82 (d, J = 7.6 Hz, 12H). IR (KBr, cm−1): 3353, 2851, 1713, 1609, 1582, 1500, 1455, 1409, 1373, 1295, 1160, 1070, 902, 816, 734, 702, 587; MALDI-TOF MS: m/z calcd for C108H176O24: 1857.2552 (100%); found: 1881.2470[M + Na]+ (100%).
Fabrication of the C4A-CL Capillary Column
The C4A-CL capillary column was fabricated by static coating method [12, 13]. Before coating, one bare fused-silica capillary column (10 m × 0.25 mm, i.d.) was pretreated with a saturated solution of sodium chloride in methanol for the inner surface roughening of the capillary column. The roughening procedure was performed with reference to the methods in Ref [14, 15]. First, 6 mL of the saturated solution of sodium chloride in methanol was added to 8 mL of chloroform under stirring, and then, another 8 mL of chloroform and 0.6 mL of methanol were added. Then, the obtained suspension of sodium chloride in the solvent mixture was passed through the capillary column and stayed for 1 h. Afterwards, the solution was removed and the column was conditioned up to 200 °C and held for 2 h under nitrogen atmosphere. The C4A-CL was dissolved in the solution of dichloromethane with the concentration of 1.5 mg mL−1 (0.15%, w/v). Then, the pretreated column was statically coated with the C4A-CL solution at 40 °C by one end of the column sealed and the other end connected to a vacuum pump to remove the solvent. Further conditioning of the coated column was performed with a temperature program: 40 °C for 30 min, then conditioned up to 160 °C at 1 °C min−1, and held at the final temperature for 7 h under nitrogen. The as-prepared C4A-CL column was used for the following work.
Results and Discussion
Characterization of the C4A-CL Stationary Phase and Column
The inherent thermal stability of the C4A-CL stationary phase was evaluated by thermal gravimetric analysis (TGA). As shown in Fig. 2a, the C4A-CL stationary phase showed about 5% weight loss at 263 °C, suggesting its good thermal stability and feasibility as the stationary phase for GC separations. As shown in Fig. 2b, the Golay curve relating the heights equivalent to a theoretical plate (HETP) with flow rates were determined by naphthalene at 120 °C, and the minimum HETP of 0.26 mm was observed at 0.5 mL min−1 corresponding to the column efficiency of 3877 plates m−1. The higher column efficiency can be attributed to the good solubility of the C4A-CL stationary phase in the solvent for column fabrication, facilitating its uniform coating on the capillary wall. Figure 2c presents the SEM cross-sectional images of the C4A-CL column, confirming its good coating. The film thickness (df, μm) of the C4A-CL column was about 0.10 μm obtained by the empirical formula df = (dc× c)/400, where dc is the capillary inner diameter (μm), c is the concentration of the stationary phase solution (%, w/v) [16]. Polarity of the C4A-CL stationary phase was evaluated by McReynolds constants of five probe solutes, namely benzene, 1-butanol, 2-pentanone, 1-nitropropane, and pyridine, which were determined at 120 °C [17, 18]. Table 1 lists the general polarity and average polarity of the C4A-CL stationary phase in comparison to the commercial polysiloxane stationary phase, suggesting its moderate polarity as GC stationary phase. Abraham system constants can be used for quantitative evaluation of the specific intermolecular interactions between the given stationary phase and diverse probe analytes, and can be determined on the basis of Abraham’s linear solvation parameter model [19, 20]. The Abraham system constants of the C4A-CL stationary phase were determined at three temperatures (60 °C, 80 °C, and 100 °C) and the results are provided in Table 2. Table S1 (Supporting information) provides the solutes used in this work and their solute descriptors. Table 2 indicates that the major interactions of the C4A-CL stationary phases are H-bonding basicity (a), dipole–dipole (s), and dispersion (l) interactions. Observably, C4A-CL stationary phase exhibits strong H-bonding basicity interactions due to its hydroxyl functional groups.
Separation Capability and Retention Behaviors
Separation capability and retention behaviors of the C4A-CL column were evaluated by GC separation of different analytes of great variety, including nonpolar (n-alkanes), low-to-medium polar (bromoalkanes, esters, and ketones), polar analytes (alcohols), and the mixture of 28 analytes with varying polarity. On the above basis, the C4A-CL column was further explored for its resolving capability for different types of isomers mixtures. Also, the DB-17 column was employed for the separation.
Separation the Linear Analytes with Same Carbon Numbers
Figure 3 presents the separations of the linear analytes with same carbon numbers on C4A-CL column, including the mixtures of n-alkanes, esters, ketones, alcohols, and bromoalkanes. As shown, the C4A-CL column baseline resolved (R > 1.5) all of the analytes with good peak shapes, displaying high separation performance for analytes ranging from nonpolar to polar nature. The C4A-CL stationary phase shows high resolving ability for aliphatic analytes due to its comprehensive molecular interactions including H-bonding, dipole–dipole, and dispersion interaction from its unique star-shaped conformation with different linear links.
GC separation of an-alkanes, b esters, c ketones, d alcohols, and e bromoalkanes on C4A-CL column. Peaks for (a): (1) n-hexane, (2) n-heptane, (3) n-octane, (4) n-nonane, (5) n-decane, (6) n-undecane, (7) n-dodecane, and (8) n-tridecane; Peaks for (b): (1) methyl valerate, (2) methyl hexanoate, (3) methyl heptanoate, (4) methyl octanoate, (5) methyl nonanoate, (6) methyl decanoate, (7) methyl undecanoate, and (8) methyl dodecanoate; Peaks for (c): (1) 2-pentanone, (2) 2-hexanone, (3) 2-heptanone, (4) 2-octanone, (5) 2-nonanone, (6) 2-decanone, (7) 2-undecanone, and (8) 2-dodecanone; Peaks for (d): (1) 1-pentanol, (2) 1-hexanol, (3) 1-heptanol, (4) 1-octanol, (5) 1-nonanol, (6) 1-decanol, (7) 1-undecanol, and (8) 1-dodecanol; Peaks for (e): (1) 1-bromopentane, (2) 1-bromohexane, (3) 1-bromoheptane, (4) 1-bromooctane, (5) 1-bromononane, (6) 1-bromodecane, (7) 1-bromoundecane, and (8) 1-bromodecane. Temperature program for (a–e): 40 °C for 1 min to 160 °C at 10 °C min−1 and flow rate at 0.6 mL min−1
Separation of the Mixture of 28 Analytes with Varying Polarity
Afterwards, the selectivity and retention behaviors of the C4A-CL stationary phase were investigated by utilizing a mixture consisting of 28 analytes with varying polarity. Meanwhile, the DB-17 column was utilized as the reference for the evaluation. Figure 4 shows the separations of the mixture of 28 analytes with varying polarity including n-alkanes, esters, ketones, alcohols, and bromoalkanes on the C4A-CL column (a) in comparison to the DB-17 column (b). Notably, the C4A-CL column achieved the baseline resolution (R > 1.5) for all the analytes with good peak shapes within 13 min. Regarding retention behaviors, the C4A-CL column showed reversal elution sequences for some analytes in contrast to the DB-17 column, revealing its different retention behaviors that governs its separation performance. Although the C4A-CL and DB-17 columns have close polarity, the elution order of most analytes in the composite in the two columns is significantly different. Except for alcohols, other analytes are eluted on the C4A-CL column in the order of boiling points, thereby indicating that van der Waals interactions have a dominant role between C4A-CL phase and analytes. The C4A-CL stationary phase is manifested as nonpolarity or weak polarity, which may be related to the long alkyl groups of the C4A-CL phase. In addition, the alcohol and adjacent analytes are eluted on the C4A-CL column against in the order of boiling point, such as n-decane/hexanol (peak 7/8), bromoheptane/heptanol (peak 11/12), bromooctane/octanol (peak 15/16), bromononane/nonanol (peak 18/19), bromodecane/decanol (peak 21/22), bromoundecane/undecanol (peak 24/25), and bromodecane/dodecanol (peak 27/28). Subsequent alcohol elution can be attributed to their strong H-bonding and dipole–dipole interactions with the OH groups of C4A-CL stationary phase. The results show that the C4A-CL phase exhibits the characteristics of a polar stationary phase. This finding is related to the abundant OH groups on the upper rims of the C4A-CL stationary phase. The CL chains in the stationary phase have similar structures with alcohols, and shape-fitting interactions are present between these chains. In general, C4A-CL has different retention behavior and interaction mechanisms to the analytes of varying polarities. According to the definition of the dual-nature retention behavior of GC stationary phase by Anderson et al., the amphipathic C4A-CL stationary phase has dual selectivity characteristics [21].
Separations of the mixture of 28 analytes with varying polarity on the C4A-CL column (a) in comparison to the DB-17 column (b). Peaks: (1) n-heptane, (2) valeraldehyde, (3) n-octane, (4) hexanal, (5) n-nonane, (6) heptaldehyde, (7) n-decane, (8) 1-hexanol, (9) 2-octanone, (10) methyl heptanoate, (11) 1-bromoheptane, (12) 1-heptanol, (13) 2-nonanone, (14) methyl octanoate, (15) 1-bromooctane, (16) 1-octanol, (17) 2-decanone, (18) 1-bromononane, (19) 1-nonanol, (20) methyl decanoate, (21) 1-bromodecane, (22) 1-decanol, (23) methyl undecanoate, (24) 1-bromoundecane, (25) 1-undecanol, (26) methyl laurate, (27) 1-bromodecane, and (28) 1-dodecanol. Temperature program: 40 °C (1 min) to 160 °C at 10 °C min−1 and flow rate at 0.6 mL min−1
Separation the Aliphatic and Aromatic Isomers of Varying Polarities
The above findings on its high separation performance suggested the good potential of the C4A-CL column for separations of analytes of a wide ranging polarity. In addition, we made the investigations on its capability for separations of aliphatic and aromatic isomers of varying polarities, covering alkylated benzenes and naphthalenes, halogenated benzenes, alcohols, phenols, benzaldehyde, anilines, and geometric cis-/trans-isomers. Figure 5a–j shows the separations of ten structural and positional isomers ranging from nonpolar to polar nature on the C4A-CL column. As can be seen, the C4A-CL column well resolved all the isomer mixtures in a quite short run time, less than 3 min for most of them. As shown, the C4A-CL column achieved baseline resolution for all the isomer mixtures, demonstrating its extraordinary high resolving ability for the critical analytes. Figure 5a–c illustrates the separation of the alkylated benzene and naphthalenes isomers on C4A-CL column with high resolution and good peak shapes, and the analytes eluted in order of their boiling points. Their elution sequence mainly accords with their strength of π–π interaction with the C4A-CL stationary phase. The 3D aromatic skeleton of calixarene played the crucial roles in the separation of nonpolar aromatic isomers. Figure 5c illustrates the separation of the isomer mixtures of halogenated benzenes containing dichlorobenzenes, trichlorobenzenes, and nitrochlorobenzenes on C4A-CL column. The dichlorobenzene and trichlorobenzene isomers are eluted on the C4A-CL column in the order of their boiling points. However, the nitrochlorobenzene isomers are eluted on the C4A-CL column in the order of the increasing their polarity. As a result, the separation of the aromatic compounds with weak polarity on the basis of C4A-CL column is mainly realized by π–π and dispersion interactions. Meanwhile, the separation of polar aromatic compounds is mainly controlled by H-bonding interactions. We also examined the separation capability of the C4A-CL column for isomers, such as alcohols, phenols, and benzaldehydes, which are liable to severe peak tailing and hard to resolve well. Figure 5g–j presents the separation results for the critical isomer mixtures of propanols and butanols, xylenols, carvacrol/thymol, and 2-/3-/4-methylbenzaldehydes on the C4A-CL column. The C4A-CL column exhibited high resolution for the critical isomer mixtures with good peak shapes possibly via its specific H-bonding, π–π, and dispersion interactions with the analytes. The xylenol isomers are eluted in the order of their boiling point. Meanwhile, the carvacrol/thymol and methylbenzaldehyde isomers elute in the order of the increase in their dipole moments. This result indicates that the separation of the aromatic compounds on the C4A-CL column also has dual selectivity, which is similar to the retention behavior of the aliphatic compounds mentioned above.
Separations of isomer mixtures of a propylbenzene and butylbenzene, b trimethylbenzene, c methylnaphthalene and dimethylnaphthalene, d dichlorobenzene, e trichlorobenzene, f nitrochlorobenzene, g butanol and pentanol, h xylenol, i carvacrol/thymol, and j methylbenzaldehyde on the C4A-CL column. Temperature program for all isomer mixtures: 40 °C (1 min) to 160 °C at 10 °C min−1 and flow rate at 0.6 mL min−1
Aromatic amine is a relatively important pollutant in the field of environmental analysis [22, 23]. o-Toluidine, 4-chloroaniline, and 2,6-dimethylaniline are listed in 24 types of carcinogenic aromatic amine by the European Union. The affective separation of aniline isomers is a challenge in the analysis field, and GC is a common analytic method [24, 25]. However, aniline isomers often have to be derived for effective separation, which is caused by the close physicochemical properties. Figure 6 describes the separations of the toluidine and xylidine isomers on the C4A-CL column in comparison with the DB-17 column. The C4A-CL column baseline resolves all the analytes. However, the DB-17 column completely coelutes two pairs of analytes, namely, m-toluidine/p-toluidine and 2,6-dimethylaniline/2,5-dimethylaniline. The elution order of the toluidine isomers on the C4A-CL column is ortho < para < meta, which is neither eluted in their boiling order (para < ortho) nor their polarity order (para < ortho). The high distinguishing capability and different retention behavior of the C4A-CL phase from the polysiloxane DB-17 phase can be mainly attributed to the integrated effect of π–π, H-bonding, and van der Waals interactions in combination with its unique conformation. Afterward, the resolving ability of the C4A-CL column was examined by the nine cis–trans isomer mixtures containing aliphatic and aromatic isomers, including dimethyl cyclohexanes, chloropropenes, dimethyl tetrahydrofurans, alcohols, phenols, and decahydronaphthalenes. As illustrated in Fig. 7, the C4A-CL column also achieves high resolving ability for cis–trans isomer mixtures in a short time. Overall, the above results demonstrate the high resolving capability of the C4A-CL stationary phase for diverse types of isomers with slight differences in structures and properties.
Separations of cis–trans isomer mixtures of a 1,2-dimethylcyclohexane, b 1,3-dimethylcyclohexane, c 1,4-dimethylcyclohexane, d 1,3-dichloropropene, e 1,2,3-trichloropropene, f 2,5-dimethyltetrahydrofuran, g nerol/geraniol, h nerolidol, and i decahydronaphthalene on the C4A-CL column. Temperature program for all isomer mixtures: 40 °C (1 min) to 160 °C at 10 °C min−1 and flow rate at 0.6 mL/min
Column Quality Evaluation
The minimum allowable operating temperature (MiAOT) for the C4A-CL column was determined by naphthalene over the temperature range of 45–110 °C, and the results are provided in Fig. 8a. The MiAOT was defined as the temperature where the column efficiency drops down to half of its original value at elevated temperatures [26]. As described in Fig. 8a, the column efficiency decreased gradually with the reduction of temperature and the half column efficiency occurred at about 45 °C, which is the MiAOT according to Ref. [26]. Column thermal stability of C4A-CL column was investigated by the separations of the toluidine and xylidine isomers after the column was conditioned at each temperatures (160–220 °C at the increment of 20 °C) for 7 h. Figure 8b illustrates the effect of column-conditioning temperatures on the retention times of analytes. Notably, the retention times did not show dramatic decrease (RSD < 0.4%) over the temperature range up to 220 °C. The results suggested that the C4A-CL column can be operated in the given temperature range with high separation performance. To further investigate the column quality, isothermal separations of benzenes, anilines, and phenols at different temperatures were performed, respectively (Fig. S1). It is noteworthy that anilines and phenols are tough analytes prone to peak tailing in GC separations, but the C4A-CL column achieved symmetric peaks for them with asymmetry factors (As) mostly of 0.95–1.05 (Table S2), suggesting its high-resolution performance and good column inertness. In addition, the column durability of the column in 4 months was evaluated by the separation of xylidine isomers on C4A-CL column. As shown in Table 3, the RSD % values for the analytes were in the range of 0.10–0.45% over the time period, indicating its good durability.
Applications for the Determination of Isomer Impurities in Real Samples
To testify the potential of the C4A-CL column for practical analysis, it was applied to analyze the possible minor isomer impurities in commercial reagent samples. Figure 9 shows the results for the determination of isomer impurities in the reagent samples of nerol, geraniol, cis-decahydronaphthalene, and trans-decahydronaphthalene. Table 4 lists their content results by peak area normalization method. As can be seen from Table 4, the label purity of nerol is 98%, but we measured that the content of the major component of nerol is 97% and the contents of the minor isomer impurities are 3.21% for geraniol. The measured purities of the rest real samples were in good agreement with their label purities, such as geraniol, cis-decahydronaphthalene, and trans-decahydronaphthalene. The above results demonstrated the potential of the C4A-CL column for the quick detection of minor isomers in real samples.
Conclusion
This work presents a new amphiphilic calix[4]resorcinarene material (C4A-CL) as the stationary phase for GC separations. As demonstrated, its intriguing structural characteristics endow C4A-CL with high selectivity for the polar/nonpolar aliphatic analytes. Interestingly, it shows significantly prolonged retention trend for alcohols mainly due to their strong H-bonding and dipole–dipole interactions with the OH groups of C4A-CL stationary phase. Moreover, the C4A-CL column exhibits high resolving ability for diverse types of isomers with good peak shapes. Particularly, it achieved baseline resolution of the challenging isomers of benzaldehyde, phenols, and anilines. This work demonstrates the distinguishing capability of the C4A-CL stationary phase for the aliphatic and aromatic analytes of varying polarities owing to its unique amphiphilic architecture and multiple interactions.
References
Kongor AR, Mehta VA, Modi KM, Panchal MK, Dey SA, Panchal US (2016) JainVK. Topics Curr Chem 374:28–74
Kim HJ, Lee MH, Mutihac L, Vicens J, Kim JS (2010) Chem Soc Rev 41:1173–1190
Mangia A, Pochini A, Ungaro Ŕ, Andreetti GD (1983) Anal Lett 16:1027–1036
Mňuk P, Feltl L (1995) J Chromatogr A 696:101–112
Lim HJ, Lee HS, Kim IW, Chang SH, Moon SC, Kim BE, Park JH (1998) Chromatographia 48:422–426
Gross B, Jauch J, Schurig V (1999) J Microcolumn Sep 11:313–317
Zeng ZR, Guan N, Tang XH, Lu XR (2000) Analyst 125:843–848
Delahousse G, Peulon-Agasse V, Debray JC, Vaccaro M, Cravotto G, Jabin I, Cardinael P (2013) J Chromatogr A 1318:207–216
Dash TK, Konkimalla V (2015) Mol Pharmaceut 9:2365–2379
Wu RZ, Al-Azemi TF, Bisht KS (2009) Chem Commun 14:1822–1824
Gou PF, Zhu WP, Shen ZQ (2010) J Polym Sci Part A Polym Chem 48:5643–5651
Lv Q, Zhang Q, Qi ML, Bai H, Ma Q, Meng XS, Fu RN (2015) J Chromatogr A 1404:89–94
Lv Q, Feng S, Jing LM, Zhang Q, Qi ML, Wang JL, Bai H, Fu RN (2016) J Chromatogr A 1454:114–119
Peng JL, Sun T, Wu LQ, Qi ML, Huang XB (2017) RSC Adv 7:45408–45415
Yang YH, Qi ML, Wang JL (2018) J Chromatogr A 1578:67–75
Yang XH, Li CX, Qi ML, Qu LT (2016) J Chromatogr A 1460:173–180
Xiong XH, Qi ML (2018) J Chromatogr A 1567:191–197
Sun T, Chen H, Qiao XG, Ma LF, Hu SQ, Liu XM (2018) RSC Adv 8:34102–34109
Poole CF, Poole SK (2008) J Chromatogr A 1184:254–280
Martin SD, Poole CF, Abraham MH (1998) J Chromatogr A 805:217–235
Anderson JL, Armstrong DW (2005) Anal Chem 77:6453–6462
Torbati M, Mohebbi A, Farajzadeh MA, Afshar Mogaddam MR (2018) Anal Chim Acta 1032:48–55
Haas R, Schmidt TC, Steinbach K, von Löw E (1997) Fresen J Anal Chem 359:497–501
Chen MM, Zhu GH, Wang SS, Jiang KZ, Xu JX, Liu JS, Jiang JX (2018) J Sep Sci 41:440–448
Wang SS, Cheng YY, Chen MM, Jiang KZ (2018) Eur J Mass Spectrom 24:337–343
Mayer-Helm BX, Rauter W (2005) Analyst 130:502–507
Funding
The work was supported by the National Natural Science Foundation of China (No. 21705072), Colleges and Universities in Henan Province Key Science and Research Project (No. 17A150039), and Natural Science Foundation of Liaoning Province (20180550016).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human or animal subjects.
Informed Consent
Informed consent was not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sun, T., Li, B., Li, Y. et al. Amphiphilic Star-Shaped Calix[4]resorcinarene as Stationary Phase for Capillary Gas Chromatography. Chromatographia 82, 1697–1708 (2019). https://doi.org/10.1007/s10337-019-03783-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-019-03783-0