Abstract
A liquid chromatography with electrospray ionization tandem mass spectrometry method was developed and validated for the determination of dioctyldiethylenetriamine acetate in soil and tobacco. The separation was performed on a Restek Ultra AQ C18 column (2.1 × 100 mm i.d., particle size 3 µm) at 40 °C with a gradient elution. Methanol and trifluoroacetic acid (0.1%) were used as mobile phase, and the flow rate was set at 0.3 mL min−1. A modified quick, easy, cheap, effective, rugged, and safe (QuEChERS) method was used for sample extraction and cleanup pretreatment. The recovery was tested in the real samples and calculated to be 86.3–97.4%, the relative standard deviations were 1.1–11.9%. The limits of detection and quantitation were 3.3–16.7 and 10–50 µg kg−1, respectively. The method was demonstrated to be reliable for the routine monitoring of dioctyldiethylenetriamine acetate in tobacco samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dioctyldiethylenetriamine acetate, N1-octyl-N2-(2-(octylamino)ethyl)ethane-1,2-diamine (Fig. 1), is an environmentally friendly amino acid polymer fungicide. It can combat a variety of diseases caused by fungi, bacteria and viruses. The anti-fungi mode of action includes solidifying bacteria’s protein, destroying the bacteria’s cell membrane and inhibiting bacteria’s respiration [1]. Broad-spectrum anti-fungi activities of dioctyldiethylenetriamine acetate render it widely used to control Botrytis cinerea, Fulvia fulva, Phytophthora capsici, Valsa mali, Physalospora piricola and other fungal diseases in various field crops such as tobacco, apples, rice, pepper, tomato, cotton and wheat [2, 3]. Owing to the broad application of dioctyldiethylenetriamine acetate in China, rapid and sensitive methods for its detection are required.
In 2003, a simple method for sample pretreatment called QuEChERS (quick, easy, cheap, effective, rugged and safe) based on acetonitrile extraction and partitioning, and a subsequent dispersive solid-phase extraction (DSPE) clean-up of the extracts was created [4]. In QuEChERS procedure, analytes are extracted with an aqueous miscible solvent with a high amount of salt and/or buffering agents, to induce liquid phase separation and stabilize acid and base pesticides [5]. This method covers a lot of analytes, including polar, semi-polar and non-polar pesticide residues in various food matrices [6]. QuEChERS approach has been shown in numerous laboratories to provide high-quality results, save time and labor, and lower solvent consumption [7,8,9,10,11]. Even though QuEChERS method has been employed for several kinds of chemicals and crops, it has not been reported on the extraction of dioctyldiethylenetriamine acetate residue in tobacco and soil.
Liquid chromatography coupled with tandem mass spectrometry (LC–MS/MS) detection is currently considered as the method of choice for quantitative analysis of compounds in many matrices [12]. The advantages of using LC–MS/MS are mainly higher versatility, specificity and selectivity, and enabling the detection of target analytes in the low ng/L range [13]. However, the major drawback of this technique in quantitative analysis, especially with electrospray ionization (ESI), is ion suppression/enhancement when analyzing complex matrices [14,15,16]. Therefore, a related study to evaluate matrix effects should be included in the method validation, to ensure the reliability of the data obtained.
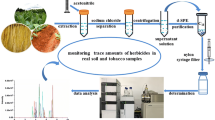
To our knowledge, there was no research on pretreatment and residue of dioctyldiethylenetriamine acetate in any crops. The aim of this work was to develop a rapid and effective liquid chromatography coupled by an ESI source to tandem mass chromatography (LC–ESI–MS/MS) method for the determination of concentration levels of dioctyldiethylenetriamine acetate in soil, green and cured tobacco leaf samples. The proposed method was successfully applied on the quantification of dioctyldiethylenetriamine acetate in actual soil, green and cured tobacco leaf samples. The results could provide some data for the pretreatment and detection method of dioctyldiethylenetriamine acetate in other crop matrix.
Experimental
Materials and Reagents
Analytical standards of dioctyldiethylenetriamine acetate (95.0% purity) and 8% aqueous solution were offered by Qinchengyixin Co., Ltd. (Beijing, China). HPLC–grade methanol and acetonitrile were purchased from Tedia Co Inc. (Phoenix, USA). Distilled water was purchased from Watson Co., Ltd. (Dongguan, China). Analytical grade magnesium sulfate (MgSO4), sodium chloride (NaCl), trifluoroacetic acid (TFA) and acetic acid were purchased from Chengdu Jinshan Chemical Reagent Co. (Chengdu, China). Syringe filter (nylon, 0.22 µm) was purchased from PeakSharp Technologies (Yibin, China). Primary secondary amine (PSA, 40–60 µm), octadecyl silane (C18, 40–60 µm) and graphitized carbon black (GCB, 60–90 µm) were purchased from Agela Technologies (Tianjin, China).
Standard Solutions
Stock standard solution of dioctyldiethylenetriamine acetate (100 µg mL−1) was prepared in methanol. The standard solutions required to obtain the analytical curves (5, 10, 50, 100, 500, 1000 and 10,000 µg L−1) were prepared from stock solution by serial dilution with methanol. Correspondingly, matrix-matched standard solutions were prepared by adding a certain volume of the standard solution to the blank extracts (soil, green and cured tobacco leaves) to each serially diluted standard solution. A matrix-matched calibration method was used to eliminate matrix effects. The concentration ranges of dioctyldiethylenetriamine acetate were 5–10,000 µg L−1 for soil, green and cured tobacco leaves. All solutions were stored in a refrigerator in the dark at − 20 °C. The stability was determined in the solvent and in the matrix. The stability of the spiked samples (1000 µg L−1) for the target compounds were evaluated monthly (stable in 1 month), and all the samples used in the stability test were stored at − 20 °C. These three matrices were obtained from the tobacco experiment base in Huishui, which were not contaminated by the target pesticide. Soil, green and tobacco leaves were put into polyethylene bags. They were transported to the laboratory and stored in the dark at − 20 °C until analysis.
Instrumentation
Chromatographic separation of dioctyldiethylenetriamine acetate was performed on a Shimadzu (Tokyo, Japan) 20AD–XR LC system, a binary solvent manager, a column oven, a solvent degasser, and an autosampler equipped with a Restek Ultra AQ C18 column (100 mm × 2.1 mm i.d., particle size 3 µm; Bellefonte, USA). The mobile phase was a mixture of methanol (A) and 0.1% TFA aqueous solution (B). The following chromatographic gradient was used: 0–3.1 min, held at 40% A + 60% B; 3.1–3.2 min, changed from 40% A + 60% B to 90% A + 10% B; 3.2–10.1 min, held at 90% A + 10% B; 10.1–13.0 min, changed from 90% A + 10% B to 40% A + 60% B; and 13.0–13.1 min, held at 40% A + 60% B. 5 µL of the sample solution was injected at 0.3 mL min−1. The column oven temperature was maintained at 40 °C to decrease viscosity, and the temperature of the sample vial holder was set at 10 °C. The retention time of dioctyldiethylenetriamine acetate was approximately 5.8 min.
A Sciex API 4000Q Trap quadrupole mass spectrometer (Foster City, CA, USA) equipped with an Ion Source Turbo Spray unit was used to quantify dioctyldiethylenetriamine acetate. Nitrogen was used as curtain, nebulizer, and collision gas. The ESI source-dependent parameter settings were as follows: curtain gas pressure, 40 psi; ion source gas 1 pressure, 60 psi; ion source gas 2 pressure, 60 psi; ion source temperature, 550 °C; interface heater, “on”; and ion spray voltage, 5500 V. Multiple reaction monitoring (MRM) in positive mode was used for the quantitative determination of dioctyldiethylenetriamine acetate. The m/z 328.5 → 156.2 was used for quantification, and m/z 328.5 → 199.2 was used for confirmation. The collision energy was set at 35.1 and 24.6 V for dioctyldiethylenetriamine acetate’s daughter ions 156.2 and 199.2, respectively.
Sample Pretreatment
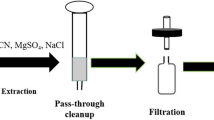
For soil sample, it was collected from 0 to 15 cm depth, air-dried and passed through a 1 mm sieve. 10 g aliquot of homogenized sample was weighed into a 50 mL polypropylene centrifuge tube with screw cap. Recovery studies for validation were carried out by spiking appropriate amounts of standards to blank samples. The tubes that contained the samples were vortexed for 1 min and allowed to stand for 2 h at room temperature to distribute the pesticide evenly. Afterwards, water (5 mL) and 20 mL of acetonitrile were added. The sample tube was vortexed for 3 min using a XW-80A vortex mixer (Kylin-Bell Lab Instruments, Haimen, China) and sonicated for 30 min using an AS3120 ultrasonic instrument (Autoscience, Tianjin, China). Subsequently, 5 g of NaCl and 5 g of MgSO4 were added. The tube was capped and immediately vortexed intensively for 1 min and then centrifuged with a TGL-10B Centrifuge (Anke, Shanghai, China) at relative centrifugal force (RCF) 4025×g for 5 min. Then, 1 mL of the upper layer was filtered through 0.22 µm nylon syringe filter and analyzed by LC–MS/MS without further clean-up.
For tobacco sample, green tobacco leaves were chopped and cured tobacco leaves were crushed after cured. An aliquot (5 g) of homogenized green tobacco leaves or (2 g) of homogenized cured tobacco leaves was weighed into a 50 mL polypropylene centrifuge tube with screw cap. Afterwards, water (5 mL) and 20 mL of methanol were added. The sample tube was vortexed for 1 min and sonicated for 20 min. 5 g of NaCl and 5 g of MgSO4 were added. The tube was capped and immediately vortexed intensively for 1 min and then centrifuged at RCF 4025×g for 5 min. Then, 1 mL of the upper layer was transferred into a single-use centrifuge tube containing 50 mg of PSA and 50 mg of C18. The tube was vortexed for 30 s and centrifuged at RCF 3220×g for 5 min. The supernatant was filtered through 0.22 µm nylon syringe filter and analyzed by LC–MS/MS.
Method Validation
The method was validated to evaluate its performance in accordance with a routine validation procedure that included the following parameters: linearity, limit of detection (LOD), limit of quantification (LOQ), matrix effects, accuracy and precision by fortifying blank fresh tobacco leaf, soil or cured tobacco leaf samples to satisfy the SANTE/11945/2015 requirements (method validation and quality control procedures for the analysis of pesticide residues in food and feed) [17]. A series of dioctyldiethylenetriamine acetate matrix-matched standard solutions (5–10,000 µg L−1 for soil, green and cured tobacco leaves) were prepared to determine the linearity of dioctyldiethylenetriamine acetate by LC–MS/MS analysis. Calibration curves were generated by plotting the peak area versus the concentration of dioctyldiethylenetriamine acetate. Linear regression analysis was performed using Microsoft Excel 2010. The matrix effect (ME) was calculated as follows: ME% = (peak area of standard in matrix − peak area of standard in solvent)/peak area of standard in solvent × 100. Positive values of the MR demonstrate enhancement, whereas negative values indicate suppression [18]. The LODs were the concentrations that produced a signal-to-noise (peak area to peak area) ratio of 3, whereas the LOQs were defined as the lowest spiked concentration levels that acceptable recoveries and precision have been attained [19]. Precision and accuracy of the methods were evaluated by recovery studies using fortified samples at three concentration levels (10, 100 and 1000 µg kg−1) for dioctyldiethylenetriamine acetate in soil, (20, 200 and 2000 µg kg−1) in green tobacco leaves and (50, 500 and 5000 µg kg−1) in cured tobacco leaves based on five replicates. For recovery studies, samples without residue (10 g soil, 5 g green tobacco leaves and 2 g cured tobacco leaves) were spiked prior to extraction by the addition of appropriate volumes of the dioctyldiethylenetriamine acetate standard solution in methanol. The treated samples were analyzed following the described procedure, and the recoveries were calculated. The precision in these conditions for repeatability, expressed as relative standard deviation (RSD), was determined by the intra- and inter-day assays [20].
Field Trials
To study the application of established methods, the field trials were performed in Guizou and Hunan. Each experiment plot was 15 m2 and each treatment was applied three times. A buffer area of 15 m2 was used to separate the plots with different treatments. Formulation of dioctyldiethylenetriamine acetate (aqueous solution, 8%) was applied at a low dosage of 60 g a.i ha−1 (the recommended high dosage) and at a high dosage of 90 g a.i ha−1 (1.5 times the recommended high dosage), every 7 days for a total of 3–4 times. Approximately, 2 kg of tobacco leaves and representative 2 kg of surface soil (from 0 to 15 cm depth) samples were collected randomly from several points in each plot at 7, 14, 21 days after last spraying. Tobacco leaves were cured by conventional processes and then crushed [21]. Both cured tobacco leaf and soil samples were placed into polyethylene bags and stored at − 20 °C until analysis.
Results and Discussion
Optimization of Chromatographic Conditions
The chromatographic conditions such as column, mobile phase and flow rate were optimized systemically in the preliminary test to improve the separation of the analyte in a reasonable analysis time [22]. Four different C18 columns, including waters BEH C18 column (100 mm × 2.1 mm i.d., particle size 1.7 µm; Milford, USA), Agilent ZORBAX SB–C18 column (150 mm × 4.6 mm i.d., particle size 5 µm; Santa Clara, USA), Phenomenex Luna C18 column (250 mm × 4.6 mm i.d., particle size 5 µm; Tianjin, China), and Restek Ultra AQ C18 column (100 mm × 2.1 mm i.d., particle size 3 µm) were compared. It was observed that the best separation results were obtained using the Restek Ultra AQ C18 column (100 mm × 2.1 mm i.d., particle size 3 µm) at the shortest time (Fig. S1). The column temperature was set at 40 °C. As to the mobile phase, we compared methanol/water, methanol/acetate acid, methanol/TFA and it was found that without acid, the peak of dioctyldiethylenetriamine acetate was very broad and cannot be separated thoroughly. To acquire sharp peak, TFA was adopted and the optimized concentration was 0.1% (Fig. S2). Gradient and isocratic LC modes were optimized for the separation of dioctyldiethylenetriamine acetate and the latter mode was chosen for better separation. As to the flow rate, we compared 0.2, 0.3, 0.4 mL min−1 and found that the best flow rate was 0.3 mL min−1 (Fig. S3).
Optimization of MS/MS Conditions
Precursor ion scan was used for screening and MRM acquisition mode was used for the analysis. Dioctyldiethylenetriamine acetate was infused into the mass spectrometer. The precursor ion and two product ions were preliminarily selected in both positive ion and negative ion modes. Due to the nitrogen atom in the chemical structure, which is more easy to get positively charged [23], dioctyldiethylenetriamine acetate exhibited better signal sensitivity in positive ion mode. Two abundant daughter ions were chosen as the quantitative (156.2) and qualitative (199.2) ion pairs after optimization of collision energy. Nitrogen was used as the nebulizer, heater, and curtain gas as well as the collision activated dissociation gas. The optimized MS conditions were as follows: positive ion mode; ion spray voltage, 5500 V, nebulizing gas, 5 L min−1; nitrogen drying gas, 12 L min−1; desolvation temperature, 550 °C; heater block temperature, 550 °C.
Optimization of Sample Pretreatment
The extraction procedure, as described above, was optimized by evaluating the performance of different combination of solvents. Different proportions of water/methanol and water/acetonitrile were used to extract dioctyldiethylenetriamine acetate from soil and tobacco matrix. The best compromise for the extraction of dioctyldiethylenetriamine acetate from soil was achieved using 5 mL water and 20 mL acetonitrile. For green and cured tobacco leaf samples, the best extractant was 5 mL of water and 20 mL of methanol (Fig. 2a). Based on QuEChERS methodology, MgSO4 and NaCl were selected to dewater, reduce the solubility of co-extracted interferences and enhance the separation of mixed solvents [24, 25]. The best combination was 5 g of each salt. The different extraction times of vortex/sonication were compared. Finally, 3 min vortex and 30 min sonication were selected for soil samples, 1 min vortex and 20 min sonication were selected for green and cured tobacco leaf samples (Fig. 2b) because it was the shortest time for each matrix that obtained acceptable recoveries range (90–100%) of dioctyldiethylenetriamine acetate.
To obtain satisfactory clean-up effect for QuEChERS pretreatment, three sorbents, PSA, C18 and GCB, were used in this work to investigate the influences on recovery in tobacco matrix. The results showed that, without the clean-up using PSA, C18 or GCB, the tobacco extracted colors were much deeper than that with sorbent; furthermore, GCB could greatly adsorb dioctyldiethylenetriamine acetate. However, when sorbent mixtures were used, the best results were obtained with 50 mg PSA and 50 mg C18 for dioctyldiethylenetriamine acetate in green and cured tobacco leaf matrices.
Method Validation
The typical chromatograms (Fig. 3) of standard and spiked samples proved that the established method was able to separate dioctyldiethylenetriamine acetate from the matrix interferences. As shown in Table 1, the linearity was evaluated by preparing different calibration curves (standard, soil, green and cured tobacco leaves) in the concentration range of 5–10,000 µg L−1. The regression equations and correlation coefficients (R2) of all the matrix-matched curves and standard solution curves indicated that satisfactory linearity was observed for dioctyldiethylenetriamine acetate (R2 > 0.999). The LODs and LOQs were estimated to be 3.3 and 10, 6.7 and 20, 16.7 and 50 µg kg−1 for dioctyldiethylenetriamine acetate in soil, green and cured tobacco leaves, respectively.
When ESI is used, the ionization of the tested compounds can be affected by the presence of co-extractives from samples [26]. The matrix effects might affect the response in a positive or negative way depending on the level of ion suppression or enhancement [27]. The accuracy and precision of the method could be greatly influenced by matrix effects. So the matrix effect was investigated in soil, green and cured tobacco leaves by comparing standard in the solvent with matrix-matched standards. If the ME value was < 0 or > 0, the matrix effect showed suppression or enhancement, respectively [17]. The results in Table 1 indicated signal suppression was observed in cured tobacco leaf samples as the ME value was − 24.7%, however, signal enhancement differences were found in soil and green tobacco leaf samples with the ME values of 39.2% and 34.4%, respectively. Therefore, external matrix-matched standards were used to eliminate the matrix effect and more realistic data could be obtained.
To evaluate the stability and precision of the established method, the intra- and inter-day recovery precisions are listed in Table 2. The average recovery of dioctyldiethylenetriamine acetate ranged from 86.3 to 94.6, 89.7–92.3 and 87.3–97.4% in soil, green and cured tobacco leaves, respectively. Intra-day precision (n = 5) was evaluated by analysis of samples at different concentration levels during the same day. Inter-day precision were determined by repeated analysis of samples at different concentration levels over three consecutive days [28, 29]. The RSD was calculated as a measurement of method repeatability. As shown in Table 2, the established method shows good repeatability with all the RSDs under 12%.
Application to Real Samples
To evaluate the feasibility of the established method for the analysis of real samples, it was applied to the determination of dioctyldiethylenetriamine acetate in soil and tobacco (144 samples of soil and 144 samples of cured tobacco leaf) collected from the trial fields in Guizhou and Hunan in 2014 and 2015. The residues in soil samples of the two experiment sites were all below the LOQ value (0.5 µg kg−1). And the results also showed that the residual levels of dioctyldiethylenetriamine acetate were < 50–710 µg kg−1 in cured tobacco leaves at harvest after the last spraying at low and high dosages in Guizhou and Hunan, China.
Conclusions
A simple and sensitive approach has been established for the qualitative and quantitative analysis of dioctyldiethylenetriamine acetate in soil, green and cured tobacco leaves by LC–MS/MS system. The optimized procedure includes a modified QuEChERS extraction followed by a cleanup with DSPE (PSA + C18). The LODs and LOQs were 3.3–16.7 and 10–50 µg kg−1, respectively. The recoveries were between 86.3 and 97.4% in all the three matrices. The new method was proved to be an important tool for the determination of dioctyldiethylenetriamine acetate residues in tobacco and soil samples. Considering the advantages and satisfactory performance of the proposed method, it could be applied for the routine monitoring of dioctyldiethylenetriamine acetate residues.
References
Ren FX, Cai CC (2010) Determination of 1.8% dioctyldiethylenetriamine acetate aqueous solutions by GC. Appl Chem 39:1098–1099
Li XS, Xu J, Pan CP, Shan WL, Li GP, Chen TC, Ye JM (2009) Qualitative analysis of dioctyldiethylenetriamine acetate and its primary impurities in technical concentrate and the quantitative analytical method for dioctyldiethylenetriamine acetate. Pest Sci Admin 30:8–13
Li LK, Yuan SK, Yan QP, Cui XL, Jiang H (2010) Study on the anti-microbial activity of Xinjunan to 15 plant pathogens. Pest Sci Admin 31:32–34
Anastassiades M, Lehotay SJ, Stajnbaher D, Schenck FJ (2003) Fast and easy multiresidue method employing acetonitrile extraction/partitioning, “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J AOAC Int 86:412–431
Cunha SC, Lehotay SJ, Mastovska K, Fernandes JO, Beatriz M, Oliveira PP (2007) Evaluation of the QuEChERS sample preparation approach for the analysis of pesticide residues in olives. J Sep Sci 30:620–632
Pareja L, Cesio V, Heinzen H, Fernández-Alba AR (2011) Evaluation of various QuEChERS based methods for the analysis of herbicides and other commonly used pesticides in polished rice by LC–MS/MS. Talanta 83:1613–1622
Schenck FJ, Hobbs JE (2004) Evaluation of the quick, easy, cheap, effective, rugged, and safe (QuEChERS) approach to pesticide residue analysis. Bull Environ Contam Toxicol 73:24–30
Li XX, Jiang YP, Shan WL, Pan CP (2010) Dissipation and residues detection of dioctyldiethylenetriamine acetate in rice plant and environment by QuEChERS method and liquid chromatography/electrospray tandem mass spectrometry. Bull Environ Contam Toxicol 84:596–601
Koesukwiwat U, Lehotay SJ, Mastovska K, Dorweiler KJ, Leepipatpiboon N (2010) Extension of the QuEChERS method for pesticide residues in cereals to flaxseeds, peanuts, and doughs. J Agric Food Chem 58:5950–5958
Marchis D, Ferro GL, Brizio P, Squadrone S, Abete MC (2012) Detection of pesticides in crops: a modified QuEChERS approach. Food Control 25:270–273
Costa FP, Caldas SS, Primel EG (2014) Comparison of QuEChERS sample preparation methods for the analysis of pesticide residues in canned and fresh peach. Food Chem 165:587–593
Eeckhaut AV, Lanckmans K, Sarre S, Smolders I, Michotte Y (2009) Validation of bioanalytical LC–MS/MS assays: evaluation of matrix effects. J Chromatogr B 877:2198–2207
Gros M, Petrović M, Barceló D (2006) Development of a multi-residue analytical methodology based on liquid chromatography—tandem mass spectrometry (LC–MS/MS) for screening and trace level determination of pharmaceuticals in surface and wastewaters. Talanta 70:678–690
Stubbings G, Bigwood T (2009) The development and validation of a multiclass liquid chromatography tandem mass spectrometry (LC–MS/MS) procedure for the determination of veterinary drug residues in animal tissue using a QuEChERS (QUick, Easy, CHeap, Effective, Rugged and Safe) approach. Anal Chim Acta 637:68–78
Sack C, Smoker M, Chamkasem N, Thompson R, Satterfield G, Masse C, Mercer G, Neuhaus B, Cassias I, Chang E, Lin Y, MacMahon S, Wong J, Zhang K, Smith RE (2011) Collaborative validation of the QuEChERS procedure for the determination of pesticides in food by LC–MS/MS. J Agric Food Chem 59:6383–6411
Gilart N, Marcé RM, Borrull F, Fontanals N (2012) Determination of pharmaceuticals in wastewaters using solid-phase extraction-liquid chromatography-tandem mass spectrometry. J Sep Sci 35:875–882
National Food Administration (2016) Method validation and quality control procedures for pesticide residues analysis in food and feed (SANTE/11945/2015), Uppsala. http://www.eurl-pesticides.eu/library/docs/allcrl/AqcGuidance_SANTE_2015_11945.pdf. Accessed 3 Mar 2018
Rahman MM, Farha W, Abd El-Aty AM, Kabir MH, Im SJ, Jung DI, Choi JH, Kim SW, Son YW, Kwon Ch, Shin HC, Shim JH (2015) Dynamic behavior and residual pattern of thiamethoxam and its metabolite clothianidin in Swiss chard using liquid chromatography–tandem mass spectrometry. Food Chem 174:248–255
Ramasubramanian T, Paramasivam M, Jayanthi R (2012) Rapid and sensitive analytical method for simultaneous determination of imidacloprid and thiamethoxam residues in soils of sugarcane ecosystem by reversed-phase HPLC. Water Air Soil Pollut 223:6045–6050
Nie YH, Zhang KK, Chen HY, Zhang W, Zhu HJ, Hu DY (2015) Dissipation and residue fate of kresoxim-methyl in tobacco leaves and soil under field conditions. Int J Environ Anal Chem 95:1338–1352
Liu JS, Liu JL (2010) Practical guide to leaf tobacco production techniques in China. China National Leaf Tobacco Corporation, Beijing
Cabrera LC, Caldas SS, Prestes OD, Primel EG, Zenella R (2016) Evaluation of alternative sorbents for dispersive solid-phase extraction clean-up in the QuEChERS method for the determination of pesticide residues in rice by liquid chromatography with tandem mass spectrometry. J Sep Sci 39:1945–1954
Xiang QY, Hashi Y, Chen ZL (2016) Simultaneous detection of eight active components in Radix Tinosporae by ultra high performance liquid chromatography coupled with electrospray tandem mass spectrometry. J Sep Sci 39:2036–2042
Xu JJ, Zhou J, Huang BF, Cai ZX, Xu XM, Ren YP (2016) Simultaneous and rapid determination of deoxynivalenol and its acetylate derivatives in wheat flour and rice by ultra high performance liquid chromatography with photo diode array detection. J Sep Sci 39:2028–2035
Yang ZZ, Wang L, Xu MC, Gu JK, Yu LS, Zeng S (2016) Simultaneous analysis of gemfibrozil, morphine, and its two active metabolites in different mouse brain structures using solid-phase extraction with ultra-high performance liquid chromatography and tandem mass spectrometry with a deuterated internal standard. J Sep Sci 39:2087–2096
Li MM, Liu XG, Dong FS, Xu J, Kong ZQ, Li YB, Zheng YQ (2013) Simultaneous determination of cyflumetofen and its main metabolite residues in samples of plant and animal origin using multi-walled carbon nanotubes in dispersive solid-phase extraction and ultrahigh performance liquid chromatography-tandem mass spectrometry. J Chromatogr A 1300:95–103
Zhang ZY, Jiang W, Jian Q, Song WC, Zheng ZT, Wang DL, Liu XJ (2015) Residues and dissipation kinetics of triazole fungicides difenoconazole and propiconazole in wheat and soil in Chinese field. Food Chem 168:396–403
Feinberg M, Boulanger B, Dewé W, Hubert P (2004) New advances in method validation and measurement uncertainty aimed at improving the quality of chemical data. Anal Bioanal Chem 380:502–514
Taverniers I, De Loose M, Bockstaele EV (2004) Trends in quality in the analytical laboratory. II. Analytical method validation and quality assurance. Trends Anal Chem 23:535–552
Funding
This study was funded by the National Key Research and Development Program of China (Grant number 2016YFD0200203-3).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Jin Gong declares that he has no conflicts of interest. Guoqiang Yang declares that he has no conflicts of interest. Weiwei Yu declares that she has no conflicts of interest. Min Huang declares that she has no conflicts of interest. Fuqin Li declares that she has no conflicts of interest. Mingjiao Jin declares that she has no conflicts of interest. Deyu Hu declares that she has no conflicts of interest. Kankan Zhang declares that he has no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gong, J., Yang, G., Yu, W. et al. The Development and Validation of a Liquid Chromatography–Tandem Mass Spectrometry Procedure for the Determination of Dioctyldiethylenetriamine Acetate Residues in Soil, Green and Cured Tobacco Leaves Using a Modified QuEChERS Approach. Chromatographia 81, 1035–1041 (2018). https://doi.org/10.1007/s10337-018-3535-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-018-3535-z