Abstract
Home-made micro-solid-phase extraction (SPE) cartridges using different adsorbent materials were tested for the desorption electrospray ionization–high-resolution mass spectrometry (DESI-HRMS) determination of explosives like 2,4,6-trinitrotoluene, cyclotrimethylene-trinitramine, cyclotetramethylene-tetranitramine, pentaerythritol tetranitrate, and trinitrophenylmethylnitramine in soil samples. Quantitation limits in the low nanogram per kilogram range proved the reliability of the method for the detection of explosives at ultra-trace levels. The reduced sample preparation allowed for low costs and high-throughput analyses. Finally, the superior extraction capability of the method was proved by obtaining DESI-HRMS responses at least five times higher than those achieved by performing DESI-HRMS analyses of solid–liquid extracts spotted onto commercial polytetrafluoroethylene slides.

DESI-HRMS analysis of explosives from soil samples
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The rapid detection of ultra-trace explosives is of paramount importance in the field of forensic sciences. Different methods capable of direct sampling have been developed: although ion mobility spectrometry [1, 2] is one of the most commonly used techniques for explosive analysis, recently, ambient ionization approaches like direct analysis in real-time mass spectrometry [3–5] and desorption electrospray ionization–mass spectrometry (DESI-MS) [6–9] have been successfully applied with advantages in terms of reduced or absent sample preparation. The potential for field detection of explosives has been also proved by implementing DESI sources on fieldable mass spectrometers [10, 11]. In order to enhance selectivity and specificity of DESI-MS, both tandem mass spectrometry and reactive DESI experiments can be performed. In particular, reactive DESI has been proved to be useful in the detection of cyclotrimethylene-trinitramine (RDX) and cyclotetramethylene-tetranitramine (HMX) using chloride-doped spray [12], whereas hexamethylene triperoxide diamine and triacetone triperoxide have been detected using complexation reactions with alkali metals [6]. However, when complex matrices like soil need to be analyzed, both sensitivity and possible contamination of the ion source due to the blowing up of dust particles still remain unsolved problems. Sensitivity of DESI-MS strictly depends also on both the compounds and the surface to be analyzed: typically, detection limits in the picogram range can be obtained, but femtogram levels of 2,4,6-trinitrotoluene (TNT) have been detected on nonporous surfaces [12]. However, it has also to be noticed that when complex matrices have to be analyzed, usually analytes are not homogeneously distributed on the surface. Although, recently, an innovative method based on a combination of thermal desorption with a direct current atmospheric pressure glow discharge source has been proposed to directly analyze semi-volatile explosives TNT and RDX at trace levels [13], solvent extraction followed by pre-concentration before instrumental analysis is one of the most common approaches for explosive enrichment. Solid-phase extraction (SPE) is widely accepted as a valid alternative to time-consuming and laborious liquid–liquid extraction. Different materials can be used as adsorbents in SPE process: from octadecyl silica to various polymeric sorbents [14], and from molecularly imprinted polymers [15, 16] to graphene [17]. Micro-sample preparation techniques are particularly appealing for forensic applications: miniaturized solid-phase extraction (micro-SPE) cartridges containing graphitized carbon black have been used for the surface-assisted laser desorption ionization time-of-flight mass spectrometry screening of both sulfonamides, human medicines, and organophosphate esters from water and urine samples [18] and pesticides from water [19]. Finally, a SPE-DESI-MS approach using a styrene-divinylbenzene reversed-phase sulfonated (SDB-RPS) membrane has been evaluated by Mulligan and coworkers for the determination of RDX in water samples [20]. Quantitation is another important matter for DESI-MS analysis: signal instability is one of the most common phenomena preventing reliability of data. The use of rough surfaces is another important parameter to avoid washing effects able to remove samples from DESI supports. Additional difficulties in the achievement of repeatable signals can be observed when mass spectrometers are not equipped with automated sampling devices. To solve all these problems, the use of an appropriate internal standard is required.
The aim of this study was the development of a micro-SPE-DESI-MS method for the quantitation of explosives like TNT, RDX, trinitrophenylmethyl-nitramine (Tetryl), and pentaerythritol tetranitrate (PETN) from soil samples collected at a bombing scene.
Home-made micro-extraction cartridges were used for explosive enrichment, disassembled and used as DESI surfaces. The performance of different surfaces like Empore™ C18 Disk, Empore™ SDB-RPS Disk, Empore™ SDB-XC Disk, and graphitized carbon black (GCB) was compared. Finally, reliability of the developed method was assessed by developing and validating a micro-SPE-DESI-MS method for the quantification of the investigated compounds at trace and ultra-trace levels.
Methods and materials
Chemicals
Acetonitrile, acetone, methanol, and hydrochloric acid (37 % v/v) were from Sigma-Aldrich (Milan, Italy). HMX, RDX, PETN, Tetryl, TNT, and 2,4,6-trinitrophenol (TNP, used as internal standard (IS)) were supplied by Reparto Carabinieri Investigazioni Scientifiche of Rome.
Stock solutions of explosives (1000 mg/L) were prepared using acetonitrile as solvent and stored in the dark at 4 °C. Working solutions were prepared daily by dilution from the stock solutions in water. TNP was used as an internal standard at 10 μg/kg.
Empore™ C18, Empore™ SDB-RPS, and Empore™ SDB-XC 47-mm disks were purchased from IVA Analysentechnik e. K. (Meerbusch, Germany). GCB membranes were from Laboratori Analitici di Ricerca Associati (Formello, Rome, Italy).
Micro-SPE
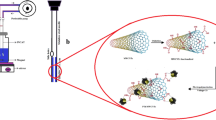
The home-made micro-SPE traps were obtained by the manual insertion of pre-cut 2.5-mm disks of Empore™ C18, Empore™ SDB-RPS, Empore™ SDB-XC, and graphitized carbon black membranes in 100-μL tips (Fig. 1). The micro-SPE cartridges were conditioned with 100 μL of methanol and then loaded with 1.5 mL of sample extract. Finally, the micro-SPE devices were disassembled, anchored on a glass slide, and 1 μL of a CH3OH:H2O (50:50) solution containing 0.05 % HCl was added on the surface. Samples were then submitted to DESI-HRMS analysis.
Confirmatory analyses were carried out by performing additional GC-MS analyses. TNT and Tetryl were used as model compounds. Briefly, the micro-traps were loaded with 1.5 mL of aqueous solutions containing known amounts of TNT and Tetryl. Then, the micro-SPE cartridges were eluted with 500 μL of different solvents (methanol, acetonitrile, water:methanol (1:1, v/v), water:acetonitrile (1:1, v/v), methanol:acetonitrile (1:1, v/v), and methanol:acetonitrile:water (1:1:1, v/v), and 1 μL of the extract was submitted to GC-MS analysis).
DESI-HRMS analysis
All the analyses were carried out using a LTQ Orbitrap XL hybrid FTMS instrument (ThermoFisher Scientific Inc., San Jose, CA, USA), equipped with an Omni Spray™ ion source (Prosolia Inc.) and operating in the negative ion mode.
The experimental conditions were as follows: solvent flow, 2 μL/min; spray voltage, 3 kV; tube lens voltage, −65 V; capillary voltage, −15 V; capillary temperature, 150 °C; nitrogen pressure, 8.5 bar; incident angle, 37°; collection angle, 53°; tip-to-surface distance (d 1) 1.6 mm; sniffer-to-surface distance (d 2) 0.5 mm; and tip-to-sniffer distance (d 3) 3 mm. Different spraying solvents were used: methanol, acetonitrile, water:methanol (1:1, v/v), water:acetonitrile (1:1, v/v), methanol:acetonitrile (1:1, v/v), and methanol:acetonitrile:water (1:1:1, v/v). The sample plate was positioned on a moveable 1-D stage.
Preliminarily, full-scan accurate mass spectra in the 150–750-amu range were acquired to determine appropriate masses for each analyte. Identification and quantitation of target compounds was performed using the accurate mass of the analytes within a mass window of 5 ppm. Quantitation was performed by using the extracted ion chromatograms of the m/z value corresponding both to the chloride adducts for HMX, RDX, PETN, and Tetryl and to the deprotonated molecule for TNT and TNP, respectively. Signal acquisition and data processing were performed using the Xcalibur 2.0 software (Thermo Finningan).
GC-MS analysis
An HP 6890 Series Plus gas chromatograph (Agilent Technologies, Milan, Italy) equipped with a MSD 5973 mass spectrometer (Agilent Technologies) was used for GC-MS analysis. Helium was used as the carrier gas at a constant flow rate of 1 mL/min; the gas chromatograph was operated in splitless mode with a Programmable Temperature Vaporization (PTV) injector (Agilent Technologies) maintained at the temperature of 200 °C and equipped with a PTV multi-baffled liner (I.D. 1.5 mm, Agilent Technologies). Chromatographic separation was performed on a 30 m × 0.25 mm, df 0.25 μm HP-5MS capillary column (Agilent Technologies). The following GC oven temperature program was applied: 50 °C, 10 °C/min to 200 °C, 200 °C for 5 min, and 10 °C/min to 250 °C. Transfer line and source were maintained at the temperature of 280 and 150 °C, respectively. Preliminarily, full-scan electron ionization (EI) data were acquired to determine appropriate masses for operating in selected-ion monitoring (SIM) mode under the following conditions: ionization energy, 70 eV; mass range, 35–300 amu; and scan time, 3 scan/s. The mass spectrometer was finally operated in time-scheduled SIM mode by applying a delay time of 6 min and by recording the current of the following ions: m/z 63, 89, and 210 for TNT and m/z 194, 225, and 242 for Tetryl.
For all the investigated analytes, the corresponding ion ratios were used for confirmation purposes. A dwell time of 30 ms was used for all the ions. All the analyses were performed by setting the electron multiplier voltage at 2000 V.
Signal acquisition and processing were performed using the HP Chemstation (Agilent Technologies).
Method validation
Method validation was performed according to EURACHEM guidelines [21] following the same procedure reported in previous studies [22, 23]. Not contaminated soil samples were used as blank matrix.
Real sample analysis
Two soil samples taken from post-blast scenarios (about 1 m from the explosion) were provided by Reparto Carabinieri Investigazioni Scientifiche of Rome. The same procedure used in a previous study with some modification was used for the extraction of explosives from samples [24]. After a preliminary drying step, 0.5 g of sample was added in 4-mL vial containing 2 mL of a mixture water:acetone (8:2) and submitted to sonication at room temperature for 1 h. After centrifugation, the supernatant was filtered using a 0.45-μm teflon membrane and loaded onto the micro-SPE traps. After the addition of the IS, 1.5 mL of the extract was submitted to micro-SPE-DESI-HRMS analysis.
Results and discussion
With the aim of investigating the capabilities of micro-SPE for the DESI-HRMS determination of some explosives in soil samples, preliminary experiments were carried out in order to evaluate the best instrumental conditions for explosive detection. As already described in many studies [12, 13], different reagents can be used to promote complexation with the analytes, thus allowing to increase both sensitivity and selectivity. In this study, the addition of both NaCl and HCl on the micro-SPE supports prior to DESI-HRMS analysis proved to be particularly useful for the detection of RDX, HMX, PETN, and Tetryl by generating chloride adducts. The most significant adduct ions in the negative ion DESI spectra corresponded to [RDX + 35Cl]−, [2RDX + 35Cl]−, [HMX + 35Cl]−, [PETN + 35Cl]−, [Tetryl + 35Cl]−, and to the deprotonated ions [TNT-H]− and [TNP-H]−. Table 1 lists the chemical structures, the m/z values, and the mass accuracy of the investigated analytes. Another important parameter affecting sensitivity was related to the geometrical configuration of the DESI source. Our findings proved that by setting an incident angle (α) of 37° and a tip-to-surface distance (d 1) of 1.6 mm, a further improvement in sensitivity could be achieved (Fig. 2). Obviously, HRMS proved to be of pivotal importance for the univocal identification of the analytes: in fact, taking into account the absence of a chromatographic separation, only the specificity of accurate mass and the full-spectrum acquisition in HRMS could allow the correct identification of the investigated explosives. Additional experiments were also carried out in order to evaluate the proper amount of soil to be used for DESI-HRMS experiments. A rough estimation of robustness, recovery, detection, and quantitation limits using different soil samples representative of various environments was carried out, thus suggesting that 0.5 g of soil was sufficient to achieve reliable quantitation limits. Finally, as for recovery, no significant differences were found when explosives were added to different blank soil samples. This behavior could be explained taking into account the good solubility of the analytes in the acetone–water mixture used for the solid–liquid extraction due to the presence of the nitro-groups.
Micro-SPE cartridges
To evaluate the capability of micro-SPE-DESI-HRMS for explosive analysis in soil samples, three different types of Empore™ SPE membranes (C18, SDB-XC, and SDB-RPS) as well as a GCB membrane were used for analyte enrichment. The micro-traps were prepared by the insertion of a small circle (2.5 mm in diameter) of each membrane inside the tip of a micropipette using a polyethylene disk as support. A simple system easy to be assembled and disassembled was realized: the elution of the analytes from the micro-SPE traps can be avoided, and after disassembling, the small membranes can be placed onto glass slides for direct DESI-MS analysis. If regularly aligned, as in the case of commercial PTFE slides, the devices are suitable for high-throughput analyses, thus being useful for both screening and quantitative purposes. In fact, since chromatographic separation of the investigated analytes is not required, a noticeable reduction of the analysis times can be achieved.
Preliminary experiments were carried out in order to select the best spray for DESI-HRMS analyses. Taking into account the high variability of the responses due to the DESI process, the optimization step was performed using an internal standard (TNP), thus allowing to obtain a better repeatability. Different spray compositions were tested, as described in the DESI-HRMS analysis section, showing different results in connection with the utilized substrates. The best results were achieved by using the C18 micro-traps with the MeOH:ACN:H2O (1:1:1) spray composition (Fig. 3). The observed behavior could be explained taking into account the nature of the interactions among the sorbents and the analytes: if on the one hand C18 membranes are recommended for nonpolar interactions to extract semi-volatile and non-volatile compounds, stronger interactions (π–π and dipole–dipole) could be established by using the SDB-XC and SDB-RPS sorbents which are characterized by a porous styrene-divinylbenzene copolymer. Probably, under these conditions, the power of the DESI spray was not sufficient to obtain the complete desorption of the analytes from the membrane. As for GCB, no DESI-MS signals were observed.
In order to confirm the results achieved by DESI-HRMS, additional experiments were carried out by performing GC-MS analyses on the micro-SPE extracts. In this case, TNT and Tetryl were used as model compounds. The results achieved were similar to those obtained by DESI-MS analyses, thus confirming that the best results could be obtained by using the C18 membrane and the mixture MeOH:ACN:H2O (1:1:1) as elution solvent. By contrast to DESI-HRMS, micro-SPE-GC-MS responses were obtained also by using the GCB membranes. In fact, whereas no signals were obtained by performing DESI-MS analyses, in this case, the best extraction yield was obtained by using the mixture methanol:acetonitrile (1:1) as SPE eluent (Fig. 4). On the basis of these findings, the absence of DESI-HRMS signals could be explained taking into account both the strong interactions among the analytes and the substrate and the highest thickness of the GCB membrane with respect to those of the Empore disks (1 vs 0.5 mm). Under these circumstances, the spray composition does not possess sufficient power to guarantee the desorption of the analytes from the DESI support.
A comparison with the DESI-HRMS analysis of solid–liquid extractions was also performed: a blank soil sample was spiked with 10 ng of each explosive and submitted both to the micro-SPE-DESI-HRMS analysis and to a solid–liquid extraction procedure. In the latter case, 1 μL of the extract was then spotted onto the surface of PTFE slides for the subsequent DESI-HRMS analysis. The achieved results showed that by using the micro-SPE procedure, DESI-HRMS responses at least five times higher than those achieved by spotting the PTFE slides could be obtained. This behavior can be explained taking into account the highest enrichment factor achieved on the small surface of the micro-membranes. On the basis of these findings, validation of the C18 micro-SPE-DESI-HRMS method was carried out.
Method validation
The micro-SPE-DESI-HRMS method was validated and applied for the quantification of the investigated analytes in soil samples. Excellent results were obtained with LOD and LOQ values in the low nanogram per kilogram range (Table 2), thus proving the potential of the method for the determination of these compounds at ultra-trace levels. Good linearity was proved in the 0.06–100-μg/kg range for RDX, 0.1–100 μg/kg for HMX, 0.6–100 μg/kg for PETN, and Tetryl and 0.6–100 μg/kg for TNT by applying Mandel’s fitting test. Method precision was assessed by testing two amount levels, i.e., 2 and 60 μg/kg for each analyte. Good results were obtained both in terms of intra-day repeatability and intermediate precision: relative standard deviation (RSD) values lower than 5 % at the highest concentration and lower than 6 % at the lowest one were calculated for intra-day repeatability, whereas intermediate precision was evaluated verifying homoscedasticity and performing ANOVA on the data acquired over 3 days. ANOVA showed that mean values were not significantly different among the 3 days, obtaining p values >0.05. RSD lower than 17 % at both concentration levels was calculated. Extraction recoveries ranging from 96 ± 8 % to 98 ± 9 % (n = 3) were calculated at the same concentration levels used for the evaluation of precision, thus showing the good efficiency of the developed method in terms of extraction recovery as well as of precision.
Real samples
Finally, the developed method was applied for the analysis of two different soil samples taken from post-blast scenarios by Reparto Carabinieri Investigazioni Scientifiche of Rome, thus demonstrating the capability of the micro-SPE-DESI-HRMS method of detecting explosives at low microgram per kilogram levels. More precisely, RDX was the only compound detected in the first sample at the amount of 10.6 ± 1 μg/kg, whereas in the second sample, TNT and RDX were found at 4.8 ± 0.2 and 7.2 ± 1.4 μg/kg, respectively. The achieved results suggested the hypothesis that both the explosive composition 4 (an explosive containing mainly RDX, dioctyl sebacate, or dioctyl adipate as plasticizers and polyisobutylene as binder) and composition B (containing RDX, TNT, and paraffin wax) were used for the criminal attack.
Conclusions
In the present work, home-made micro-SPE cartridges were developed and successfully used for the DESI-HRMS analysis of explosives in soil samples taken from post-blast scenarios. The developed method allowed for rapid analyses with minimal sample preparation. Quantitation of the analytes by using the internal standard method was also proved, thus confirming suitability of DESI-MS not only for screening approaches, but also for high-throughput quantitative purposes, thus being really advantageous also when large areas need to be investigated.
References
Lee J, Park S, Cho SG, Goh EM, Lee S, Koh SS, Kim J (2014) Talanta 120:64–70
Mattarozzi M, Bianchi F, Bisceglie F, Careri M, Mangia A, Mori G, Gregori A (2011) Anal Bioanal Chem 399:2741–2746
Clemons K, Dake J, Sisco E, Verbeck GF IV (2013) Forensic Sci Int 231:98–101
Rowell F, Seviour J, Lim AY, Elumbaring-Salazar CG, Loke J, Ma L (2012) J Forensic Sci International 221:84–91
Sisco E, Dalke J, Bridge C (2013) Forensic Sci Int 232:160–168
Cotte-Rodriguez I, Hernandez-Soto H, Chen H, Cooks RG (2008) Anal Chem 80:1512–1519
Zhao M, Zhang S, Yang C, Xu Y, Wen Y, Sun L, Zhang X (2008) J Forensic Sci 53:807–811
Morelato M, Beavis A, Ogle A, Doble P, Kirkbride P, Roux C (2012) Forensic Sci Int 217:101–106
Morelato M, Beavis A, Kirkbride P, Roux C (2013) Forensic Sci Int 226:10–21
Wells JM, Roth MJ, Keil AD, Grossenbacher JW, Justes DR, Patterson GE, Barket DJJ Jr (2008) Am Soc Mass Spectrom 19:1419–1424
Sanders NL, Kothari S, Huang G, Salazar G, Cooks RG (2010) Anal Chem 82:5313–5316
Cotte-Rodriguez I, Takats Z, Talaty N, Chen H, Cooks RG (2005) Anal Chem 77:6755–6764
Ma L, Xin B, Chen Y (2012) Analyst 137:1730–1736
Tachon R, Pichon V, Le Borgne MB, Minet JJ (2008) J Chromatogr A 1185:1–8
Tang K, Gu X, Luo Q, Chen S, Wu L, Xiong J (2014) Food Chem 150:106–112
Kubo T, Kuroda K, Tominaga Y, Lordel S, Chapuis-Hugon F, Eudes V, Pichon V (2011) Anal Bioanal Chem 399:449–458
Qian Liu Q, Shi J, Zeng L, Wang T, Cai Y, Jiang G (2011) J Chromatogr A 1218:197–204
Shariatgorji M, Amini N, Thorsen G, Crescenzi C, Ilag LL (2008) Anal Chem 80:5515–5523
Amini N, Shariatgorji M, Crescenzi C, Thorsen G (2010) Anal Chem 82:290–296
Mulligan CC, MacMillan DK, Noll RJ, Cooks RG (2007) RG Rapid Commun Mass Spectrom 21:3729–3736
EURACHEM Guide (1998) The fitness for purpose of analytical methods: a laboratory guide to method validation and related topics, 1st English ed., LGC (Teddington) Ltd., http://www.eurachem.ul.pt/
Bianchi F, Basini G, Grolli S, Conti V, Bianchi F, Grasselli F, Careri M (2013) Ramoni R Anal Bioanal Chem 405:1067–1075
Bianchi F, Giannetto M, Mori G, D’Agostino G, Careri M, Mangia A (2012) Anal Bioanal Chem 403:2411–2418
Holmgren E, Ek S (2012) Colmsjo A J Chromatogr A 1222:109–115
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in the topical collection celebrating ABCs 13th Anniversary.
Rights and permissions
About this article
Cite this article
Bianchi, F., Gregori, A., Braun, G. et al. Micro-solid-phase extraction coupled to desorption electrospray ionization–high-resolution mass spectrometry for the analysis of explosives in soil. Anal Bioanal Chem 407, 931–938 (2015). https://doi.org/10.1007/s00216-014-8208-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-014-8208-7