Abstract
Phytopathogenic fungi, causal agents of some of the world’s most serious plant diseases, can significantly reduce yields during large-scale agricultural production. Among the numerous hydrolytic enzymes they produce for nutritional and/or pathogenicity purposes, hydrolases and proteases are required for their growth and survival. The present review focuses on extracellular and/or secretory proteases from phytopathogenic fungi. Several extracellular proteases have been identified that contribute to fungal growth, infection structure formation, cell wall degradation, proteolytic processing of pathogenesis-related proteins and that act as elicitors of defense responses. In this review, the positive correlation between protease secretion and disease aggressiveness and/or necrosis is highlighted. The involvement of various fungal proteases in pathogenic mechanisms makes them potential targets for designing protease inhibitors that may provide an improved way to combat plant diseases, which in turn will reduce dependence on fungicides.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phytopathogenic fungi are a serious threat to plant health, causing a plethora of diseases that contribute substantially to overall losses in agricultural yield. In addition, ~10 % of all known fungal species can cause plant disease, affecting more than 10,000 plant species (Horbach et al. 2011; Kubicek et al. 2014). Cell-wall-degrading enzymes are important for determining the ability of a pathogen to colonize a host plant (Annis and Goodwin 1997; Juge 2006; Lebeda et al. 2001; Nakajima and Akutsu 2014). There is also considerable evidence implicating pectinases (Annis and Goodwin 1997; Nakajima and Akutsu 2014) and to a lesser extent cellulases (Doi and Kosugi 2004) and xylanases as virulence factors (Annis and Goodwin 1997; Nakajima and Akutsu 2014). Initially, when pathogenic fungi encounter a host, they produce various cell-wall-degrading enzymes such as glycanases and proteases to fragment the plant cell wall polymers, thus facilitating penetration into the host cells (Chu et al. 2015; Doi and Kosugi 2004; Kubicek et al. 2014; Jashni et al. 2015a). Apart from their role in cell wall degradation, proteases synthesized by plant pathogenic fungi have also been proposed as possible virulence factors in plant–pathogen interactions (Jashni et al. 2015a; Slavokhotova et al. 2014) (Table 1). Moreover, plant cell walls possess several proteins, glycoproteins, and structural proteins. Therefore, proteolytic enzymes play an important role in pathogenesis (Jashni et al. 2015b; Saitoh et al. 2009). However, most saprophytic fungi produce proteolytic enzymes for their nutritional requirements. Thus, the type and level of protease production determine the saprophytic or pathogenic characteristics of fungi. The role of these enzymes in the process of pathogenesis has been the subject of intensive research. This review aims to discuss the various features of proteases secreted by phytopathogenic fungi and their role in virulence.
Classifications of proteases
Proteases are one of the most important industrial enzymes, accounting for nearly 65 % of the total worldwide enzyme sales (Kasana et al. 2011; Souza et al. 2015). The protease (E.C. 3.4) group of enzymes hydrolyzes peptide bonds [Nomenclature Committee of the International Union of Biochemistry and Molecular Biology (NC-IUBMB)]. Proteases are classified based on their hydrolytic process (endopeptidases and exopeptidases), pH (acidic, alkaline and neutral proteases), or functional group at the active site (aspartic, cysteine, glutamic, metallo, serine, threonine, and unknown (NC-IUBMB). According to the MEROPS peptide database, proteolytic enzymes are grouped into 12 clans and 35 families based on amino acid sequence similarity and catalytic mechanism (Rawlings et al. 2012). Most of the fungal proteolytic enzymes are represented largely by serine proteases, named for the nucleophilic Ser residue at the active site (Asp-His-Ser). Based on their substrate specificity, they were divided into two major families of serine-like proteases such as trypsin-like (TLPs) and subtilisin-like proteases (SLPs) (Rawlings et al. 2012). The specificity of TLPs is restricted to the C-terminal side positively charged residues (Lys and Arg) because of the Asp 189 residue located in the binding pocket. SLPs cleave peptide bonds located on the C-terminal side of large hydrophobic residues (Phe, Trp, Met, Tyr and Leu) as a result of the hydrophobic makeup of the pocket (Rawlings et al. 2012). Moreover, enzyme activity has been determined using synthetic substrates. It is evident that Phytophthora infestans exoproteinases most effectively hydrolyze N-α-benzoyl-l-Arg-pNa (BAPNA) (a substrate for TLPs) and to a lesser extent N-carbobenzyloxy-l-Ala-l-Ala-l-Leu-pNa (Z-AALPNA) (a substrate for SLPs). At the same time, exoproteinases did not act on the substrates for chymotrypsin-and elastase-like proteinases (N-succinyl-glycyl–glycyl-l-phenylalanine p-nitroanilide [Suc-GGFPNA] and N-acetyl-l-alanyl-l-alanyl-l-alanyl p-nitroanilide [Ac-AAAPNA], respectively), nor did aminopeptidases [l-leucine p-nitroanilide (LPNA)]. The enzymes secreted by Fusarium culmorum hydrolyze Z-AALPNA very efficiently and to a lesser extent BAPNA. They have low activity toward substrates for chymotrypsin-like and elastase-like proteinases and for aminopeptidases (Valueva et al. 2011, 2015). Thus, the proteases from phytopathogenic fungi have unique properties and deserve further study.
Protease production and phytopathogenicity
Phytopathogenic fungal proteases can be produced in various conditions and ways. Depending on culture conditions, different forms of the same protease can be expressed (Inácio et al. 2015). Many species of phytopathogenic fungi produce different proteases in a variety of culture media and host tissues (Chandrasekaran et al. 2014; He et al. 2015; Valueva et al. 2015). Casein is a traditional substrate used to determine proteolytic activity, and hemoglobin, rennin, azo-casein, serum albumin, gelatin, yeast and other proteinaceous substrates have been used to a lesser degree (Chandrasekaran et al. 2014; Valueva et al. 2011, 2015). According to Zaferanloo et al. (2014), the optimum condition for protease production of Alternaria alternata is 37 °C, pH 7.0 using soybean as substrate. The optimum condition for protease production in Alternaria solani is 27 °C, pH 8.5 with casein as the substrate (Chandrasekaran and Sathiyabama 2014; Chandrasekaran et al. 2014, 2016). The addition of heat-stable potato tuber proteins to the culture medium is vital for secretion of exoprotease from Thanatephorus cucumeris and F. culmorum (Valueva et al. 2011). Recently, Mandujano-González et al. (2013) identified the 41-kDa extracellular aspartyl protease Eap1 from Sphacelotheca reiliana in both solid and liquid culture media. Similarly, in Ustilago maydis, non-aspartyl acid extracellular protease pumAe was found under acidic conditions in the culture medium (Mercado-Flores et al. 2003). According to Feldman et al. (2014), the oomycete P. infestans secretes serine and metalloproteases into culture media; metalloproteases are more abundant. These studies suggest that different nutritional sources are essential for the differential production of various proteases.
Fungal proteases are thought to be important during different aspects of the infection process, including adhesion to host cells, initial penetration of the plant cell wall and colonization (Brown et al. 2001; Movahedi and Heale 1990; Olivieri et al. 2004; Soberanes-Gutiérrez et al. 2015). A correlation between proteolytic activity in the culture medium and in the infected plant has been shown for several phytopathogenic fungi to establish disease (Bindschedler et al. 2003; Pekkarinen et al. 2002). According to Movahedi and Heale (1990), 38–39 kDa aspartyl acid proteases were detected in the culture medium of Botrytis cinerea and in carrot tissue infected by the fungus, but not in uninfected carrot tissue. Further, cell death investigations by Movahedi and Heale (1990) for B. cinerea suggested that aspartic protease might be the most important factor contributing to the initial stage of infection. Significant extracellular proteolytic activity in Spunta potato tubers during infection by Fusarium solani (Olivieri et al. 2004) confirms proteases as a key regulator of infection and immunity-priming factor in plant hosts. An investigation of the genetic basis of proteolytic activity in Sclerotinia sclerotiorum showed the expression of genes acp1 and asps responsible for the production of acid protease and aspartyl protease, respectively (Poussereau et al. 2001). In a similar study, Rolland et al. (2009) identified BcACP1, a member of the G1 family of protease, produced by B. cinerea during infection. An array of proteases such as aspartic proteases, cysteine proteases, and serine proteases were produced by Glomerella acutata (a causal agent of soft rot in fruits) (Gregori et al. 2010), showing the involvement of several proteases in disease development. Recently, ten Have et al. (2010) demonstrated that aspartic proteases are the key factors of pathogenicity in B. cinerea. It is clear from recent studies of several phytopathogenic fungi that the importance of proteases in the disease process may depend upon the specific plant–pathogen interaction (Figueiredo et al. 2014; Jashni et al. 2015a; Soberanes-Gutiérrez et al. 2015; ten Have et al. 2010).
Furthermore, the necessity of extracellular proteases in phytopathogenicity was confirmed by mutational studies in several fungal species such as Pyrenopeziza brassicae, Verticillium dahliae, Nakataea oryzae, U. maydis and F. oxysporum (Ball et al. 1991; Dobinson et al. 2004; Saitoh et al. 2009; Jashni et al. 2015a; Soberanes-Gutiérrez et al. 2015). An extracellular protease was reported to be a significant pathogenicity factor of Colletotrichum coccodes, and its removal by mutagenesis converts a virulent pathogen into a nonpathogenic endophyte (Redman and Rodriguez 2002), while in Bipolaris zeicola disruption of the ALP1 gene, which encodes a TLP, had no distinct effect on virulence (Murphy and Walton 1996). Saitoh et al. (2009) confirmed that that the SPM1-encoded SLP is a major protease for autophagy that might affect endocytosis during pathogenesis of the rice blast fungus N. oryzae.
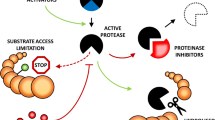
The secretion of protease inhibitors (PI) during plant–pathogen interactions implies the existence of protein targets in the fungal pathogens. The lack of protease activity in phytopathogenic fungus correlates with the lack of proteases in most fungi. Transient expression of serine protease inhibitor gene NmIMSP in susceptible tobacco leads to enhanced resistance to the oomycetes Peronospora hyoscyami f. sp. tabacina and the Phytophthora nicotianae (Silva et al. 2013). A PI mixture obtained from potato sprouts is strongly inhibitory to proteases, spore germination, hyphal elongation and necrotic activity of B. cinerea (Turra 2006). Moreover, PLPKI (potato serine protease inhibitor I family) inhibited the activity of proteases secreted by two pathogens of potato, the oomycete P. infestans and the fungus Thanatephorus cucumeris, but not of the proteases of nonpathogenic Thanatephorus N2. Furthermore, a clear correlation between PLPKI activity and the degree of horizontal resistance against P. infestans was corroborated on potato clones at varying degrees of resistance to this oomycete. The fact that plants have developed ways to sense proteases (either directly or through the products of their actions) highlights their importance in pathogenesis. In response to perception, a series of signalling events takes place (involving protease inhibitors, antimicrobial peptides, PR proteins, mitogen-activated protein kinase [MAPK] signaling, and interactions between the products of plant resistance [R] and pathogen avirulence [avr] genes), that is shared among various elicitors and eventually leads to the deployment of defence mechanisms, again implicating proteases in pathogenicity (Cheng et al. 2015; Ryan 1990; Slavokhotova et al. 2014).
Role of proteases in plant–fungi interactions
Table 1 lists phytopathogenic fungi that secrete a broad spectrum of proteolytic enzymes during penetration and colonization of host tissues. Proteolysis, a vital process for cell life, plays an important role in different physiological functions such as post-secretion protein processing, germination, sporulation, aerial mycelium and appressorium formation, nutrition, and adaptation to different environmental conditions (Chandrasekaran and Sathiyabama 2014; Tucker and Talbot 2001). In plants, fungal proteolytic mechanisms are responsible for removal of abnormal or nonfunctional proteins, activation/inactivation of specific proteins and autolytic processes. In addition, proteases increase the permeability of the plant plasma membrane, suggesting that they may have a critical role in phytopathogenesis (Soberanes-Gutiérrez et al. 2015). Polypeptides released upon the action of proteases secreted from phytopathogenic fungi may act as elicitors, (damage-associated molecular patterns), which are subsequently recognized by corresponding immune receptors and are known to trigger MAPK signaling. A metalloprotease from the rice blast fungus Pyricularia grisea (now P. oryzae) was shown to act as an avirulence factor (AVR-Pita) by directly binding to a plant resistance gene product (Pi-ta) and stimulating a signaling cascade that leads to resistance in a classic example of a gene-for-gene interaction (Jia et al. 2000). Olivieri et al. (2002) demonstrated that the fungus F. solani f. sp. eumartii secretes 30 kDa SLP with the ability to degrade pathogenesis related (PR) proteins as well as specific polypeptides of intercellular washing fluids and cell wall proteins. A study on a novel extracellular elicitor protein produced by the strawberry pathogen Sarocladium strictum confirmed that the proteolytic activity of the AsES elicitor is required for the induction of defense responses in plants (Chalfoun et al. 2013). Other reports suggest that pathogens might overcome the deleterious effects of plant chitinases by secreting proteases that modify them. A recently discovered Zn-metalloprotease from Fusarium verticillioides, fungalysin Fv-cmp, cleaves the Gly–Cys peptide bond between two domains of corn class IV chitinases ChitA and ChitB (Slavokhotova et al. 2014). Fungalysin-mediated proteolytic degradation of class IV chitinases is also found in Arabidopsis (Naumann and Price 2012).
Proteases as markers of phytopathogenicity
Positive correlations between certain protease activities and disease aggressiveness have been reported in several plant pathogenic fungi. A cerevisin (homologous to proteinase B from S. cerevisiae) mutant of V. dahliae with decreased secretion of low molecular weight proteins known to be virulence factors was generated to assess the effects on virulence. At 21 days after inoculation with the cerevisin mutant, cotton seedlings did not have noticeable symptoms. Even though the role of cerevisin in the production and secretion of these polypeptides remains to be elucidated, the experiments highlight the indirect involvement of cerevisin in the virulence of V. dahliae (He et al. 2015). Soberanes-Gutiérrez et al. (2015) reported that the knockout of the PEP4 gene in U. maydis reduced virulence of U. maydis on maize. Nonpathogenic mutants of the fungus Pyrenopeziza brassicae do not have the ability to produce extracellular cysteine proteinase (Ball et al. 1991). Staples and Mayer (1995) showed that an increase in the level of aspartic protease activity in B. cinerea led to an increase in virulence. Magnaporthiopsis poae expresses a SLP (Mp1) in infected roots, and its level of expression is positively correlated with the severity of symptoms (Sreedhar et al. 1999). Olivieri et al. (2004) demonstrated a correlation between proteolytic activity detected in intercellular washings with the size of lesions caused by F. eumartii in susceptible potato tubers, but not in the resistant. Higher proteolytic activity in roots infected with F. solani f. sp. phaseoli correlated with the processing of class IV chitinase (Lange et al. 1996). Recently, Jashni et al. (2015b) reported that both secreted metalloprotease and a serine protease were responsible for reduced chitinase activity in tomato. Because these chitinases have antifungal properties, the deletion of both encoding genes (FoMep1 and FoSep1) rendered F. oxysporum f. sp. lycopersici unable to cleave and inactivate specific chitinases and thus less virulent on tomato.
Dunaevsky et al. (2001, 2006) reported that the production of TLPs is characteristic of plant pathogens. Further, the validity of TLPs as a marker in fungal phytopathogenicity was proved to be due to conserved motifs around the active site residues (Dubovenko et al. 2010). Nevertheless, SLPs are the key virulence factors in nematophagy (Zhao et al. 2005) and responsible for pathogenesis in saprophytes such as A. solani (Valueva et al. 2013). In the plant pathogens V. albo-atrum, V. dahliae, and Neocosmospora haematococca, the protease activities detected were trypsin-like, with only trace levels of SLP (St. Leger et al. 1997). A comparative study of extracellular proteases of six species of mycelial fungi, including three phytopathogens (A. alternata, B. cinerea, and Alternaria botrytis) and three saprophytes (Penicillium chrysogenum, Penicillium glabrum and Trichoderma harzianum) showed that SLPs are likely present in all surveyed fungal species (Dunaevsky et al. 2006). More virulent F. sporotrichioides exhibited greater extracellular TLP activity than the less virulent Gibberella gordonii (Dunaevsky et al. 2008). Some fungi such as T. harzianum and other saprotrophic fungi secrete solely SLPs. However, A. alternata and other phytopathogens produce TLPs as well (Valueva et al. 2015). Kudryavtseva et al. (2008, 2013) found that Pleurotus pulmonarius, which grows on dead timber, secretes subtilisin but not trypsin. Pleurotus ostreatus, which grows in living hosts, secretes extracellular trypsin throughout its development, suggesting that successful colonization of living tissues requires TLP (Inácio et al. 2015). By using SLPs, a pathogenic fungus mainly affects the physiological integrity of host during penetration and colonization (Chalfoun et al. 2013; Dunaevsky et al. 2006; Figueiredo et al. 2014). There is evidence of extensive gene duplication and loss within the SLP family in fungal lineages, which has been correlated with differences in fungal lifestyles. These data indicate that the production of TLPs is a distinct feature of phytopathogenic fungi, whereas the production of SLPs is a specific feature of saprotrophic fungi (Dubovenko et al. 2010; Kudryavtseva et al. 2008). In some fungi, SLPs either can play a general nutritive role, or may play specific roles in cell metabolism or as pathogenicity or virulence factors. Thus, the presence of significant extracellular TLPs and/or SLPs activity in phytopathogenic fungi may be considered as an indicator of their phytopathogenicity.
Conclusion and future perspectives
In this review, the role in pathogenicity of proteases from phytopathogenic fungi was discussed, including during host–pathogen interactions and signaling involved in priming an immune response and the fact that the energy requirements of phytopathogens are met after the degradation of plant cell wall proteins. Identification and characterization of fungal proteases will be a key aspect in the generation of new molecular markers for phytopathogenicity. Isolation and analysis of genes that encode proteases will promote further investigation of their genetics, regulation, and evolution. In the case of redundant gene families, studies on multiple protease mutants will be needed to characterize their role in virulence. Advanced transcriptome and proteome analyses will facilitate identification of important proteases and their specific inhibitors for further functional analysis. Progress in this research area will allow plant pathologists to design more efficient strategies to generate pathogen-resistant plants. Metabolomics and expression profiling will help to identify specific targets of fungal proteases important in pathogenesis, thus opening new paths for the development of more resistant crops and the progress of sustainable agricultural practices.
References
Annis SL, Goodwin PH (1997) Recent advances in the molecular genetics of plant cell wall-degrading enzymes produced by plant pathogenic fungi. Eur J Plant Pathol 103:1–14
Ball AM, Ashby AM, Daniels MJ, Ingram DS, Johnstone K (1991) Evidence for the requirement of extracellular protease in the pathogenic interaction of Pyrenopeziza brassicae with oilseed rape. Physiol Mol Plant Pathol 38:147–161
Bindschedler LV, Sanchez P, Dunn S, Mikan J, Thangavelu M, Clarkson JM, Cooper RM (2003) Deletion of the SNP1 trypsin protease from Stagonospora nodorum reveals another major protease expressed during infection. Fungal Genet Biol 38:43–53
Brown RL, Chen ZY, Cleveland TE, Cotty PJ, Cary JW (2001) Variation in in vitro α-amylase and protease activity is related to the virulence of Aspergillus flavus isolates. J Food Protect 64:401–404
Chalfoun NR, Grellet-Bournonville CF, Martínez-Zamora MG, Díaz-Perales A, Castagnaro AP, Díaz-Ricci JC (2013) Purification and characterization of AsES protein: a subtilisin secreted by Acremonium strictum is a novel plant defense elicitor. J Biol Chem 288:14098–14113
Chandrasekaran M, Sathiyabama M (2014) Production, partial purification and characterization of protease from a phytopathogenic fungi Alternaria solani (Ell. and Mart.) Sorauer. J Basic Microbiol 54:763–774
Chandrasekaran M, Chandrasekar R, Sa T, Sathiyabama M (2014) Serine protease identification (in vitro) and molecular structure predictions (in silico) from a phytopathogenic fungus, Alternaria solani. J Basic Microbiol 54:S210–S218
Chandrasekaran M, Chandrasekar R, Chun S, Sathiyabama M (2016) Isolation, characterization and molecular three-dimensional structural predictions of metalloprotease from a phytopathogenic fungus, Alternaria solani (Ell. & Mart.) Sor. J Biosci Bioeng 122:131–139
Cheng Z, Li JF, Niu Y, Zhang XC, Woody OZ, Xiong Y, Djonoć S, Millet Y, Bush J, McConkey BJ, Sheen J, Ausubel FM (2015) Pathogen-secreted proteases activate a novel plant immune pathway. Nature 521:213–216
Chu J, Li WF, Cheng W, Lu M, Zhou KH, Zhou HQ, Li FG, Zhou CZ (2015) Comparative analyses of secreted proteins from the phytopathogenic fungus Verticillium dahliae in response to nitrogen starvation. Biochim Biophys Acta 1854:437–448
de Souza PM, de Assis Bittencourt ML, Caprara CC, de Freitas M, de Almeida RPC, Silveira D, Fonseca YM, Filho EXF, Junior AP, Magalhães PO (2015) A biotechnology perspective of fungal proteases. Braz J Microbiol 46:337–346
Dobinson KF, Grant SJ, Kang S (2004) Cloning and targeted disruption, via Agrobacterium tumefaciens-mediated transformation, of a trypsin protease gene from the vascular wilt fungus Verticillium dahliae. Curr Genet 45:104–110
Doi RH, Kosugi A (2004) Cellulosomes: plant-cell-wall degrading enzyme complexes. Nature Rev Microbiol 2:541–551
Dubovenko AG, Dunaevsky YE, Belozersky MA, Oppert B, Lord JC, Elpidina EN (2010) Trypsin-like proteins of the fungi as possible markers of pathogenicity. Fungal Biol 114:151–159
Dunaevsky YE, Golubeva EA, Gruban TN, Beliakova GA, Belozersky MA (2001) Regulation of secretion of extracellular proteases by filamentous fungus Botrytis cinerea Fr. J Russ Phytopathol Soc 2:39–44
Dunaevsky YE, Gruban TN, Belia Kova GA, Belozersky MA (2006) Extracellular proteinases of filamentous fungi as potential markers of phytopathogenesis (in Russian). Mikrobiologia 75:747–751
Dunaevsky YE, Matveeva AR, Fatkhullina GN, Belyakova GA, Kolomiets TM, Kovalenko ED, Belozersky MA (2008) Extracellular proteases of mycelia fungi as participants of pathogenic process. Russ J Bioorg Chem 34:286–289
Feldman ML, Andreu AB, Korgan S, Lobato MC, Huarte M, Walling LL, Daleo GR (2014) PLPKI: a novel serine protease inhibitor as a potential biochemical marker involved in horizontal resistance to Phytophthora infestans. Plant Breed 133:275–280
Figueiredo A, Monteiro F, Sebastiana M (2014) Subtilisin-like proteases in plant–pathogen recognition and immune priming: a perspective. Front Plant Sci 5:739
Gregori R, Guidarelli M, Mari M (2010) Preliminary studies on partial reduction of Colletotrichum acutatum infection by proteinase inhibitors extracted from apple skin. Physiol Mol Plant Pathol 74:303–308
He XJ, Li XL, Li YZ (2015) Disruption of Cerevisin via Agrobacterium tumefaciens-mediated transformation affects microsclerotia formation and virulence of Verticillium dahliae. Plant Pathol 64:1157–1167
Horbach R, Navarro-Quesada AR, Knogge W, Deising HB (2011) When and how to kill a plant cell: infection strategies of plant pathogenic fungi. J Plant Physiol 168:51–62
Inácio FD, Ferreira RO, Araujo CAV, Brugnari T, Castoldi R, Peralta RM, Souza CGM (2015) Proteases of wood rot fungi with emphasis on the genus Pleurotus. BioMed Res Int 2015. doi:10.1155/2015/290161
Jashni MK, Mehrabi R, Collemare J, Mesarich CH, de Wit PJ (2015a) The battle in the apoplast: further insights into the roles of proteases and their inhibitors in plant–pathogen interactions. Front Plant Sci 6:584
Jashni MK, Dols HMI, Iida Y, Boeren S, Beenen HG, Mehrabi R, Collemare J, de Wit PJ (2015b) Synergistic action of a metalloprotease and a serine protease from Fusarium oxysporum f. sp. lycopersici cleaves chitin-binding tomato chitinases, reduces their antifungal activity and enhances fungal virulence. Mol Plant Microbe Interact 28:996–1008
Jia Y, McAdams SA, Bryan GT, Hershey HP, Valent B (2000) Direct interaction of resistance gene and avirulence gene products confers rice blast resistance. EMBO J 19:4004–4014
Juge N (2006) Plant protein inhibitors of cell wall degrading enzymes. Trends Plant Sci 11:359–367
Kasana RC, Salwan R, Yadav SK (2011) Microbial proteases: detection, production, and genetic improvement. CRC Crit Rev Microbiol 37:262–276
Kubicek CP, Starr TL, Glass NL (2014) Plant cell wall-degrading enzymes and their secretion in plant-pathogenic fungi. Annu Rev Phytopathol 52:427–451
Kudryavtseva OA, Dunaevsky YE, Kamzolkina OV, Belozersky MA (2008) Fungal proteolytic enzymes: features of the extracellular proteases of xylotrophic basidiomycetes. Microbiology 77:643–653
Kudryavtseva NN, Sofyin AV, Revina TA, Gvozdeva EL, Ievleva EV, Valueva TA (2013) Secretion of proteolytic enzymes by three phytopathogenic microorganisms. Appl Biochem Microbiol 49:514–520
Lange J, Mohr U, Wiemken A, Boller T, Vögeli-Lange R (1996) Proteolytic processing of class IV chitinase in the compatible interaction of bean roots with Fusarium solani. Plant Physiol 111:1135–1144
Lebeda A, Luhova L, Sedlarova D, Jancova D (2001) The role of enzymes in plant–fungal pathogens interactions. J Plant Dis Protect 108:89–111
Mandujano-González V, Arana-Cuenca A, Anducho-Reyes MÁ, Téllez-Jurado A, González-Becerra AE, Mercado-Flores Y (2013) Biochemical study of the extracellular aspartyl protease Eap1 from the phytopathogen fungus Sporisorium reilianum. Protein Expr Purif 92:214–222
Mercado-Flores Y, Hernández-Rodríguez C, Ruiz-Herrera J, Villa-Tanaca L (2003) Proteinases and exopeptidases from the phytopathogenic fungus Ustilago maydis. Mycologia 95:327–339
Movahedi S, Heale JB (1990) Purification and characterization of an aspartic proteinase secreted by Botrytis cinerea Pers ex. Pers in culture and in infected carrots. Physiol Mol Plant Pathol 36:289–302
Murphy JM, Walton JD (1996) Three extracellular proteases from Cochliobolus carbonum: cloning and targeted disruption of ALP1. Mol Plant Microbe Interact 9:290–297
Nakajima M, Akutsu K (2014) Virulence factors of Botrytis cinerea. J Gen Plant Pathol 80:15–23
Naumann TA, Price NP (2012) Truncation of class IV chitinases from Arabidopsis by secreted fungal proteases. Mol Plant Pathol 13:1135–1139
Olivieri F, Zanetti ME, Oliva CR, Covarrubias AA, Casalongué CA (2002) Characterization of an extracellular serine protease of Fusarium eumartii and its action on pathogenesis related proteins. Eur J Plant Pathol 108:63–72
Olivieri FP, Maldonado S, Tonón CV, Casalongué CA (2004) Hydrolytic activities of Fusarium solani and Fusarium solani f. sp. eumartii associated with the infection process of potato tubers. J Phytopathol (Berlin) 152:337–344
Pekkarinen AI, Jones BL, Niku-Paavola ML (2002) Purification and properties of an alkaline proteinase of Fusarium culmorum. Eur J Biochem 269:798–807
Poussereau N, Creton S, Brillon-Grand G, Rascle C, Fevre M (2001) Regulation of acp1, encoding a non-aspartyl acid protease expressed during pathogenesis of Sclerotinia sclerotiorum. Microbiology 147:717–726
Rawlings ND, Barrett AJ, Bateman A (2012) MEROPS: the database of proteolytic enzymes, their substrates and inhibitors. Nucl Acids Res 40:D343–D350
Redman RS, Rodriguez RJ (2002) Characterization and isolation of an extracellular serine protease from the tomato pathogen Colletotrichum coccodes (Wallr.), and its role in pathogenicity. Mycol Res 106:1427–1434
Rolland S, Bruel C, Rascle C, Girard V, Billon-Grand G, Poussereau N (2009) pH controls both transcription and post-translational processing of the protease BcACP1 in the phytopathogenic fungus Botrytis cinerea. Microbiology 155:2097–2105
Ryan CA (1990) Protease inhibitors in plants: genes for improving defenses against insects and pathogens. Annu Rev Plant Pathol 28:425–449
Saitoh H, Fujisawa S, Ito A, Mitsuoka C, Berberich T, Tosa Y, Asakura M, Takano Y, Terauchi R (2009) SPM1 encoding a vacuole-localized protease is required for infection-related autophagy of the rice blast fungus Magnaporthe oryzae. FEMS Microbiol Lett 300:115–121
Silva Y, Portieles R, Pujol M, Terauchi R, Matsumura H, Serrano M, Hidalgo OB (2013) Expression of a microbial serine proteinase inhibitor gene enhances the tobacco defense against oomycete pathogens. Physiol Mol Plant Pathol 84:99–106
Slavokhotova AA, Naumann TA, Price NPJ, Rogozhin EA, Andreev YA, Vassilevski AA, Odintsova TI (2014) Novel mode of action of plant defense peptides—hevein like antimicrobial peptides from wheat inhibit fungal metalloproteases. FEBS J 281:4754–4764
Soberanes-Gutiérrez CV, Juárez-Montiel M, Olguín-Rodríguez O, Hernández-Rodríguez C, Ruiz-Herrera J, Villa-Tanaca L (2015) The pep4 gene encoding proteinase A is involved in dimorphism and pathogenesis of Ustilago maydis. Mol Plant Pathol 16:837–846
Sreedhar L, Kobayashi DY, Bunting TE, Hillman BI, Belanger FC (1999) Fungal proteinase expression in the interaction of the plant pathogen Magnaporthe poae with its host. Gene 235:121–129
St. Leger RJ, Joshi L, Roberts DW (1997) Adaptation of proteases and carbohydrases of saprophytic, phytopathogenic and entomopathogenic fungi to the requirements of their ecological niches. Microbiology 143:1983–1992
Staples RC, Mayer AM (1995) Putative virulence factors of Botrytis cinerea acting as a wound pathogen. FEMS Microbiol Lett 134:1–7
ten Have A, Espino JJ, Dekkers E, Van Sluyter SC, Brito N, Kay J, González C, van Kan JAL (2010) The Botrytis cinerea aspartic proteinase family. Fungal Genet Biol 47:53–65
Tucker SL, Talbot NJ (2001) Surface attachment and pre-penetration stage development by plant pathogenic fungi. Annu Rev Phytopathol 39:385–417
Turra D (2006) Expression profiles of potato proteinase inhibitors potentially useful for the control of phytopathogenic fungi. J Plant Pathol 88:S3
Valueva TA, Kudryavtseva NN, Sof’in AV, Revina TA, Gvozdeva EL, Ievleva EV (2011) Comparative analyses of exoproteinases produced by three phytopathogenic microorganisms. J Pathogens 2011. doi:10.4061/2011/947218
Valueva TA, Kudryavtseva NN, Gvozdeva EL, Sof’in AV, Iľina NA, Pobedinskaya MA, Elansky SN (2013) Serine proteinases secreted by two isolates of the fungus Alternaria solani. J Basic App Sci 9:105–115
Valueva TA, Kudryavtseva NN, Sof’i AV, Zaitchik BT, Pobedinskaya MA, Kokaeva LY, Elansky SN (2015) Serine exoproteinases secreted by the pathogenic fungi of Alternaria genus. J Plant Pathol Microb 6:272
Zaferanloo B, Quang TD, Daumoo S, Ghorbani MM, Mahon PJ, Palombo EA (2014) Optimization of protease production by endophytic fungus, Alternaria alternata, isolated from an Australian native plant. World J Microbiol Biotechnol 30:1755–1762
Zhao ML, Huang JS, Mo MH, Zhang KQ (2005) A potential virulence factor involved in fungal pathogenicity: serine-like protease activity of nematophagous fungus Clonostachys rosea. Fungal Divers 19:217–234
Acknowledgments
This article was supported by the KU Brain Pool (2016–2017) of Konkuk University, Seoul Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chandrasekaran, M., Thangavelu, B., Chun, S.C. et al. Proteases from phytopathogenic fungi and their importance in phytopathogenicity. J Gen Plant Pathol 82, 233–239 (2016). https://doi.org/10.1007/s10327-016-0672-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10327-016-0672-9