Abstract
Pesticides contribute to human welfare by reducing vector-borne diseases and protecting crops against pests. Insecticides are the most widely employed pesticides for agricultural, domestic, and industrial pest control. However, some insecticides such as synthetic pyrethroids, analogs of the natural pyrethrin, persist in the environment and result in different hostile effects on nontarget organisms. Due to a continuous increase in the use of pyrethroids and their widespread application, different generations and types of pyrethroids have been frequently reported from environmental media, biota, and residential areas. Synthetic pyrethroids are observed to be less toxic to mammal and birds, relatively toxic to amphibians, and highly toxic to aquatic organisms including fish. Here, we review the occurrence, fate, biotransformation, and bioavailability of pyrethroids in waters. We also present biomarkers used to evidence toxicological effects of pyrethroids on fish. Toxic effects include oxidative stress and damage such as production of reactive oxygen species and lipid peroxidation; neurological behavioral inconsistencies; developmental effects such as delayed development and signaling; biochemical alterations of protein, glucose, and enzymes; hematological changes in white blood cells, red blood cells, and hemoglobin; physiological effects on metabolism and heart function; histopathological changes in the brain, liver, and gills; molecular toxicity including DNA damage, micronuclei induction, and altered gene or mRNA expression; and reproductive or endocrine disruption, e.g., disrupted pathways and signaling. Mechanisms of toxicity and control measures are also discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
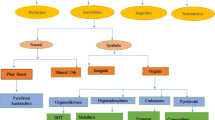
The human race made enormous progress; however, revolutionary achievements are coupled with environmental off-putting factors such as vast use and release of drugs, heavy metals, fertilizers, and pesticides (Stankovic et al. 2014; Ullah et al. 2016a; Vieira et al. 2017; Afridi et al. 2018; Ali and Khan 2018; Khristoforova et al. 2018; Ullah and Li 2018). Pesticides are employed to repel, deter, or kill target organisms such as insects, algae, fungi, and bacteria in agricultural fields, public places such as homes, hospitals, and parks, industries, and public health programs (Ullah et al. 2019). With advancements in the field of pesticides chemistry, the numbers of pesticides are growing continuously. Different types of pesticides are used for targeting different types and/or species of organisms. The use of these pesticides is a major reason of elevating the standard of human life by different ways such as protecting the crops in the fields and stored food, destroying breeding site of different diseases causing insects, controlling harmful microorganisms including bacteria and viruses, and vanishing exasperating flies (Gill et al. 2018; Ullah et al. 2018a).
Different classes of pesticides
Pesticides are synthesized commercially and used under different names, belonging to different types and classes. The different classes of pesticides are employed based on their target organisms such as virucides against viruses, avicides against birds, algicides against algae, fungicides against fungi, nematicides against nematodes, rodenticides against rodents, herbicides against herbs, bactericides against bacteria, and acaricides or insecticides against insects (Regnery et al. 2018; Singh et al. 2018a; Valle et al. 2018). Among different classes of pesticides, insecticides are the most widely employed ones and attribute to about 80% of the use of the total pesticide (Ullah et al. 2018b). There are different registered classes of insecticides including organochlorines, carbamates, organophosphates, formamidines, organotins, organosulfurs, avermectins, neonicotinides, ryanodine, and rotenone, among others (Ullah et al. 2016b, c; Yang et al. 2018). However, one of the late introduced and most widely employed classes of insecticides is synthetic pyrethroids.
Introduction to pyrethroids
Pyrethroids are derived synthetically from pyrethrins, which are extracted from the flower of a plant, Chrysanthemum cinerariaefolium (Ullah 2015). Pyrethrins are insecticidal in nature due to the presence of ketoalcoholic esters of strongly lipophilic pyrethroic and chrysanthemic acids, having the capability of rapidly penetrating into insect bodies and leading to toxicosis. However, being highly sensitive to light natural pyrethrins break down within a few hours and cannot bioaccumulate in a sufficient concentration or amount to kill insects. With the help of modified structures, formulations, and stereochemistry, thousands of synthetic pyrethroids are developed. These modifications include cyano group addition, mixing of optical and geometric isomers, halogenation of the cyclopropane side chain of the pyrethrin molecule, adding different solvents and carriers, and different technical grade formulations (Kaviraj and Gupta 2014). These pyrethroids have a wide range of chemical and biological properties and performance; therefore, suitable pyrethroids are employed in agricultural fields, industries, parks, orchards, and homes (Ullah et al. 2018b).
Biotransformation and environmental fate of synthetic pyrethroids
The routes for the elimination of synthetic pyrethroids in the environmental media include microbial degradation, photodegradation, volatilization, and hydrolysis (Gan et al. 2005). However, in the biological systems, pyrethroids are detoxified by two pathways—esterase-dependent hydrolytic reaction and oxidative reaction mediated by cytochrome P450s. The main factors recognized for nontarget organisms’ susceptibility against synthetic pyrethroids are toxicokinetic factors. Synthetic pyrethroids are degraded through esterase-based hydrolysis followed by cytochrome P450s-based oxidation easily; therefore, they are relatively less toxic to mammals (Gammon et al. 2012). However, pyrethroids are highly toxic to fish because they lack hydrolase and therefore cannot swiftly detoxify synthetic pyrethroids hydrolytically like mammals (Yang et al. 2016). The only metabolic pathway of synthetic pyrethroids in fish is oxidative reaction catalyzed by cytochrome P450s. Different non-specific metabolites of the synthetic pyrethroids have been recognized, such as 3-phenoxybenzoic acid, 3-phenoxybenzaldehyde, 3-phenoxybenzyl alcohol, 3-phenoxybenzoic acid, and 3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylic acid. The transformation and degradation of compounds in the environment depend upon their physicochemical properties (Singh et al. 2016). Synthetic pyrethroids have the property of hydrophobicity and are insoluble in water with an n-octanol distribution coefficient 6.6 in water. They are stable and persist in the aquatic sediments and soil (Gammon et al. 2012). Figure 1 shows some toxicological impacts, some metabolites, environmental fate or degradation, and biotransformation of synthetic pyrethroids.
Synthetic pyrethroids (SPs) exposure leads to different toxicological impacts in the exposed organisms such as the induction of oxidative stress followed by oxidative damage, neurotoxicity, hematological toxicity, biochemical toxicity, and developmental toxicity. Some metabolites of synthetic pyrethroids have been identified, and their exposure led to different immunotoxicity, endocrine disruption, and reproductive toxicity. Pyrethroids get degraded by microbial degradation, photodegradation, hydrolysis, and volatilization. Moreover, pyrethroids are biotransformed easily by mammals through hydrolytic (esterase) and oxidative (cytochrome P450s) reactions. Therefore, pyrethroids are less toxic to them. However, fish lack hydrolase and metabolize synthetic pyrethroids through oxidative (cytochrome P450s) reaction only. Therefore, they are highly toxic to fish and other aquatic organisms
Bioavailability of synthetic pyrethroids in the aquatic environment
Synthetic pyrethroids lead to aquatic bodies through runoffs from the sprayed agricultural fields, parking lots, industries, and public health programs through spray drift to some extent; however, the main source of bioavailability in the water bodies is flowing therethrough rainstorm events. The magnitude and frequency of synthetic pyrethroids use and their precipitation patterns are observed to be critical factors governing synthetic pyrethroids transport to water bodies (Oros and Werner 2005). Moreover, the breakdown rates of the pyrethroids such as persistence on the soil surface, temperature, and canopy cover in association with their precipitation events may play a specific role in the determination of concentrations of synthetic pyrethroids in the runoffs (Palmquist et al. 2012). The concrete drainage system may transport a higher concentration of aqueous-phase pyrethroids in the urban and suburban areas as compared to earthen ditches channeled from agricultural particulate-rich runoffs (Weston and Lydy 2010).
Synthetic pyrethroids in the environment
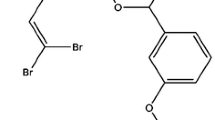
Owing to the widespread applications of synthetic pyrethroids, these are reported from various parts of the world. Table 1 shows the reported concentrations of different synthetic pyrethroids from the soil, and land organisms or their products, whereas Table 2 shows the reported concentration of different synthetic pyrethroids from sediments, water, fish, and other aquatic organisms from different countries across the globe. The range is given for individual synthetic pyrethroids, such that any of them observed in minimum and maximum concentrations. Synthetic pyrethroids are divided into two types, type I and type II. Type I pyrethroids are non-cyano pyrethroids, while type II pyrethroids contain the α-cyano group. Figure 2 shows the chemical structure of widely employed esters of synthetic pyrethroids of different generations from both type I and type II groups, whereas Fig. 3 shows the chemical structure and formulae of different esters of natural pyrethrin isolated from C. cinerariaefolium. Type II pyrethroids are considered to be more severely neuro-intoxicating as compared to type I, solely due to the presence of an α-cyano group (Soderlund et al. 2002). Table 3 shows the acute toxic concentrations of type II synthetic pyrethroids against different fish species. Synthetic pyrethroids are widely used across the globe due to their low toxicity to mammals and birds. However, synthetic pyrethroids are known to pose marked hostile effects on aquatic organisms, more specifically on fish (Assis et al. 2009). Keeping in view the toxic effects of synthetic pyrethroids on fish, different biomarkers are used to delineate their toxic impacts as well as envisaging biomarkers for future research.
Synthetic pyrethroids (SPs) induced neurotoxicity (mechanism and subtle consequences) and chemical structures of different SPs [type I (without α-cyano group) including allethrin (first generation), permethrin (second generation), resmethrin (third generation), and bifenthrin (fourth generation), while type II (with α-cyano group) including fenvalerate (third generation), cyhalothrin (fourth generation), cypermethrin (fourth generation), and deltamethrin (fourth generation)]
The isolated pyrethrins from Chrysanthemum cinerariaefolium flowers with their chemical structures and formulae—the shown pyrethrins are esters of natural pyrethrum. Mechanism of action of synthetic pyrethroids (SPs): (1) SPs exposure results in the production of excessive reactive oxygen species (ROS) that leads to detrimental effects on lipids, proteins, and DNA, (2) SPs interrupt ligand-gated channel and allow inflow of sodium in a higher concentration which leads to multiple nerve impulses and ultimately to inhibition of acetylcholinesterase and accumulation of acetylcholine, which stimulate other nerves, (3) SPs affect voltage-gated calcium channel and consequently (4) increase calcium concentration in the cytosol that consequently lead to cytotoxicity, (5) SPs inhibit receptor of γ-aminobutyric acid that consequently inhibits GABA receptor and ultimately leads to excitability and convulsion, (6) SPs disturb ATP formation/synthesis directly as well as glucose regulation is disturbed in response to cortisol regulation, (7) SPs exposure leads to the retention of acetylcholine in the synaptic gap (due to closing of synaptic cleft) which increases acetylcholine level, and (8) SPs lead to genotoxicity either by oxidative stress induction or due to accumulation of calcium in a higher concentration in the cytosol
Biomarkers of pyrethroid toxicity in fish
Biomarkers are indicators of the response of exposure to any toxicant, chemical, pollutant, or any other foreign particle. Biomarkers can be evaluated at a molecular or cellular level to community or ecosystem level. These biomarkers can substantially reveal the toxic effects of the toxicant on the exposed organism, such as toxicities on their neurology resulting in altered behavior, histopathological, morphological, anatomical, physiological, hematological, and biochemical profiles. Table 4 presents various toxic effects of pyrethroids to biomarkers in different fish species.
Pyrethroid-induced oxidative stress or damage in fish
Oxidative stress is widely employed as a sensitive biomarker in ecotoxicological assessments in order to understand the underlying hostile effects. The oxidative stress is evaluated in terms of reactive oxygen species (ROS) or free radicals’ production, increased lipid peroxidation, and altered activities of the antioxidant enzymes in response. ROS production leads to oxidative damage at the cellular level to DNA, lipids, and protein (Ullah et al. 2018a). To cope with the oxidative damage and to defend the cell against free radicals, different stress proteins such as heat-shock proteins, glucose-regulating proteins, and antioxidant enzymes including catalase, peroxidases, superoxide dismutase, glutathione reductase, glutathione-S-transferase, and glutathione peroxidase are produced. However, when the production of the free radicals exceeds the potential of the defense system of the exposed organisms, it leads to different levels of oxidative damage such as DNA damage (Ullah et al. 2017). Research revealed that synthetic pyrethroids-induced oxidative stress leads to the different type of instant toxicities as well as toxicities and weak immunity at later stages in fish. In response to the oxidative stress, the fish adapt defensive mechanism by changing their antioxidant enzymatic activities such as increasing their activities to cope with the free radicals.
Exposure to different synthetic pyrethroids induced oxidative stress in different species of fish, for example, cypermethrin induced oxidative stress in different tissues of Tor putitora (Ullah et al. 2014), Labeo rohita (Ullah 2015), and Oncorhynchus mykiss (Kutluyer et al. 2015), cyhalothrin induced oxidative stress in different tissues of Cyprinus carpio (Clasen et al. 2018) and Prochilodus lineatus (Vieira and dos Reis Martinez 2018), and deltamethrin induced oxidative stress in different tissues of Cyprinus carpio (Ensibi et al. 2013), Sparus aurata (Guardiola et al. 2014), Oreochromis niloticus (Abdel-Daim et al. 2015), Danio rerio (Parlak 2018), and Hypophthalmichthys molitrix (Ullah et al. 2019).
Neurotoxicity
Pyrethroids exert toxic effects on the nervous system of the fish by affecting their sodium channels. They attached to these gated channels and delay the inactivation of the Na+ channels, which ultimately led to neuronal excitability (Ullah et al. 2019). However, recent research revealed that synthetic pyrethroids also affect the other voltage-gated channels such as calcium and chloride channels, and receptor of γ-aminobutyric acid as their secondary targets (Soderlund 2012). Disturbance to these channels leads to different neurobehavioral changes. Moreover, the neurotoxic effects lead to complex consequences such as affected energy metabolism, neuromuscular functions, neural transduction, and homeostasis. Figure 2 shows the neurotoxic effects of synthetic pyrethroids, their mechanisms, and subtle consequences. The neurotoxic effects can be in the form of disturbed voltage-gated channels, behavioral inconsistencies or alterations, and inhibition of acetylcholinesterase activity.
Behavioral inconsistencies and alterations
Research revealed that exposure to synthetic pyrethroids resulted in different behavioral inconsistencies in fish, such as sluggish movement, disturbed swimming or swimming pattern, inability to maintain their position, reduced feeding, interrupted school behavior, hypo- or hyperexcitability, dangling or irregular or erratic swimming, increased opercula movements, rapid jerky movements, loss of equilibrium, frequently surfacing, adapting vertical position, sinking to bottom, hypo- or hyperactiveness, jumping, loss of balance, motionlessness, and disturbed migratory pattern in different fish species such as Tor putitora (Ullah et al. 2014), Labeo rohita (Ullah 2015), and Clarias batrachus (Kumar et al. 2011b). The acetylcholinesterase is active at both the neural and neuromotor junctions of the muscle tissues; therefore, the neuromuscular inhibition of acetylcholinesterase leads to blocked neural transmission and increased acetylcholine at the nerve endings, which consequently lead to different behavioral inconsistencies. Therefore, these alterations are often associated with the inhibition of acetylcholinesterase activity in the brain or muscles of the fish and/or increment in the level of acetylcholine.
Inhibition of acetylcholinesterase activity
A number of research studies revealed that synthetic pyrethroids induce neurotoxic effects by inhibiting the activity of acetylcholinesterase or incrementing the level of acetylcholine in the brain of various fish tissues. The inhibition of acetylcholinesterase results in nerve impulses and makes them permeable to sodium. Synthetic pyrethroids delay the closing of sodium channels, allowing sodium inflow in a heavy concentration, which consequently leads to multiple never impulses, which in turn release a neurotransmitter, acetylcholine, leading to their higher accumulation in the nerve synapses and ultimately decreased cholinergic transmission and other neurotoxic effects. In fish, these effects are increased operculum movement, convulsions, and surfacing (Singh et al. 2018b). Deltamethrin exposure resulted in inhibition of the acetylcholinesterase in the brain of silver carp resulting in erratic swimming, vertical position adaptation, hyperactivity, and equilibrium loss as well as in the muscle tissues that resulted in the desensitization of the receptors of nicotine acetylcholine and subsequently resulted in muscular weakness and changed swimming pattern (Ullah et al. 2019).
Developmental toxicity
Synthetic pyrethroids are reported to exhibit greater acute toxic effects on the developing stages of animals as compared to adult stages (Yang et al. 2018). However, fish is highly sensitive and more susceptible to synthetic pyrethroids during their early life stages as compared to their adult stage (Yang et al. 2014). Synthetic pyrethroids also have the capability of affecting the development and growth of various animals (DeMicco et al. 2010). There is a continuously growing body of evidence, revealing the developmental toxicity of different pyrethroids on nontarget organisms, more specifically against fish, for example, exposure to bifenthrin accelerated hatching and impaired the normal morphology of Danio rerio, same as by cypermethrin, by inducing craniofacial abnormalities, pericardial edema, body curvatures, yolk edema, and crooked body (DeMicco et al. 2010; Jin et al. 2009; Shi et al. 2011). Similarly, joint exposure of Danio rerio to cypermethrin and permethrin led to different toxicities at larval stage (Yang et al. 2014), bifenthrin disturbed the dopaminergic signaling at the juvenile stage of Oncorhynchus mykiss (Crago and Schlenk 2015), cypermethrin induced different developmental deformities and altered the enzymatic activities in the developmental stages of Labeo rohita (Dawar et al. 2016), and deltamethrin induced oxidative stress leading to apoptosis and different morphological alterations in Danio rerio (Parlak 2018).
Hematological toxicity
Hematology is often assessed as a useful biomarker in eco-, aquatic, pesticides, and fisheries toxicology. Synthetic pyrethroids exposure results in different hematotoxic effects because after entering into the fish body, blood and blood-producing hematopoietic tissues are continuously exposed to the destructive effects of the respective pyrethroid. Exposure of Tor putitora to the acute concentration of cypermethrin led to an increase in white blood cells and a decrease in red blood cells (Ullah et al. 2015). Similarly, a number of studies reported different types of toxic effects on the hematological profile including white blood cells such as lymphocytes, thrombocytes, granulocytes, and monocytes, red blood cells, hemoglobin, packed cell volume, mean corpuscular volume, mean corpuscular hemoglobin concentration, and mean corpuscular hemoglobin of different fish species after exposure to different synthetic pyrethroids such as Cyprinus carpio (Velisek et al. 2009a), Catla catla (Vani et al. 2012), Rhamdia quelen (Montanha et al. 2014), and Alburnus tarichi (Özok et al. 2018) in response to cypermethrin, Catla catla (Vani et al. 2011) and Salmo trutta fario (Karatas 2016) in response to deltamethrin, and Prochilodus lineatus in response to λ-cyhalothrin (Vieira and dos Reis Martinez 2018). The alterations in the hematological parameters including red blood cells might be attributed to the inhibition of hemosynthesis or erythropoiesis, destruction of blood cells such as red blood cells (anemia), decreased genesis of the red blood cells due to hypoxia, less hemoglobin or no hemoglobin, hematopoietic system’s failure, and osmoregulatory dysfunction, whereas white blood cells may be altered due to the stimulated defense mechanism or immune system of the fish, as a compensatory response to the circulating lymphocytes by the lymphoid tissues, and tissue damage (Ullah et al. 2019).
Biochemical toxicity
Biochemical parameters are often employed as handy biomarkers to appraise the toxic effects of different exogenous compounds, toxicants, and chemicals including pesticides, heavy metals, and pharmaceutical drugs on fish. Different generations of synthetic pyrethroids have been tested against different fish species, and almost all of them resulted in varying levels of biochemical toxicity, for example, deltamethrin induced different biochemical toxicities in Hypophthalmichthys molitrix including a marked reduction in the total protein contents in the liver, gills, muscles, blood, and brain tissues, marked increase in blood glucose concentration, and significant alterations in the concentration of potassium, sodium, chloride, total bilirubin, albumin, urea, inorganic phosphate, and cholesterol in serum (Ullah et al. 2019). Similarly, the activities of metabolic enzymes including aspartate aminotransferases, alanine aminotransferases, lactate dehydrogenases, and glutamate dehydrogenases, and concentration of whole-body cortisol were significantly increased.
A number of well-documented studies revealed that different esters of synthetic pyrethroids induced different toxic impacts on the biochemical indices of various fish species, for example, permethrin altered the vitellogenin protein’s concentration in the liver of Oryzias latipes (Nillos et al. 2010), fenvalerate increased blood glucose level, serum creatinine, and triglyceride and reduced total protein, globulin, and albumin in the serum of Labeo rohita (Prusty et al. 2011), cypermethrin decreased total proteins in the muscles, gills, brain, and liver and increased blood glucose in Tor putitora (Ullah et al. 2014), and deltamethrin increased alkaline phosphatase and decreased acid phosphatase in the liver and kidney of Labeo rohita (Suvetha et al. 2015). A number of such other changes have been reported for different fish species exposed to various synthetic pyrethroids such as cypermethrin-exposed Rhamdia quelen (Montanha et al. 2014) and Brycon amazonicus (de Moraes et al. 2018) and λ-cyhalothrin-exposed Prochilodus lineatus (Vieira and dos Reis Martinez 2018).
Reproductive and endocrine disruptive toxicity
The reproductive toxic effects and endocrine disrupting potential of synthetic pyrethroids are widely studied. They are known as endocrine disruptors, for example, they interfere with the receptors of steroid hormone, and exhibit anti-mineralocorticoid, anti-glucocorticoid, and anti-estrogenic effects (Zhang et al. 2016, 2018). Bifenthrin disrupted the development of testis, inhibited the sperm maturation, delayed spermatocyte development, and reduced testosterone and 17β-estradiol in Sebastiscus marmoratus (Li et al. 2017), decreased gonadosomatic index and increased ovarian follicle diameter and 17β-estradiol in the plasma of Oncorhynchus mykiss (Forsgren et al. 2013), and significantly decreased the reproductive output of Menidia beryllina (Brander et al. 2016). Similarly, several studies documented different toxic effects of different pyrethroids on reproduction and endocrine disruption in different fish species, such as altering the dopaminergic and estrogenic pathways in Danio rerio (Bertotto et al. 2018), changing the spermatozoa quality in Oncorhynchus mykiss (Kutluyer et al. 2016), denaturing the structure of the ovaries in Heteropneustes fossilis (Monir et al. 2016), and up-regulating the vitellogenin gene expression in Oncorhynchus mykiss (Crago and Schlenk 2015), Pimephales promelas (Beggel et al. 2011), and Dario rerio (Jin et al. 2009).
Histomorphological and anatomical toxicity
Histopathological assessment in response to exogenous toxicants, environmental stressors, and abrupt deleterious environmental change is a powerful, useful, and key biomarker in ecotoxicological studies. It emerged as a key parameter in chemical risk assessment and safety studies using fish as a model organism because it is rapid and can be applied to a number of fish tissues such as kidneys, intestines, brain, gills, and liver. Moreover, it is a more sensitive biomarker than a single biochemical response because the histological changes reveal a transition of bio-organization from individual-level biochemical effect at a lower level to population-level effect at a higher level (Ullah et al. 2018a). For histopathological investigation, different important tissues of the fish are employed based on their significance and objective of the study. Gills are studied because of their involvement in different major functions including excretion, respiration, osmoregulation, acid–base balance, being primary contact organ to ambient water having the toxicants, and continuously exposed to the exogenous chemicals. Liver histopathology is often studied in aquatic toxicology because of being the detoxification center. The histopathological alterations in the intestine reveal typical stress induction in fish. Similarly, the histomorphological changes in the brain of fish can display a different level of severity, more specifically in response to synthetic pyrethroids because of their lipophilicity and efficient accumulative and absorptive capability of the fish brain.
A number of well-documented research studies demonstrated synthetic pyrethroids induced histomorphological alterations in different tissues of the exposed species of fish, for example, deltamethrin mediated different histopathological changes in the liver such as congestion, sinusoidal dilation, vacuolation, inflammatory cell accumulation, hemosiderosis, and cellular shrinkage, in the gills such as secondary lamellae folding, epithelium disruption, epithelium fusion, calcium accumulation, secondary lamellae detachment, secondary lamellae degeneration, and secondary lamellae fusion, in the brain such as spongiosis, neuronal degeneration, discoloration, and infiltration, and in the intestine such as disruption of mucosal cells, goblet cells increase, necrosis, and mucosal cells shredding of Hypophthalmichthys molitrix (Ullah et al. 2019). Similarly, a number of other histomorphological changes are observed in different tissues of different fish species in response to different synthetic pyrethroids, such as cypermethrin-exposed Tor putitora (Ullah et al. 2015) and Pangasianodon hypophthalmus (Monir et al. 2015), deltamethrin-exposed Oreochromis niloticus (Kan et al. 2012), Aphanius dispar (Al-Ghanbousi et al. 2012), Cyprinus carpio (Stará et al. 2015), Danio rerio (Parlak 2018), and Colossoma macropomum (Cunha et al. 2018), and bifenthrin-exposed Oncorhynchus mykiss (Velisek et al. 2009b).
Molecular toxicity
There is a growing body of emerging evidence, depicting the molecular toxicological impacts of synthetic pyrethroids on fish. Synthetic pyrethroids-induced DNA damage is well studied in different fish species, for example, cypermethrin induced genotoxicity in Channa punctatus (Ansari et al. 2011) and DNA damage in the gills of Prochilodus lineatus (Poletta et al. 2013) and in the erythrocyte of Labeo rohita (Ullah 2015) and λ-cyhalothrin induced DNA damage in the blood erythrocyte Prochilodus lineatus (Vieira and dos Reis Martinez 2018). Synthetic pyrethroids-mediated micronuclei induction is also well studied, for example, λ-cyhalothrin exposure led to nuclear abnormalities and induced micronuclei formation in Gambusia affinis (Gökalp Muranli and Güner 2011) and deltamethrin induced micronuclei formation in the erythrocyte of Oreochromis niloticus (Kan et al. 2012). There is also enough evidence regarding synthetic pyrethroids-mediated alterations in gene/mRNA expression, for example, permethrin altered VTG-mRNA expression in the hepatocytes of Oncorhynchus mykiss (Nillos et al. 2010). Moreover, research revealed that synthetic pyrethroids up-regulate or down-regulate several transcripts or genes, for example, bifenthrin down-regulated several estrogen-associated transcripts in Menidia beryllina (Brander et al. 2016).
Mechanism of action of synthetic pyrethroids
Synthetic pyrethroids adapt different mechanisms of toxicity; however, the primary mechanism is neurotoxicity or intoxicating the nervous system of the fish. Figure 2 shows a summary of neurotoxicity induction in response to synthetic pyrethroids and the leading subtle consequences. The schematic presentation of the mechanism of action of synthetic pyrethroids is provided in Fig. 3. Synthetic pyrethroids such as cypermethrin form cyanohydrin, which is decomposed into aldehydes and cyanides and subsequently results in the production of reactive oxygen species (Ullah et al. 2018b). Reactive oxygen species induce lipid peroxidation, increase oxidative stress leading to oxidative damage, and increase the concentration of calcium in the cytosol which in turn leads to cytotoxicity and genotoxicity in fish (Ullah 2015). Synthetic pyrethroids layer on the nerve cells and hinder the sodium channels during repolarization, which lead to an unconstrained depolarization and disturbed transmission of the driving forces. The adverse impacts of the synthetic pyrethroids are mainly attributed to their neurotoxic effects linked with the pathological retention of acetylcholine in the synaptic gaps and inhibition of acetylcholinesterase, giving rise to multiple nerve impulses and consequently leading to decreased cholinergic transmission. Moreover, exposure to synthetic pyrethroids results in trans-activated p53 leading to induction of MiR-200 and consequently resulting in apoptosis. Similarly, synthetic pyrethroids change the mitochondrial proteome, leading to mitochondrial dysfunction and subsequently leading to apoptosis, whereas the induced oxidative stress ultimately results in nigrostriatal dopaminergic neurodegeneration (Ullah et al. 2018b).
Control and prevention of synthetic pyrethroids
In order to minimize the use of synthetic pyrethroids, an adaptation of proper strategies and practicing proper management should be ensured. Synthetic pesticides should be phased out gradually and continuously till completely phased out. In order to reduce potential risks, ecological farming should be adopted instead of industrial agriculture. Multi-level approaches should be adapted for crops protection, rather than exclusively depending on pesticides. This will elevate landscape heterogeneity, increase suitable habitats for pollinators, and control pests naturally or biologically. Vegetation should be actively managed, which will increase functional biodiversity. Crops should be types-wise and cultivar-wise rotated to increase soil fertility and make crops resistant to the pest. Natural agents should be used for bio-control such as the introduction of beneficial insects, viruses, bacteria, and nematodes. This will also improve crop protection (Douglas and Tooker 2015).
Control and preventive measures against pesticides
Pesticides should be employed according to the regulation and should be used by following the stipulated regulations. Pesticides risk assessment and safety, biopesticides use in agriculture, and biotechnological advancement of agriculture should be exclusively included in future plans. The advanced form of constructed wetlands should be employed, which emerged as a more reliable management approach and treatment system for alleviating different nonpoint sources of pesticides including agricultural runoffs and draining. Through this advance system of wetlands, pesticides are evacuated via different processes such as biological processes including plant absorption or metabolism, physical processes including absorption, sedimentation, co-precipitation, and precipitation, chemical processes, e.g., hydrolysis, photolysis, cation exchange, oxidation, and reduction, and biochemical processes such as microbial deprivation (Vymazal and Březinová 2015).
Pesticides use should be avoided and restricted at local and home level via using lesser or no cosmetics. Biological pest management should be adapted. Pesticides should be locked and stored in childproof containers, cupboards, or cabinets. Pesticides with the least hazardous impacts and dangers should be used. The pesticides users, exposed masses, applicators, dealers, and farmers should be educated properly. They should be guided regarding manufacturers’ suggestions, instructions, protection equipment, and avoiding exposure of pregnant women, infants, toddlers, and children to the pesticides. Similarly, at the community level, organic farming and integrated pest management should be adopted at public buildings such as schools, hospitals, and public parks and mass awareness programs including workshops, seminars, and symposia should be arranged. The government should instruct children, pesticides dealers, pesticides applicators, pesticides users, and general masses about the hostile effects of pesticides, at the national level. The environmental protection and public health organizations should monitor and regularly assess pesticides concentration in the local environmental media. They should restrict the use of illegal and banned pesticides. Regular pesticide-based poisoning surveillance and epidemiological studies should be a part of their plan. Permissible limits for the pesticides should be established. These organizations should try to restrict pesticide use within their defined limits and should establish pesticide poisoning control and emergency center.
Conclusion
Pyrethroids have been reported from the soil, cash crops, land or terrestrial organisms, water, sediments, and aquatic organisms including fish. Therefore, it is threatening at a fish biodiversity standpoint, as pyrethroids implicated population decline of fish has been confirmed by various studies in the past. Moreover, different aquatic- and ecotoxicological studies revealed the severe toxic effects of synthetic pyrethroids on fish at various biological levels such as at molecular, cellular, histological, organismal, and population level. These studies provide a future window for further studies. To comprehensively appraise the hostile impacts of synthetic pyrethroids and explain the underlying mechanism more deeply, studies that can possibly link these different levels of biological impacts are highly recommended. Furthermore, toxicological studies regarding individual enantiomers of the pyrethroids should be undertaken. The knowledge from such experiments that are based on the enantioselective toxicity and chirality of the pyrethroids will help in developing environment-friendly pyrethroids. This will also enrich activity of pyrethroids against target insects without posing severe hostilities on nontarget organisms including fish.
References
Abdel-Daim MM, Abdelkhalek NKM, Hassan AM (2015) Antagonistic activity of dietary allicin against deltamethrin-induced oxidative damage in freshwater Nile tilapia; Oreochromis niloticus. Ecotoxicol Environ Saf 111:146–152. https://doi.org/10.1016/j.ecoenv.2014.10.019
Afridi AJ, Zuberi A, Yousafzai AM, Maria KM, Ullah S (2018) Hemp (Marijuana) reverted copper-induced toxic effects on the essential fatty acid profile of Labeo rohita and Cirrhinus mrigala. Mol Biol Rep. https://doi.org/10.1007/s11033-018-4483-2
Akinrotimi O, Gabriel U, Ariweriokuma S (2012) Haematotoxicity of cypermethrin to African catfish (Clarias gariepinus) under laboratory conditions. J Environ Eng Technol 1:20–25
Akoto O, Gavor S, Appah MK, Apau J (2015) Estimation of human health risk associated with the consumption of pesticide-contaminated vegetables from Kumasi, Ghana. Environ Monit Assess 187:244. https://doi.org/10.1007/s10661-015-4471-0
Aldana-Madrid ML, Valenzuela-Quintanar AI, Silveira-Gramont MI, Rodríguez-Olibarría G, Grajeda-Cota P, Zuno-Floriano FG, Miller MG (2011) Residual pyrethroids in fresh horticultural products in Sonora, Mexico. Bull Environ Contam Toxicol 87:436. https://doi.org/10.1007/s00128-011-0391-z
Al-Ghanbousi R, Ba-Omar T, Victor R (2012) Effect of deltamethrin on the gills of Aphanius dispar: a microscopic study. Tissue Cell 44:7–14. https://doi.org/10.1016/j.tice.2011.09.003
Ali H, Khan E (2018) Bioaccumulation of non-essential hazardous heavy metals and metalloids in freshwater fish. Risk to human health. Environ Chem Lett 16:903–917. https://doi.org/10.1007/s10311-018-0734-7
Alonso MB, Feo ML, Corcellas C, Vidal LG, Bertozzi CP, Marigo J, Secchi ER, Bassoi M, Azevedo AF, Dorneles PR, Torres JPM, Lailson-Brito J, Malm O, Eljarrat E, Barceló D (2012) Pyrethroids: a new threat to marine mammals? Environ Int 47:99–106. https://doi.org/10.1016/j.envint.2012.06.010
Amin KA, Hashem KS (2012) Deltamethrin-induced oxidative stress and biochemical changes in tissues and blood of catfish (Clarias gariepinus): antioxidant defense and role of alpha-tocopherol. BMC Vet Res 8:45. https://doi.org/10.1186/1746-6148-8-45
Andrade FH, Figueiroa FC, Bersano PR, Bissacot DZ, Rocha NS (2010) Malignant mammary tumor in female dogs: environmental contaminants. Diagn Pathol 5:45. https://doi.org/10.1186/1746-1596-5-45
Ansari BA, Ahmad MK (2010) Toxicity of synthetic pyrethroid Lambda cyhalothrin and neem based pesticide Neem gold on Zebra fish Danio rerio (Cyprinidae). Glob J Environ Res 4:151–154
Ansari RA, Rahman S, Kaur M, Anjum S, Raisuddin S (2011) In vivo cytogenetic and oxidative stress-inducing effects of cypermethrin in freshwater fish, Channa punctata Bloch. Ecotoxicol Environ Saf 74:150–156. https://doi.org/10.1016/j.ecoenv.2010.08.036
Assis HCS, Nicareta L, Salvo LM, Klemz C, Truppel JH, Calegari R (2009) Biochemical biomarkers of exposure to deltamethrin in freshwater fish, Ancistrus multispinis. Brazil Arch Biol Technol 52:1401–1407. https://doi.org/10.1590/S1516-89132009000600012
Atamanalp M, Erdoğan O (2010) Alterations of HSP70 gene expression in rainbow trout (Oncorhyncus mykiss) exposed to deltamethrin. Turk J Vet Anim Sci 34:359–363. https://doi.org/10.3906/vet-0808-7
Aydın R, Köprücü K, Dörücü M, Köprücü SŞ, Pala M (2005) Acute toxicity of synthetic pyrethroid cypermethrin on the common carp (Cyprinus carpio L.) embryos and larvae. Aquacult Int 13:451–458. https://doi.org/10.1007/s10499-005-0615-5
Ayoola S, Ajani E (2008) Histopathological effects of cypermethrin on juvenile African catfish (Clarias gariepinus). World J Biol Res 1:1–14
Aznar-Alemany Ò, Eljarrat E, Barceló D (2017) Effect of pyrethroid treatment against sea lice in salmon farming regarding consumers’ health. Food Chem Toxicol 105:347–354. https://doi.org/10.1016/j.fct.2017.04.036
Aznar-Alemany Ò, Eljarrat E, Barceló D (2019) Pyrethroid accumulation in farmed salmon, Aquaculture Europe 2019. World Aquaculture Society, Berlin
Bedi JS, Gill JPS, Aulakh RS, Kaur P (2015) Pesticide residues in Bovine Milk in Punjab, India: spatial variation and risk assessment to human health. Arch Environ Contam Toxicol 69:230–240. https://doi.org/10.1007/s00244-015-0163-6
Beggel S, Connon R, Werner I, Geist J (2011) Changes in gene transcription and whole organism responses in larval fathead minnow (Pimephales promelas) following short-term exposure to the synthetic pyrethroid bifenthrin. Aquat Toxicol 105:180–188. https://doi.org/10.1016/j.aquatox.2011.06.004
Begum G (2009) Enzymes as biomarkers of cypermethrin toxicity: response of Clarias batrachus tissues ATPase and glycogen phosphorylase as a function of exposure and recovery at sublethal level. Toxicol Mech Methods 19:29–39. https://doi.org/10.1080/15376510802205650
Bertotto LB, Richards J, Gan J, Volz DC, Schlenk D (2018) Effects of bifenthrin exposure on the estrogenic and dopaminergic pathways in zebrafish embryos and juveniles. Environ Toxicol Chem 37:236–246. https://doi.org/10.1002/etc.3951
Bhattacharya M, Kaviraj A (2009) Toxicity of the pyrethroid pesticide fenvalerate to freshwater catfish Clarias gariepinus: lethality, biochemical effects and role of dietary ascorbic acid. J Environ Sci Health Part B 44:578–583. https://doi.org/10.1080/03601230903000602
Bhutia D, Rai BK, Pal J (2013) Detection of multiple cytochrome P450 in hepatic tissue of Heteropneustes fossilis (Bloch) exposed to cypermethrin. Proc Zool Soc 66:14–19
Bibi N, Zuberi A, Naeem M, Ullah I, Sarwar H, Atika B (2014) Evaluation of acute toxicity of Karate and its sub-lethal effects on protein and acetylcholinestrase activity in Cyprinus carpio. Int J Agric Biol 16:731–737
Bonansea RI, Wunderlin DA, Amé MV (2016) Behavioral swimming effects and acetylcholinesterase activity changes in Jenynsia multidentata exposed to chlorpyrifos and cypermethrin individually and in mixtures. Ecotoxicol Environ Saf 129:311–319. https://doi.org/10.1016/j.ecoenv.2016.03.043
Borges A, Scotti LV, Siqueira DR, Zanini R, Amaral F, Jurinitz DF, Wassermann GF (2007) Changes in hematological and serum biochemical values in jundiá Rhamdia quelen due to sub-lethal toxicity of cypermethrin. Chemosphere 69:920–926. https://doi.org/10.1016/j.chemosphere.2007.05.068
Brander SM, He G, Smalling KL, Denison MS, Cherr GN (2012) The in vivo estrogenic and in vitro anti-estrogenic activity of permethrin and bifenthrin. Environ Toxicol Chem 31:2848–2855. https://doi.org/10.1002/etc.2019
Brander SM, Jeffries KM, Cole BJ, DeCourten BM, White JW, Hasenbein S, Fangue NA, Connon RE (2016) Transcriptomic changes underlie altered egg protein production and reduced fecundity in an estuarine model fish exposed to bifenthrin. Aquat Toxicol 174:247–260. https://doi.org/10.1016/j.aquatox.2016.02.014
Bro E, Devillers J, Millot F, Decors A (2016) Residues of plant protection products in grey partridge eggs in French cereal ecosystems. Environ Sci Pollut Res 23:9559–9573. https://doi.org/10.1007/s11356-016-6093-7
Brodeur JC, Malpel S, Anglesio AB, Cristos D, D’Andrea MF, Poliserpi MB (2016) Toxicities of glyphosate- and cypermethrin-based pesticides are antagonic in the tenspotted livebearer fish (Cnesterodon decemmaculatus). Chemosphere 155:429–435. https://doi.org/10.1016/j.chemosphere.2016.04.075
Cai D, Chen J, Fu J, Zheng Y, Song Y, Yan J, Ding G (2011) Study on contamination of endocrine disrupting chemicals in aquatic environment of Qiantang River. Wei sheng yan jiu = J Hyg Res 40:481–484
Carpenter KD, Kuivila KM, Hladik ML, Haluska T, Cole MB (2016) Storm-event-transport of urban-use pesticides to streams likely impairs invertebrate assemblages. Environ Monit Assess 188:345. https://doi.org/10.1007/s10661-016-5215-5
Chau N, Sebesvari Z, Amelung W, Renaud F (2015) Pesticide pollution of multiple drinking water sources in the Mekong Delta, Vietnam: evidence from two provinces. Environ Sci Pol Res 22:9042–9058. https://doi.org/10.1007/s11356-014-4034-x
Chen XY, Li XL, Huang HW, Huang XR, Liu LL, Zeng CF (2015) Determination of pyrethroids residues in cultural water and aquatic product tissues by gas chromatography. J Anhui Agric Sci 43:96–98
Chinen K, Lau S-L, Nonezyan M, McElroy E, Wolfe B, Suffet IH, Stenstrom MK (2016) Predicting runoff induced mass loads in urban watersheds: linking land use and pyrethroid contamination. Water Res 102:607–618. https://doi.org/10.1016/j.watres.2016.06.040
Clasen B, Loro VL, Murussi CR, Tiecher TL, Moraes B, Zanella R (2018) Bioaccumulation and oxidative stress caused by pesticides in Cyprinus carpio reared in a rice-fish system. Sci Total Environ 626:737–743. https://doi.org/10.1016/j.scitotenv.2018.01.154
Corcellas C, Eljarrat E, Barceló D (2015) First report of pyrethroid bioaccumulation in wild river fish: a case study in Iberian river basins (Spain). Environ Int 75:110–116. https://doi.org/10.1016/j.envint.2014.11.007
Costin D, Staicu AC, Dinu D, Huculeci R, Costache M, Dinischioutu A (2007) Biochemical and histological effects of deltamethrin exposure on the gills of Carassius auratus Gibelio (Pisces Cyprinidae). Sci Pap Anim Sci Biotechnol 40:65–72
Crago J, Schlenk D (2015) The effect of bifenthrin on the dopaminergic pathway in juvenile rainbow trout (Oncorhynchus mykiss). Aquat Toxicol 162:66–72. https://doi.org/10.1016/j.aquatox.2015.03.005
Cunha F, Sousa N, Santos RFB, Meneses JO, do Couto MVS, de Almeida FTC, de Sena Filho JG, Carneiro PCF, Maria AN, Fujimoto RY (2018) Deltamethrin-induced nuclear erythrocyte alteration and damage to the gills and liver of Colossoma macropomum. Environ Sci Pollut Res 25:15102–15110. https://doi.org/10.1007/s11356-018-1622-1
Dallegrave A, Pizzolato TM, Barreto F, Eljarrat E, Barceló D (2016) Methodology for trace analysis of 17 pyrethroids and chlorpyrifos in foodstuff by gas chromatography–tandem mass spectrometry. Anal Bioanal Chem 408:7689–7697. https://doi.org/10.1007/s00216-016-9865-5
Datta M, Kaviraj A (2011) Acute toxicity of the synthetic pyrethroid pesticide fenvalerate to some air breathing fishes. Toxicol Environ Chem 93:2034–2039. https://doi.org/10.1080/02772248.2011.626416
David M, Sangeetha J, Shrinivas J, Harish E, Naik V (2015) Effects of deltamethrin on haematological indices of indian major carp, Cirrhinus mrigala (Hamilton). Int J Pure App Zool 3:37–43
Dawar FU, Zuberi A, Azizullah A, Khan Khattak MN (2016) Effects of cypermethrin on survival, morphological and biochemical aspects of rohu (Labeo rohita) during early development. Chemosphere 144:697–705. https://doi.org/10.1016/j.chemosphere.2015.09.007
de Moraes FD, Venturini FP, Rossi PA, Avilez IM, da Silva de Souza NE, Moraes G (2018) Assessment of biomarkers in the neotropical fish Brycon amazonicus exposed to cypermethrin-based insecticide. Ecotoxicology 27:188–197. https://doi.org/10.1007/s10646-017-1884-2
DeMicco A, Cooper KR, Richardson JR, White LA (2010) Developmental neurotoxicity of pyrethroid insecticides in zebrafish embryos. Toxicol Sci off J Soc Toxicol 113:177–186. https://doi.org/10.1093/toxsci/kfp258
Dey C, Saha S (2014) A comparative study on the acute toxicity bioassay of dimethoate and lambda-cyhalothrin and effects on thyroid hormones of freshwater teleost fish Labeo rohita (Hamilton). Int J Environ Res 8:1085–1092
Domagalski JL, Weston DP, Zhang M, Hladik M (2010) Pyrethroid insecticide concentrations and toxicity in streambed sediments and loads in surface waters of the San Joaquin Valley, California, USA. Environ Toxicol Chem 29:813–823. https://doi.org/10.1002/etc.106
Douglas MR, Tooker JF (2015) Large-scale deployment of seed treatments has driven rapid increase in use of neonicotinoid insecticides and preemptive pest management in US field crops. Environ Sci Technol 49:5088–5097. https://doi.org/10.1021/es506141g
Duong HT, Kadokami K, Pan S, Matsuura N, Nguyen TQ (2014) Screening and analysis of 940 organic micro-pollutants in river sediments in Vietnam using an automated identification and quantification database system for GC–MS. Chemosphere 107:462–472. https://doi.org/10.1016/j.chemosphere.2014.01.064
Elfman L, Tooke NE, Patring JDM (2011) Detection of pesticides used in rice cultivation in streams on the island of Leyte in the Philippines. Agric Water Manag 101:81–87. https://doi.org/10.1016/j.agwat.2011.09.005
El-Sayed YS, Saad TT (2008) Subacute intoxication of a deltamethrin-based preparation (Butox® 5% EC) in Monosex Nile Tilapia, Oreochromis niloticus L. Basic Clin Pharmacol Toxicol 102:293–299. https://doi.org/10.1111/j.1742-7843.2007.00157.x
Ensibi C, Perez-Lopez M, Rodríguez FS, Miguez-Santiyan M, Yahya MD, Hernández-Moreno D (2013) Effects of deltamethrin on biometric parameters and liver biomarkers in common carp (Cyprinus carpio L.). Environ Toxicol Pharmacol 36:384–391. https://doi.org/10.1016/j.etap.2013.04.019
Ensminger MP, Budd R, Kelley KC, Goh KSJ (2013) Pesticide occurrence and aquatic benchmark exceedances in urban surface waters and sediments in three urban areas of California, USA, 2008–2011. Environ Monit Assess 185:3697–3710. https://doi.org/10.1007/s10661-012-2821-8
Essumang DK, Asare EA, Dodoo DK (2013) Pesticides residues in okra (non-target crop) grown close to a watermelon farm in Ghana. Environ Monit Assess 185:7617–7625. https://doi.org/10.1007/s10661-013-3123-5
Fang Y, Chen P, Bian J, Zhong W, Zhu L (2012) Levels and toxicity assessment of pyrethroids in the surface sediments of Taihu Lake and Liaohe River. Acta Sci Circ 32:2600–2606
Feo ML, Eljarrat E, Barceló D (2010a) A rapid and sensitive analytical method for the determination of 14 pyrethroids in water samples. J Chromatogr A 1217:2248–2253. https://doi.org/10.1016/j.chroma.2010.02.018
Feo ML, Ginebreda A, Eljarrat E, Barceló D (2010b) Presence of pyrethroid pesticides in water and sediments of Ebro River Delta. J Hydrol 393:156–162. https://doi.org/10.1016/j.jhydrol.2010.08.012
Fernandez-Alvarez M, Llompart M, Lamas JP, Lores M, Garcia-Jares C, Cela R, Dagnac T (2008) Simultaneous determination of traces of pyrethroids, organochlorines and other main plant protection agents in agricultural soils by headspace solid-phase microextraction–gas chromatography. J Chromatogr A 1188:154–163. https://doi.org/10.1016/j.chroma.2008.02.080
Fernández-Ramos C, Šatínský D, Solich P (2014) New method for the determination of carbamate and pyrethroid insecticides in water samples using on-line SPE fused core column chromatography. Talanta 129:579–585. https://doi.org/10.1016/j.talanta.2014.06.037
Forsgren KL, Riar N, Schlenk D (2013) The effects of the pyrethroid insecticide, bifenthrin, on steroid hormone levels and gonadal development of steelhead (Oncorhynchus mykiss) under hypersaline conditions. Gen Comp Endocrinol 186:101–107. https://doi.org/10.1016/j.ygcen.2013.02.047
Frank DF, Miller GW, Harvey DJ, Brander SM, Geist J, Connon RE, Lein PJ (2018) Bifenthrin causes transcriptomic alterations in mTOR and ryanodine receptor-dependent signaling and delayed hyperactivity in developing zebrafish (Danio rerio). Aquat Toxicol 200:50–61. https://doi.org/10.1016/j.aquatox.2018.04.003
Gammon DW, Chandrasekaran A, ElNaggar SF (2012) Chapter 5 comparative metabolism and toxicology of pyrethroids in mammals. In: Marrs TC (ed) Mammalian toxicology of insecticides. The Royal Society of Chemistry, Cambridge, pp 137–183. https://doi.org/10.1039/9781849733007-00137
Gan J, Lee SJ, Liu WP, Haver DL, Kabashima JN (2005) Distribution and persistence of pyrethroids in runoff sediments. J Environ Qual 34:836–841. https://doi.org/10.2134/jeq2004.0240
Gao ZX, Ao KH, Li B, Bao RH, Zeng QH, Deng CY (2012) Determination of tetramethrin and cyhalothrin residues in mutton tissues by RP-HPLC with solid-phase extraction. Anal Abstr 31:116–119
Gill JPK, Sethi N, Mohan A, Datta S, Girdhar M (2018) Glyphosate toxicity for animals. Environ Chem Lett 16:401–426. https://doi.org/10.1007/s10311-017-0689-0
Gökalp Muranli FD, Güner U (2011) Induction of micronuclei and nuclear abnormalities in erythrocytes of mosquito fish (Gambusia affinis) following exposure to the pyrethroid insecticide lambda-cyhalothrin. Mutat Res/Gen Toxicol Environ Mutagen 726:104–108. https://doi.org/10.1016/j.mrgentox.2011.05.004
Gopala Rao N, Balakrishna Naik K, Srinivasa Rao G (2017) Haematological changes in the fish Cyprinus carpio exposed to a synthetic pyrethroid [Class I], permethrin and its 25% EC. Curr Trends Technol Sci 6:759–763
Gu A, Shi X, Yuan C, Ji G, Zhou Y, Long Y, Song L, Wang S, Wang X (2010) Exposure to fenvalerate causes brain impairment during zebrafish development. Toxicol Lett 197:188–192. https://doi.org/10.1016/j.toxlet.2010.05.021
Guardiola F, Gónzalez-Párraga P, Meseguer J, Cuesta A, Esteban M (2014) Modulatory effects of deltamethrin-exposure on the immune status, metabolism and oxidative stress in gilthead seabream (Sparus aurata L.). Fish Shellfish Immunol 36:120–129. https://doi.org/10.1016/j.fsi.2013.10.020
Güner U (2016) Behavioral differentiation induced by insecticide Lambda-cyhalothrin in Mosquito Fish, Gambusia affinis. LIMNOFISH-J Limnol Freshw Fish Res 2:11–17. https://doi.org/10.17216/LimnoFish-5000128861
Hamed HS (2016) Ameliorative effects of Spirulina platensis on deltamethrin-induced biochemical alterations and oxidative stress in the African catfish; Clarias gariepinus. Open J Mar Sci 6:1–10. https://doi.org/10.4236/ojms.2016.61001
Haque S, Mondal K (2016) Evaluation of acute toxicity and behavioural studies of Tilapia (Oreochromis niloticus) exposed to cypermethrin. J Environ Sociobiol 13:55–58
Haverinen J, Vornanen M (2016) Deltamethrin is toxic to the fish (crucian carp, Carassius carassius) heart. Pest Biochem Physiol 129:36–42. https://doi.org/10.1016/j.pestbp.2015.10.014
Hernández-Guzmán FA, Macías-Zamora JV, Ramírez-Álvarez N, Alvarez-Aguilar A, Quezada-Hernández C, Fonseca AP (2017) Treated wastewater effluent as a source of pyrethroids and fipronil at Todos Santos Bay, Mexico: its impact on sediments and organisms. Environ Toxicol Chem 36:3057–3064. https://doi.org/10.1002/etc.3875
Hossain M, Chowdhury MAZ, Pramanik MK, Rahman M, Fakhruddin A, Alam MK (2015) Determination of selected pesticides in water samples adjacent to agricultural fields and removal of organophosphorus insecticide chlorpyrifos using soil bacterial isolates. Appl Water Sci 5:171–179. https://doi.org/10.1007/s13201-014-0178-6
Jabeen F, Chaudhry AS, Manzoor S, Shaheen T (2015) Examining pyrethroids, carbamates and neonicotenoids in fish, water and sediments from the Indus River for potential health risks. Environ Monit Assess 187:29. https://doi.org/10.1007/s10661-015-4273-4
Jaensson A, Scott AP, Moore A, Kylin H, Olsén KH (2007) Effects of a pyrethroid pesticide on endocrine responses to female odours and reproductive behaviour in male parr of brown trout (Salmo trutta L.). Aquat Toxicol 81:1–9. https://doi.org/10.1016/j.aquatox.2006.10.011
Jayaprakash C, Shettu N (2013) Changes in the hematology of the freshwater fish, Channa punctatus (Bloch) exposed to the toxicity of deltamethrin. J Chem Pharma Res 5:178–183
Jin M, Zhang X, Wang L, Huang C, Zhang Y, Zhao M (2009) Developmental toxicity of bifenthrin in embryo-larval stages of zebrafish. Aquat Toxicol 95:347–354. https://doi.org/10.1016/j.aquatox.2009.10.003
Johnson RM, Ellis MD, Mullin CA, Frazier M (2010) Pesticides and honey bee toxicity—USA. Apidologie 41:312–331. https://doi.org/10.1051/apido/2010018
Kan Y, Cengiz EI, Ugurlu P, Yanar M (2012) The protective role of vitamin E on gill and liver tissue histopathology and micronucleus frequencies in peripheral erythrocytes of Oreochromis niloticus exposed to deltamethrin. Environ Toxicol Pharmacol 34:170–179. https://doi.org/10.1016/j.etap.2012.03.009
Karatas T (2016) Effects of deltamethrin on some haematological parameters of brown trout (Salmo trutta fario). Ind J Anim Res 50:89–92. https://doi.org/10.18805/ijar.5540
Kaviraj A, Gupta A (2014) Biomarkers of type II synthetic pyrethroid pesticides in freshwater fish. Biomed Res Int 2014:7. https://doi.org/10.1155/2014/928063
Kemmerich M, Rizzetti TM, Martins ML, Prestes OD, Adaime MB, Zanella R (2015) Optimization by central composite design of a modified QuEChERS method for extraction of pesticide multiresidue in sweet pepper and analysis by ultra-high-performance liquid chromatography–tandem mass spectrometry. Food Anal Methods 8:728–739. https://doi.org/10.1007/s12161-014-9951-2
Khalili M, Khaleghi SR, Hedayati A (2012) Acute toxicity test of two pesticides diazinon and deltamethrin, on swordtail fish (Xiphophorus helleri). Glob Vet 8:541–545
Khristoforova NK, Tsygankov VY, Lukyanova ON, Boyarova MD (2018) High mercury bioaccumulation in Pacific salmons form the sea of Okhotsk and the Bering sea. Environ Chem Lett 16:575–579. https://doi.org/10.1007/s10311-018-0704-0
Kim Y, Jung J, Oh S-R, Choi K (2008) Aquatic toxicity of cartap and cypermethrin to different life stages of Daphnia magna and Oryzias latipes. J Environ Sci Health Part B 43:56–64. https://doi.org/10.1080/03601230701735029
Kittusamy G, Kandaswamy C, Kandan N, Subramanian MJ (2014) Pesticide residues in two frog species in a paddy agroecosystem in Palakkad District, Kerala, India. Bull Environ Contam Toxicol 93:728–734. https://doi.org/10.1007/s00128-014-1351-1
Koc ND, Muslu MN, Kayhan FE, Colak S (2009) Histopathological changes in ovaries of zebrafish (Danio rerio) following administration of deltamethrin. Fresenius Environ Bul 18:1872–1878
Köprücü K, Aydın R (2004) The toxic effects of pyrethroid deltamethrin on the common carp (Cyprinus carpio L.) embryos and larvae. Pest Biochem Physiol 80:47–53. https://doi.org/10.1016/j.pestbp.2004.05.004
Köprücü SŞ, Köprücü K, Ural MS (2006) Acute toxicity of the synthetic pyrethroid deltamethrin to fingerling European Catfish, Silurus glanis L. Bull Environ Contam Toxicol 76:59–65. https://doi.org/10.1007/s00128-005-0889-3
Kuivila KM, Hladik ML, Ingersoll CG, Kemble NE, Moran PW, Calhoun DL, Nowell LH, Gilliom RJ (2012) Occurrence and potential sources of pyrethroid insecticides in stream Sediments from seven U.S, Metropolitan Areas. Environ Sci Technol 46:4297–4303. https://doi.org/10.1021/es2044882
Kumar A, Sharma B, Pandey RS (2007) Preliminary evaluation of the acute toxicity of cypermethrin and λ-cyhalothrin to Channa punctatus. Bull Environ Contam Toxicol 79:613–616. https://doi.org/10.1007/s00128-007-9282-8
Kumar A, Sharma B, Pandey RS (2011a) Cypermethrin induced alterations in nitrogen metabolism in freshwater fishes. Chemosphere 83:492–501. https://doi.org/10.1016/j.chemosphere.2010.12.062
Kumar A, Sharma B, Pandey RS (2011b) Assessment of acute toxicity of λ-cyhalothrin to a freshwater catfish, Clarias batrachus. Environ Chem Lett 9:43–46. https://doi.org/10.1007/s10311-009-0244-8
Kumari B, Madan V, Kathpal T (2008) Status of insecticide contamination of soil and water in Haryana, India. Environ Monit Assess 136:239–244. https://doi.org/10.1007/s10661-007-9679-1
Kutluyer F, Erişir M, Benzer F, Öğretmen F, İnanan BE (2015) The in vitro effect of Lambda-cyhalothrin on quality and antioxidant responses of rainbow trout Oncorhynchus mykiss spermatozoa. Environ Toxicol Pharmacol 40:855–860. https://doi.org/10.1016/j.etap.2015.09.018
Kutluyer F, Benzer F, Erişir M, Öğretmen F, İnanan BE (2016) The in vitro effect of cypermethrin on quality and oxidative stress indices of rainbow trout Oncorhynchus mykiss spermatozoa. Pest Biochem Physiol 128:63–67. https://doi.org/10.1016/j.pestbp.2015.10.001
Lao W, Tiefenthaler L, Greenstein DJ, Maruya K, Bay S, Ritter K, Schiff K (2012) Pyrethroids in Southern California coastal sediments. Environ Toxicol Chem 31:1649–1656. https://doi.org/10.1002/etc.1867
Li C, Chen L (2013) Determination of pyrethroid pesticides in environmental waters based on magnetic titanium dioxide nanoparticles extraction followed by HPLC analysis. Chromatographia 76:409–417. https://doi.org/10.1007/s10337-013-2393-y
Li H, Tyler Mehler W, Lydy MJ, You J (2011) Occurrence and distribution of sediment-associated insecticides in urban waterways in the Pearl River Delta, China. Chemosphere 82:1373–1379. https://doi.org/10.1016/j.chemosphere.2010.11.074
Li W, Tai L, Liu J, Gai Z, Ding G (2014) Monitoring of pesticide residues levels in fresh vegetable form Heibei Province, North China. Environ Monit Assess 186:6341–6349. https://doi.org/10.1007/s10661-014-3858-7
Li Z, Nie J, Lu Z, Xie H, Kang L, Chen Q, Li A, Zhao X, Xu G, Yan Z (2016) Cumulative risk assessment of the exposure to pyrethroids through fruits consumption in China—based on a 3-year investigation. Food Chem Toxicol 96:234–243. https://doi.org/10.1016/j.fct.2016.08.012
Li J, Luo F, Liu L, Ruan J, Wang N (2017) Exposure to bifenthrin disrupts the development of testis in male Sebastiscus marmoratus. Acta Oceanol Sin 36:57–61. https://doi.org/10.1007/s13131-017-1001-7
Liu F-h, Zhang S (2014) Ecological risk of organophosphorous and synthetic pyrethroid pesticides in water from Nanfeihe River. J Hefei Univ Technol (Nat Sci) 37:1499–1504
Liu T-f, Zhang L, D-f Y, Dong M-h, Gu J-r (2015) Determination of pyrethroid pesticides residues in tea-planted soil. Jiangsu J Agric Sci 31:935–941
Liu Y, Li S, Ni Z, Qu M, Zhong D, Ye C, Tang F (2016) Pesticides in persimmons, jujubes and soil from China: residue levels, risk assessment and relationship between fruits and soils. Sci Total Environ 542:620–628. https://doi.org/10.1016/j.scitotenv.2015.10.148
Liu X, Zhang Q, Li S, Mi P, Chen D, Zhao X, Feng X (2018) Developmental toxicity and neurotoxicity of synthetic organic insecticides in zebrafish (Danio rerio): a comparative study of deltamethrin, acephate, and thiamethoxam. Chemosphere 199:16–25. https://doi.org/10.1016/j.chemosphere.2018.01.176
Lu JL (2011) Insecticide residues in eggplant fruits, soil, and water in the largest eggplant-producing area in the Philippines. Water Air Soil Pollut 220:413–422. https://doi.org/10.1007/s11270-011-0778-9
Ma Y, Chen L, Lu X, Chu H, Xu C, Liu W (2009) Enantioselectivity in aquatic toxicity of synthetic pyrethroid insecticide fenvalerate. Ecotoxicol Environ Saf 72:1913–1918. https://doi.org/10.1016/j.ecoenv.2009.07.005
Mahboob S, Niazi F, AlGhanim K, Sultana S, Al-Misned F, Ahmed Z (2015) Health risks associated with pesticide residues in water, sediments and the muscle tissues of Catla catla at Head Balloki on the River Ravi. Environ Monit Assess 187:81. https://doi.org/10.1007/s10661-015-4285-0
Majumder R, Kaviraj A (2017) Cypermethrin induced stress and changes in growth of freshwater fish Oreochromis niloticus. Int Aquat Res 9:117–128. https://doi.org/10.1007/s40071-017-0161-6
Malhat FM, Haggag MN, Loutfy NM, Osman MAM, Ahmed MT (2015) Residues of organochlorine and synthetic pyrethroid pesticides in honey, an indicator of ambient environment, a pilot study. Chemosphere 120:457–461. https://doi.org/10.1016/j.chemosphere.2014.08.032
Marshall S, Sharley D, Jeppe K, Sharp S, Rose G, Pettigrove V (2016) Potentially toxic concentrations of synthetic pyrethroids associated with low density residential land use. Front Environ Sci 4:75. https://doi.org/10.3389/fenvs.2016.00075
Mawussi G, Scorza Júnior R, Dossa E, Akouété Alaté K-K (2014) Insecticide residues in soil and water in coastal areas of vegetable production in Togo. Environ Monit Assess 186:7379. https://doi.org/10.1007/s10661-014-3934-z
Meenambal M, Pugazhendy K, Vasantharaja C, Venkatesan S (2012) Chelating properties of delonix elata against cypermethrin induced oxitative stress and antioxidant enzyme activity in Cyprinus carpio (Linn). Int J Pharm Biol Arch 3:237–243
Mishra D, Srivastav SK, Srivastav AK (2005) Effects of the insecticide cypermethrin on plasma calcium and ultimobranchial gland of a teleost, Heteropneustes fossilis. Ecotoxicol Environ Saf 60:193–197. https://doi.org/10.1016/j.ecoenv.2003.12.020
Monir MS, Ashaf-Ud-Doulah M, Rahman MK, Akhter JN, Hossain MR (2015) Effect of cypermethrin on the histoarchitecture of gills and liver of a freshwater catfish, Pangasianodon hypophthalmus. Asian J Med Biol Res 1:641–647. https://doi.org/10.3329/ajmbr.v1i3.26488
Monir MS, Ashaf-Ud-Doulah M, Rahman MK, Akhter JN, Hossain MR, Sultana S (2016) Histoarchitecture changes in the ovary of Stinging catfish, Shing (Heteropneustes fossilis) under cypermethrin toxicity. Asian Aust J Biosci Biotechnol 1:47–53
Montanha FP, Fredianelli AC, Wagner R, Sacco SR, Rocha DCC, Pimpão CT (2014) Clinical, biochemical and haemathological effects in Rhamdia quelen exposed to cypermethrin. Arquivo Brasileiro de Medicina Veterinária e Zootecnia 66:697–704. https://doi.org/10.1590/1678-41625934
Moreno-Villa ED, Aldana L, Silveira-Gramont MI, Rodríguez-Olibarría G, Valenzuela-Quintanar AI, Meza-Montenegro M (2012) Analysis of pyrethroids in soil and water in agricultural areas and urban valleys Yaqui and Mayo. Revista Internacional de Contaminacion Ambiental 28:303–310
Murugan A, Swarnam T, Gnanasambandan S (2013) Status and effect of pesticide residues in soils under different land uses of Andaman Islands, India. Environ Monit Assess 185:8135–8145. https://doi.org/10.1007/s10661-013-3162-y
Mushigeri S, David M (2004) Accumulation of fenvalerate and related changes in lactate and succinate dehydrogenases activity in functionally different tissues of the freshwater fish, Cirrhinus mrigala (Hamilton). J Basic Clin Physiol Pharmacol 15:143–152. https://doi.org/10.1515/JBCPP.2004.15.3-4.143
Nema S, Bhargava Y (2018) Quantitative assessment of cypermethrin induced behavioural and biochemical anomalies in adult zebrafish. Neurotoxicol Teratol 68:57–65. https://doi.org/10.1016/j.ntt.2018.05.003
Nesser GA, Abdelbagi AO, Hammad AMA, Tagelseed M, Laing MD (2016) Levels of pesticides residues in the White Nile water in the Sudan. Environ Monit Assess 188:374. https://doi.org/10.1007/s10661-016-5367-3
Nillos MG, Chajkowski S, Rimoldi JM, Gan J, Lavado R, Schlenk D (2010) Stereoselective biotransformation of permethrin to estrogenic metabolites in fish. Chem Res Toxicol 23:1568–1575. https://doi.org/10.1021/tx100167x
Olutona GO, Olatunji SO, Obisanya JF (2016) Downstream assessment of chlorinated organic compounds in the bed-sediment of Aiba Stream, Iwo, South-Western, Nigeria. SpringerPlus 5:67. https://doi.org/10.1186/s40064-016-1664-0
Oros DR, Werner I (2005) Pyrethroid insecticides: an analysis of use patterns, distributions, potential toxicity and fate in the Sacramento-San Joaquin Delta and Central Valley. San Francisco Estuary Institute Oakland, CA
Özok N, OĞuz AR, Kankaya E, Yeltekin AC (2018) Hemato-biochemical responses of Van fish (Alburnus tarichi Guldenstadt, 1814) during sublethal exposure to cypermethrin. Hum Ecol Risk Assess Int J 24:2240–2246. https://doi.org/10.1080/10807039.2018.1443389
Pacífico da Silva I, Oliveira FAS, Pedroza HP, Gadelha ICN, Melo MM, Soto-Blanco B (2015) Pesticide exposure of honeybees (Apis mellifera) pollinating melon crops. Apidologie 46:703–715. https://doi.org/10.1007/s13592-015-0360-3
Palmquist K, Salatas J, Fairbrother A (2012) Pyrethroid insecticides: use, environmental fate, and ecotoxicology. In: Perveen F (ed) Insecticides-advances in integrated pest management. InTech Open, London. https://doi.org/10.5772/29495
Papadakis E-N, Tsaboula A, Kotopoulou A, Kintzikoglou K, Vryzas Z, Papadopoulou-Mourkidou E (2015) Pesticides in the surface waters of Lake Vistonis Basin, Greece: occurrence and environmental risk assessment. Sci Total Environ 536:793–802. https://doi.org/10.1016/j.scitotenv.2015.07.099
Papadakis E-N, Tsaboula A, Vryzas Z, Kotopoulou A, Kintzikoglou K, Papadopoulou-Mourkidou E (2018) Pesticides in the rivers and streams of two river basins in northern Greece. Sci Total Environ 624:732–743. https://doi.org/10.1016/j.scitotenv.2017.12.074
Paradis D, Bérail G, Bonmatin J-M, Belzunces LP (2014) Sensitive analytical methods for 22 relevant insecticides of 3 chemical families in honey by GC–MS/MS and LC–MS/MS. Anal Bioanal Chem 406:621–633. https://doi.org/10.1007/s00216-013-7483-z
Parlak V (2018) Evaluation of apoptosis, oxidative stress responses, AChE activity and body malformations in zebrafish (Danio rerio) embryos exposed to deltamethrin. Chemosphere 207:397–403. https://doi.org/10.1016/j.chemosphere.2018.05.112
Pawar B, Jaralli J, Shendge A (2009) Toxicity and impact of deltamethrin on glycogen level of freshwater fish Puntius chrysopterus (Mc Clelland). J Exp Zool Ind 12:319–323
Piner P, Üner N (2012) Oxidative and apoptotic effects of lambda-cyhalothrin modulated by piperonyl butoxide in the liver of Oreochromis niloticus. Environ Toxicol Pharmacol 33:414–420. https://doi.org/10.1016/j.etap.2012.01.001
Poletta GL, Gigena F, Loteste A, Parma MJ, Kleinsorge EC, Simoniello MF (2013) Comet assay in gill cells of Prochilodus lineatus exposed in vivo to cypermethrin. Pest Biochem Physiol 107:385–390. https://doi.org/10.1016/j.pestbp.2013.10.007
Prusty AK, Kohli MPS, Sahu NP, Pal AK, Saharan N, Mohapatra S, Gupta SK (2011) Effect of short term exposure of fenvalerate on biochemical and haematological responses in Labeo rohita (Hamilton) fingerlings. Pest Biochem Physiol 100:124–129. https://doi.org/10.1016/j.pestbp.2011.02.010
Qi H, Ma P, Li H, You JJ (2015) Assessment of sediment risk in the north end of Tai Lake, China: integrating chemical analysis and chronic toxicity testing with Chironomus dilutus. Arch Environ Contam Toxicol 69:461–469. https://doi.org/10.1007/s00244-015-0162-7
Qiang C-k, Feng W-j, Hu C-x, Wang S-y, Zhou B-y, Wang S-s, Qin Y-h (2013) Characteristics and evaluation of pesticide residues in surface soils and grapes from main grape-producing areas of Xuzhou city. Acta Agric Zhejiangensis 25:293–297
Rafique N, Tariq SR, Ahmed D (2016) Monitoring and distribution patterns of pesticide residues in soil from cotton/wheat fields of Pakistan. Environ Monit Assess 188:695. https://doi.org/10.1007/s10661-016-5668-6
Raja V, Velmurugan B, Selvanayagam M, Ambrose T (2010) Investigation of acute toxicity of synthetic pyrethroid Fenvalerate in fish Cyprinus carpio. Pollut Res 29:27–30
Rathnamma V, Vijayakumar M, Philip GJ (2009) Effect of deltamethrin on pyruvate and lactate of freshwater fish Labeo rohita. J Ecotoxicol Environ Monit 19:129–134
Rawn DFK, Judge J, Roscoe V (2010) Application of the QuEChERS method for the analysis of pyrethrins and pyrethroids in fish tissues. Anal Bioanal Chem 397:2525–2531. https://doi.org/10.1007/s00216-010-3786-5
Regnery J, Friesen A, Geduhn A, Göckener B, Kotthoff M, Parrhysius P, Petersohn E, Reifferscheid G, Schmolz E, Schulz RS, Schwarzbauer J, Brinke M (2018) Rating the risks of anticoagulant rodenticides in the aquatic environment: a review. Environ Chem Lett. https://doi.org/10.1007/s10311-018-0788-6
Ren Q, Zhang T, Li S, Ren Z, Yang M, Pan H, Xu S, Qi L, Chon T-S (2016) Integrative characterization of toxic response of zebra fish (Danio rerio) to deltamethrin based on AChE activity and behavior strength. Biomed Res Int 2016:7309184. https://doi.org/10.1155/2016/7309184
Riederer AM, Smith KD, Barr DB, Hayden SW, Hunter RE, Ryan PB (2010) Current and historically used pesticides in residential soil from 11 homes in Atlanta, Georgia, USA. Arch Environ Contam Toxicol 58:908–917. https://doi.org/10.1007/s00244-009-9439-z
Rodríguez-Estrada J, Sobrino-Figueroa AS, Martínez-Jerónimo F (2016) Effect of sublethal α-cypermethrin exposure on main macromolecules concentration, energy content, and malondialdehyde concentration in free-feeding Danio rerio larvae. Fish Physiol Biochem 42:859–868. https://doi.org/10.1007/s10695-015-0180-4
Saha S, Kaviraj A (2013) Dietary ascorbic acid as a means to counter the stress of cypermethrin on the growth of freshwater catfish Heteropneustes fossilis. Toxicol Ind Health 29:468–473. https://doi.org/10.1177/0748233712436642
Sakr S, Jamal S, Lail A (2005) Fenvalerate induced histopathological and histochemical changes in the liver of the catfish, Clarias gariepinus. J Appl Sci Res 1:263–267
Sangchan W, Bannwarth M, Ingwersen J, Hugenschmidt C, Schwadorf K, Thavornyutikarn P, Pansombat K, Streck T (2014) Monitoring and risk assessment of pesticides in a tropical river of an agricultural watershed in northern Thailand. Environ Monit Assess 186:1083–1099. https://doi.org/10.1007/s10661-013-3440-8
Sapana Devi M, Gupta A (2014) Sublethal toxicity of commercial formulations of deltamethrin and permethrin on selected biochemical constituents and enzyme activities in liver and muscle tissues of Anabas testudineus. Pest Biochem Physiol 115:48–52. https://doi.org/10.1016/j.pestbp.2014.08.004
Sarıkaya R (2009) Investigation of acute toxicity of alpha-cypermethrin on adult nile tilapia (Oreochromis niloticus L.). Turk J Fish Aquat Sci 9:85–89
Satyavardhan K (2013) A comparative toxicity evaluation and behavioral observations of fresh water fishes to Fenvalerate. Middle East J Sci Res 13:133–136. https://doi.org/10.5829/idosi.mejsr.2013.13.2.813
Sewell IG, McKenzie J 2006 Gamma-cyhalothrin: acute toxicity to zebra fish (Brachydanio rerio), SafePharm laboratories project no. 2119/0015. The Dow Chemical Company report 050608
Shaluei F, Hedayati A, Kolangi H, Jahanbakhshi A, Baghfalaki M (2012) Evaluation of the acute toxicity of cypermethrin and its effect on behavioral responses of Caspian roach (Rutilus rutilus caspicus) and silver carp (Hypophthalmicthys molitrix). Glob Vet 9:215–219. https://doi.org/10.5829/idosi.gv.2012.9.2.63235
Shi X, Gu A, Ji G, Li Y, Di J, Jin J, Hu F, Long Y, Xia Y, Lu C, Song L, Wang S, Wang X (2011) Developmental toxicity of cypermethrin in embryo-larval stages of zebrafish. Chemosphere 85:1010–1016. https://doi.org/10.1016/j.chemosphere.2011.07.024
Singh RN (2017) Acute toxicity of synthetic pyrethroid cypermethrin to an indigenous major carp, Cirrhinus mrigala (Ham.). Ind J Sci Res 15:132–137
Singh PB, Singh V (2008) Cypermethrin induced histological changes in gonadotrophic cells, liver, gonads, plasma levels of estradiol-17β and 11-ketotestosterone, and sperm motility in Heteropneustes fossilis (Bloch). Chemosphere 72:422–431. https://doi.org/10.1016/j.chemosphere.2008.02.026
Singh S, Chaudhary R, Gaur K (2007) A study of toxicity and behaviour of freshwater fish, Channa punctatus (Bloch) after intoxication of carbamate and synthetic pyrethroid fenvalerate. J Ecophysiol Occup Health 7:39–43
Singh SK, Singh SK, Yadav RP (2010) Toxicological and biochemical alterations of cypermethrin (synthetic pyrethroids) against freshwater Teleost fish Colisa fasciatus at different season. World J Zool 5:25–32
Singh J, Singh S, Datta S, Dutta J, Dhanjal DS, Saini A, Singh J (2015) Toxicological effects of Lambda-cyhalothrin on liver, kidney and testis of Indian catfish Clarias batrachus. Toxicol Int 22:128–136
Singh S, Singh N, Kumar V, Datta S, Wani AB, Singh D, Singh K, Singh J (2016) Toxicity, monitoring and biodegradation of the fungicide carbendazim. Environ Chem Lett 14:317–329. https://doi.org/10.1007/s10311-016-0566-2
Singh S, Kumar V, Chauhan A, Datta S, Wani AB, Singh N, Singh J (2018a) Toxicity, degradation and analysis of the herbicide atrazine. Environ Chem Lett 16:211–237. https://doi.org/10.1007/s10311-017-0665-8
Singh S, Tiwari RK, Pandey RS (2018b) Evaluation of acute toxicity of triazophos and deltamethrin and their inhibitory effect on AChE activity in Channa punctatus. Toxicol Rep 5:85–89. https://doi.org/10.1016/j.toxrep.2017.12.006
Soderlund DM (2012) Molecular mechanisms of pyrethroid insecticide neurotoxicity: recent advances. Arch Toxicol 86:165–181. https://doi.org/10.1007/s00204-011-0726-x
Soderlund DM, Clark JM, Sheets LP, Mullin LS, Piccirillo VJ, Sargent D, Stevens JT, Weiner ML (2002) Mechanisms of pyrethroid neurotoxicity: implications for cumulative risk assessment. Toxicol Ind Health 171:3–59. https://doi.org/10.1016/S0300-483X(01)00569-8
Srinivasa Rao S, Balakrishna Naik K, Satyanarayana S, Gopala Rao N (2018) Haematological changes induced by the deltamethrin a synthetic pyrethroid technical grade and 11% EC (Decis) in the fish Ctenopharyngodon idella (Valenciennes). J Innov Pharm Biol Sci 5:128–134
Srivastav AK, Srivastava SK, Mishra D, Srivastav SK (2010) Deltamethrin-induced alterations in serum calcium and prolactin cells of a freshwater teleost, Heteropneustes fossilis. Toxicol Environ Chem 92:1857–1864. https://doi.org/10.1080/02772248.2010.482063
Stalin SI, Kiruba S, Das SSM (2008) A comparative study on the toxicity of a synthetic pyrethroid, deltamethrin and a neem based pesticide, azadirachtin to Poecilia reticulata Peters 1859 (Cyprinodontiformes: Poeciliidae). Turk J Fish Aquat Sci 8:1–5
Stankovic S, Kalaba P, Stankovic AR (2014) Biota as toxic metal indicators. Environ Chem Lett 12:63–84. https://doi.org/10.1007/s10311-013-0430-6
Stará A, Zuskova E, Machova J, Priborsky J, Velisek J (2015) Effects of acute exposure to deltamethrin and recovery time on common carp (Cyprinus carpio L.). Neuro Endocrinol Lett 36:133–140
Sun B-Q, Wang F, Li H-Z, You JJ (2015) Occurrence and toxicity of sediment-associated contaminants in Guangzhou College City and its adjacent areas: the relationship to urbanization. Arch Environ Contam Toxicol 68:124–131. https://doi.org/10.1007/s00244-014-0097-4
Sun D, Wei Y, Li H, Yi X, You J (2016) Insecticides in sediment cores from a rural and a suburban area in South China: a reflection of shift in application patterns. Sci Total Environ 568:11–18. https://doi.org/10.1016/j.scitotenv.2016.05.202
Suvetha L, Saravanan M, Hur J-H, Ramesh M, Krishnapriya K (2015) Acute and sublethal intoxication of deltamethrin in an Indian major carp, Labeo rohita: hormonal and enzymological responses. J Basic Appl Zool 72:58–65. https://doi.org/10.1016/j.jobaz.2015.04.005
Szpyrka E (2014) Assessment of consumer exposure related to improper use of pesticides in the region of southeastern Poland. Environ Monit Assess 187:4140. https://doi.org/10.1007/s10661-014-4140-8
Tandon S, Srivastav P, Mukherjee S, Saharan N (2005) Effect of deltamethrin and fenvalerate (short term exposure) on the growth and feed conversion of Indian major carp, Catla catla fingerlings. J Aquat Biol 20:177–183
Tiwari S, Tiwari R, Singh A (2012) Impact of cypermethrin on fingerlings of common edible carp (Labeo rohita). Sci World J 2012:1–7. https://doi.org/10.1100/2012/291395
Tu W, Xu C, Lu B, Lin C, Wu Y, Liu W (2016) Acute exposure to synthetic pyrethroids causes bioconcentration and disruption of the hypothalamus–pituitary–thyroid axis in zebrafish embryos. Sci Total Environ 542:876–885. https://doi.org/10.1016/j.scitotenv.2015.10.131
Ullah S (2015) Protective role of vitamin C against cypermethrin induced toxicity in Labeo rohita (Ham.): biochemical aspects, M. Phil. thesis, Department of Animal Sciences, Quaid-i-Azam University, Islamabad, Pakistan
Ullah S, Li Z (2018) Hydro-electric power in the Panjkora basin at the expense of environmental deterioration and biodiversity loss—immediate action required for mitigation. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-018-3610-x
Ullah R, Zuberi A, Ullah S, Ullah I, Dawar FU (2014) Cypermethrin induced behavioral and biochemical changes in mahseer, Tor putitora. J Toxicol Sci 39:829–836. https://doi.org/10.2131/jts.39.829
Ullah R, Zuberi A, Naeem M, Ullah S (2015) Toxicity to hematology and morphology of liver, brain and gills during acute exposure of Mahseer (Tor putitora) to cypermethrin. Int J Agric Biol 17:199–204
Ullah S, Hasan Z, Dhama K (2016a) Toxic effects of endosulfan on behaviour, protein contents and antioxidant enzyme system in gills, brain, liver and muscle tissues of rohu, Labeo rohita. Int J Pharmacol 12:1–10. https://doi.org/10.3923/ijp.2016.1.10
Ullah S, Begum M, Ahmad S, Dhama K (2016b) Genotoxic effect of endosulfan at sublethal concentrations in Mori (Cirrhinus mrigala) fish using single cell gel electrophoresis (comet) assay. Int J Pharmacol 12:169–176. https://doi.org/10.3923/ijp.2016.169.176
Ullah S, Begum M, Dhama K, Ahmad S, Hassan S, Alam I (2016c) Malathion induced DNA damage in freshwater fish, Labeo rohita (Hamilton, 1822) using alkaline single cell gel electrophoresis. Asian J Anim Vet Adv 11:98–105. https://doi.org/10.3923/ajava.2016.98.105
Ullah S, Hasan Z, Zorriehzahra MJ, Ahmad S (2017) Diagnosis of endosulfan induced DNA damage in rohu (Labeo rohita, Hamilton) using comet assay. Iran J Fish Sci 16:138–149
Ullah S, Li Z, Hasan Z, Khan SU, Fahad S (2018a) Malathion induced oxidative stress leads to histopathological and biochemical toxicity in the liver of rohu (Labeo rohita, Hamilton) at acute concentration. Ecotoxicol Environ Saf 161:270–280. https://doi.org/10.1016/j.ecoenv.2018.06.002
Ullah S, Zuberi A, Alagawany M, Farag MR, Dadar M, Karthik K, Tiwari R, Dhama K, Iqbal HM (2018b) Cypermethrin induced toxicities in fish and adverse health outcomes: its prevention and control measure adaptation. J Environ Manag 206:863–871. https://doi.org/10.1016/j.jenvman.2017.11.076
Ullah S, Li Z, Ul Arifeen MZ, Khan SU, Fahad S (2019) Multiple biomarkers based appraisal of deltamethrin induced toxicity in silver carp (Hypophthalmichthys molitrix). Chemosphere 214:519–533. https://doi.org/10.1016/j.chemosphere.2018.09.145
Ural MŞ, Sağlam N (2005) A study on the acute toxicity of pyrethroid deltamethrin on the fry rainbow trout (Oncorhynchus mykiss Walbaum, 1792). Pest Biochem Physiol 83:124–131. https://doi.org/10.1016/j.pestbp.2005.04.004
Valle AL, Mello FCC, Alves-Balvedi RP, Rodrigues LP, Goulart LR (2018) Glyphosate detection: methods, needs and challenges. Environ Chem Lett. https://doi.org/10.1007/s10311-018-0789-5
Vani T, Saharan N, Mukherjee SC, Ranjan R, Kumar R, Brahmchari RK (2011) Deltamethrin induced alterations of hematological and biochemical parameters in fingerlings of Catla catla (Ham.) and their amelioration by dietary supplement of vitamin C. Pest Biochem Physiol 101:16–20. https://doi.org/10.1016/j.pestbp.2011.05.007
Vani T, Saharan N, Roy SD, Ranjan R, Pal AK, Siddaiah GM, Kumar R (2012) Alteration in haematological and biochemical parameters of Catla catla exposed to sub-lethal concentration of cypermethrin. Fish Physiol Biochem 38:1577–1584. https://doi.org/10.1007/s10695-012-9650-0
Vasantharaja C, Pugazhendy K, Venkatesan S, Meenambal M, Prabakaran S, Jayachandran K (2012) Acute toxicity of cypermethrin and its impact on biochemical alteration in the freshwater fish Cirrhinus mrigala (Hamilton) and protective effect of Cardiospermum helicacabum (Linn). Int J Pharma Biol Arch 3:146–152
Velisek J, Svobodova Z, Machova J (2009a) Effects of bifenthrin on some haematological, biochemical and histopathological parameters of common carp (Cyprinus carpio L.). Fish Physiol Biochem 35:583–590. https://doi.org/10.1007/s10695-008-9258-6
Velisek J, Svobodova Z, Piackova V (2009b) Effects of acute exposure to bifenthrin on some haematological, biochemical and histopathological parameters of rainbow trout (Oncorhynchus mykiss). Vet Med 54:131–137
Velíšek J, Jurčíková J, Dobšíková R, Svobodová Z, Piačková V, Máchová J, Novotný L (2007) Effects of deltamethrin on rainbow trout (Oncorhynchus mykiss). Environ Toxicol Pharmacol 23:297–301. https://doi.org/10.1016/j.etap.2006.11.006
Velmurugan B, Selvanayagam M, Cengiz EI, Unlu E (2007) Histopathology of lambda-cyhalothrin on tissues (gill, kidney, liver and intestine) of Cirrhinus mrigala. Environ Toxicol Pharmacol 24:286–291. https://doi.org/10.1016/j.etap.2007.07.001
Vengayil DT, Singh J, Singh AL, Das V, Singh P (2011) Bioaccumulation of carbamate and pyrethroid insecticides in fishes of the river Gomti at Jaunpur during breeding season. J Ecophysiol Occup Health 11:1–8. https://doi.org/10.18311/jeoh/2011/2243
Venturini FP, de Moraes FD, Rossi PA, Avilez IM, Shiogiri NS, Moraes G (2018) A multi-biomarker approach to lambda-cyhalothrin effects on the freshwater teleost matrinxa Brycon amazonicus: single-pulse exposure and recovery. Fish Physiol Biochem. https://doi.org/10.1007/s10695-018-0566-1
Vieira CED, dos Reis Martinez CB (2018) The pyrethroid λ-cyhalothrin induces biochemical, genotoxic, and physiological alterations in the teleost Prochilodus lineatus. Chemosphere 210:958–967. https://doi.org/10.1016/j.chemosphere.2018.07.115
Vieira JCS, Braga CP, de Oliveira G, de Lima LA, de Queiroz JV, Cavecci B, Bittarello AC, Buzalaf MAR, Zara LF, de Magalhaes PP (2017) Identification of protein biomarkers of mercury toxicity in fish. Environ Chem Lett 15:717–724. https://doi.org/10.1007/s10311-017-0644-0
Vymazal J, Březinová T (2015) The use of constructed wetlands for removal of pesticides from agricultural runoff and drainage: a review. Environ Int 75:11–20. https://doi.org/10.1016/j.envint.2014.10.026
Wang S (2013) The study on determination of pyrethroid pesticide residues in tilapia and Water Central South University of Forestry and Technology, Hu’nan, China
Wang W, Cai DJ, Shan ZJ, Chen WL, Poletika N, Gao XW (2007) Comparison of the acute toxicity for gamma-cyhalothrin and lambda-cyhalothrin to zebra fish and shrimp. Regul Toxicol Pharmacol 47:184–188. https://doi.org/10.1016/j.yrtph.2006.09.002
Wang X, We Z, Yao W, Yang L, Song X, Gong Z (2010) Determination of 20 pesticides in esturine and coastal seawater by SPE column enrichment-gas chromatography. Anal Instrum 6:64–71
Wang N, Yi L, Shi L, Kong D, Cai D, Wang D, Shan Z (2012) Pollution level and human health risk assessment of some pesticides and polychlorinated biphenyls in Nantong of Southeast China. J Environ Sci 24:1854–1860. https://doi.org/10.1016/S1001-0742(11)61004-8
Wang W, Huang CJ, Zhang MC, Zhou Q, Li AM (2013) Study on status of regional water pollution by pesticides in China. Environ Prot Sci 39:5–9
Weston DP, Lydy MJ (2010) Urban and agricultural sources of pyrethroid insecticides to the Sacramento-San Joaquin Delta of California. Environ Sci Technol 44:1833–1840. https://doi.org/10.1021/es9035573
Weston DP, You J, Lydy MJ (2004) Distribution and toxicity of sediment-associated pesticides in agriculture-dominated water bodies of California’s Central Valley. Environ Sci Technol 38:2752–2759. https://doi.org/10.1021/es0352193
Weston DP, Asbell AM, Hecht SA, Scholz NL, Lydy MJ (2011) Pyrethroid insecticides in urban salmon streams of the Pacific Northwest. Environ Pollut 159:3051–3056. https://doi.org/10.1016/j.envpol.2011.04.008
Weston DP, Asbell AM, Lesmeister SA, Teh SJ, Lydy MJ (2014) Urban and agricultural pesticide inputs to a critical habitat for the threatened delta smelt (Hypomesus transpacificus). Environ Toxicol Chem 33:920–929. https://doi.org/10.1002/etc.2512
Wongsa N, Burakham R (2012) A simple solid-phase extraction coupled to high-performance liquid chromatography—UV detection for quantification of pyrethroid residues in fruits and vegetables. Food Anal Met 5:849–855. https://doi.org/10.1007/s12161-011-9317-y
Woudneh MB (2006) Quantitative determination of pyrethroids, pyrethrins, and piperonyl butoxide in surface water by high-resolution gas chromatography/high-resolution mass spectrometry. J Agric Food Chem 54:6957–6962. https://doi.org/10.1021/jf0609431
Xu C, Tu W, Lou C, Hong Y, Zhao M (2010) Enantioselective separation and zebrafish embryo toxicity of insecticide beta-cypermethrin. J Environ Sci 22:738–743. https://doi.org/10.1016/S1001-0742(09)60171-6
Xu C, Li X, Jin M, Sun X, Niu L, Lin C, Liu W (2018) Early life exposure of zebrafish (Danio rerio) to synthetic pyrethroids and their metabolites: a comparison of phenotypic and behavioral indicators and gene expression involved in the HPT axis and innate immune system. Environ Sci Pollut Res 25:12992–13003. https://doi.org/10.1007/s11356-018-1542-0
Yang L, Wen Y-Y, Gong Z-B (2010) Determination of pyrethroid pesticides in estuarine and coastal sediments by accelerated solvent extraction and liquid chromatography–tandem mass spectrometry. Chin J Analyt Chem 38:968–972. https://doi.org/10.3724/SP.J.1096.2010.00968
Yang Y, Ma H, Zhou J, Liu J, Liu W (2014) Joint toxicity of permethrin and cypermethrin at sublethal concentrations to the embryo-larval zebrafish. Chemosphere 96:146–154. https://doi.org/10.1016/j.chemosphere.2013.10.014
Yang Y, Ye X, He B, Liu J (2016) Cadmium potentiates toxicity of cypermethrin in zebrafish. Environ Toxicol Chem 35:435–445. https://doi.org/10.1002/etc.3200
Yang Y, Wu N, Wang C (2018) Toxicity of the pyrethroid bifenthrin insecticide. Environ Chem Lett 16:1377–1391. https://doi.org/10.1007/s10311-018-0765-0
Yao L-X, Huang L-X, Li G-L, Yang B-M, He Z-H, Zhou C-M, Guo B (2011) State of pesticide residue in litchi orchard soils in Guangxi and Fujian, China. Chin J Eco-Agric 19:907–911. https://doi.org/10.3724/SP.J.1011.2011.00907
Yildirim MZ, Benlı AÇK, Selvı M, Özkul A, Erkoç F, Koçak O (2006) Acute toxicity, behavioral changes, and histopathological effects of deltamethrin on tissues (gills, liver, brain, spleen, kidney, muscle, skin) of Nile tilapia (Oreochromis niloticus L.) fingerlings. Environ Toxicol 21:614–620. https://doi.org/10.1002/tox.20225
Yılmaz M, Gül A, Erbaşlı K (2004) Acute toxicity of alpha-cypermethrin to guppy (Poecilia reticulata, Pallas, 1859). Chemosphere 56:381–385. https://doi.org/10.1016/j.chemosphere.2004.02.034
Yu X, Yang H (2017) Pyrethroid residue determination in organic and conventional vegetables using liquid–solid extraction coupled with magnetic solid phase extraction based on polystyrene-coated magnetic nanoparticles. Food Chem 217:303–310. https://doi.org/10.1016/j.foodchem.2016.08.115
Yun P, Liu Y, Yang L, Lu Y (2015) The application of freezing-drying method for determination of 7 kinds of pyrethroid pesticides residues in dried jujube by gas chromatography. J Food Saf Qual 6:3855–3862
Yurtkuran Z, Saygı Y (2013) Assessment of pesticide residues in Karaboğaz Lake from Kızılırmak Delta, Turkey. Bull Environ Contam Toxicol 91:165–170. https://doi.org/10.1007/s00128-013-1037-0
Zhang J, Gao H, Peng B, Li S, Zhou Z (2011) Comparison of the performance of conventional, temperature-controlled, and ultrasound-assisted ionic liquid dispersive liquid–liquid microextraction combined with high-performance liquid chromatography in analyzing pyrethroid pesticides in honey samples. J Chromatogr A 1218:6621–6629. https://doi.org/10.1016/j.chroma.2011.07.102
Zhang J, Zhang J, Liu R, Gan J, Liu J, Liu W (2016) Endocrine-disrupting effects of pesticides through interference with human glucocorticoid receptor. Environ Sci Technol 50:435–443. https://doi.org/10.1021/acs.est.5b03731
Zhang J, Huang X, Liu H, Liu W, Liu J (2018) Novel pathways of endocrine disruption through pesticides interference with human mineralocorticoid receptors. Toxicol Sci 162:53–63. https://doi.org/10.1093/toxsci/kfx244
Zhao L, Lai Z, LI X, Wang C, Shui F, Zheng Y, Yang W (2013) Contamination and toxicity evaluation of pyrethroids in sediments of the Pearl River Estuary. Ecol Environ Sci 22:1408–1413
Zhao C, Peng S, Chen T, Xie Y, Zhao X, Wang J (2016) Ecological risk assessment of sediment-associated permethrin and esfenvalerate in Chaohu Lake and Taihu Lake watersheds. Acta Sci Circumst 36:1080–1091. https://doi.org/10.13671/j.hjkxxb.2015.0579
Zheng S, Chen B, Qiu X, Chen M, Ma Z, Yu X (2016) Distribution and risk assessment of 82 pesticides in Jiulong River and estuary in South China. Chemosphere 144:1177–1192. https://doi.org/10.1016/j.chemosphere.2015.09.050
Zhu H, Zhang D, J-z Z, G-m W, B-b T (2015) Determination of multiple pesticides and their metabolites in orchard soil by liquid chromatography–tandem mass spectrometry (LC–MS/MS). J Southwest Univ (Nat Sci) 11:144–150
Acknowledgements
The author S. Ullah has been supported by the Chinese Scholarship Council for his Ph.D. study.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ullah, S., Li, Z., Zuberi, A. et al. Biomarkers of pyrethroid toxicity in fish. Environ Chem Lett 17, 945–973 (2019). https://doi.org/10.1007/s10311-018-00852-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-018-00852-y