Abstract
Anticoagulant rodenticides are used worldwide to control commensal rodents for hygienic and public health reasons. As anticoagulants act on all vertebrates, risk is high for unintentional poisoning of terrestrial and aquatic wildlife. Causative associations have been demonstrated for the unintended poisoning of terrestrial nontarget organisms. However, behavior and fate of anticoagulant rodenticides in the aquatic environment have received minimal attention in the past despite considerable acute toxicity of several anticoagulants to aquatic species such as fish. In light of recent regulatory developments in the European Union concerning rodenticides, we critically review available information on the environmental occurrence, fate, and impact of anticoagulant rodenticides in the aquatic environment and identify potential risks and routes of exposure as well as further research needs. Recent findings of anticoagulant rodenticides in raw and treated wastewater, sewage sludge, estuarine sediments, suspended particulate matter, and liver tissue of freshwater fish in the low ng/L and µg/kg range, respectively, demonstrate that the aquatic environment experiences a greater risk of anticoagulant rodenticide exposure than previously thought. While the anticoagulant’s mechanism of action from the molecular through cellular levels is well understood, substantial data gaps exist regarding the understanding of exposure pathways and potential adverse effects of chronic exposure with multiple active ingredients. Anticoagulants accumulating in aquatic wildlife are likely to be transferred in the food chain, causing potentially serious consequences for the health of wildlife and humans alike.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In developed countries, rodenticides are primarily used to control commensal rodents such as brown rat (Rattus norvegicus), roof rat (R. rattus), and house mice (Mus spp.) for hygienic and public health reasons, in agricultural animal husbandry, in the food industry, and to a lesser extent for storage and material protection. Rodents pose a hazard to human health because they carry and transmit a vast array of diseases to humans and their domesticated animals (Battersby 2015). A particular problem in industrialized countries is the high number of brown rats in sewer systems of cities, where they find shelter and food. Sewer systems may also serve as hidden pathways for rats to move freely and undiscovered between their nests and potential food sources. Although rats in sewers are not a problem by themselves as they do not damage properly installed and intact pipes, they roam between subsurface and surface, and their population must be controlled to prevent health risks or costly damage (Lund 2015).
There are many different biocidal products registered as rodenticides worldwide. They can be grouped together depending on their mode of application, e.g., poisoned bait, poisonous gas, contact foam, as well as speed of action, i.e., acute, subacute, and chronic (Buckle and Eason 2015). Anticoagulant rodenticides are the most effective and commonly used active ingredients of these biocidal products and fall into the category of slow-acting compounds. Anticoagulant rodenticides inhibit the vitamin K epoxide reductase enzyme involved in the blood coagulation process of warm-blooded vertebrates (mammals, birds) and thereby disrupt the recycling of vitamin K1 (phylloquinone). All anticoagulant rodenticides are either derivatives of 4-hydroxycoumarin or indane-1,3-dione and are structurally similar, but variations exist in their toxicity to target rodents. The exact mechanism of inhibition of clotting caused by hydroxycoumarin-related anticoagulation is described elsewhere (Buckle and Eason 2015; Rattner and Mastrota 2018). An effective dose of anticoagulant rodenticide must be ingested to have a sufficiently prolonged effect in blocking the vitamin K cycle and causing failure of the blood clotting mechanism. Poisoned animals die via internal hemorrhage. Active ingredients such as warfarin, coumatetralyl, and chlorophacinone that were commercialized between 1950 and 1970 are categorized as first-generation anticoagulant rodenticides. The more potent hydroxycoumarin derivatives difenacoum, brodifacoum, bromadiolone, and flocoumafen as well as the thiocoumarin derivative difethialone were developed and marketed in the mid-seventies and mid-eighties, respectively, to overcome warfarin resistance in rodents and are known as second-generation anticoagulant rodenticides. In recent years, ready-to-use loose, paste, and solid bait formulations are predominantly used during chemical rodent control. Bait formulations containing first-generation anticoagulant rodenticides generally require multiple feeding of target organisms until a lethal effect is achieved whereas second-generation anticoagulant rodenticides are more toxic and single feeding is often sufficient for a lethal dose. The delayed action of anticoagulant rodenticides prevents the development of conditioned taste aversion or bait shyness by rodents (Buckle and Eason 2015).
As anticoagulant rodenticides act on all vertebrates, risk is high for unintentional poisoning of wildlife and domesticated animals. Wildlife exposure generally occurs via three pathways: through direct ingestion of rodenticide bait by nontarget species (primary exposure), by take-up of primarily or secondarily exposed individuals through predators or scavengers (secondary and tertiary exposure), or from consumption of terrestrial or aquatic organisms that have been exposed to anticoagulant rodenticides via emissions to the environment (secondary poisoning via environmental emissions). Invertebrates may also be at risk from primary poisoning as a result of bait applications (Liu et al. 2015). Pathways and important aspects of wildlife exposure to anticoagulant rodenticides in the aquatic environment are illustrated in Fig. 1. Second-generation anticoagulant rodenticides were classified as potentially persistent, bioaccumulative, and toxic substances and their release into the environment should be minimized. Despite the consideration of ‘candidates for substitution’ under European Union legislation, economic relevance of anticoagulant rodenticides in the rodenticide market is still high as no chemical alternatives that are sufficiently effective but less critical are currently approved. However, recent developments gear toward their substitution with less critical active substances. In addition, the implementation of a third generation to minimize ecotoxicological risks associated with the use of second-generation anticoagulant rodenticides without losing their efficacy was suggested (Damin-Pernik et al. 2016, 2017). Currently, alpha-bromadiolone is under evaluation as a new active substance within product type 14 (rodenticides) by the European Chemicals Agency. One important aspect in this development is that the economic viability of anticoagulant rodenticide use for rodent control depends not only on the cost of bait but also on the mode of application and required risk mitigation practices (Jacob and Buckle 2018). In principle, a wide range of risk mitigation measures must be deployed when anticoagulant rodenticides are used.
Pathways and aspects of wildlife exposure to anticoagulant rodenticides (AR) in the aquatic environment adapted from Geduhn (2015). Wildlife exposure generally occurs via three pathways: through direct ingestion of rodenticide bait by nontarget species (primary exposure), by take-up of primarily or secondarily exposed individuals through predators or scavengers (secondary and tertiary exposure), or from consumption of terrestrial or aquatic organisms that have been exposed to anticoagulant rodenticides via emissions to the environment (secondary poisoning via environmental emissions)
Environmental exposure to anticoagulant rodenticides may result during manufacture of the active substance, formulation of the biocidal product, application of baits (intended and improper use, respectively), and (inadequate) disposal of baits. Two recently published edited books attempt to gather available information on the environmental risks associated with rodent control using anticoagulant rodenticides and provide comprehensive information on their chemistry and toxicology as well as their environmental impact on terrestrial nontarget wildlife (Buckle and Smith 2015; van den Brink et al. 2018). However, surprisingly little is known about the environmental fate of active ingredients after their release from baits, rodent carcasses and feces during outdoor rodent control in urban and suburban settings, e.g., in and around sewer systems, open space near shorelines, or around buildings and constructions. With the exception of sewers and burrows, deployment of anticoagulant rodenticide containing bait during outdoor rodent control usually happens by using tamper-resistant bait stations to minimize exposure to nontarget organisms and the environment. Nevertheless, a diffuse release of active ingredients and respective transformation and metabolic residues from rodents and other nontarget wildlife via urine and feces may be anticipated around controlled areas. Some second-generation anticoagulant rodenticides are mainly excreted as unchanged compounds, whereas the metabolic transformation of warfarin and chlorophacinone in rats is governed by hydroxylation (Larsen 2003). Baits approved for use in sewers usually consist of wax or fat. As the active ingredients are not chemically bound to the bait material in these product formulations, they will be released upon disintegration of bait blocks during prolonged exposure to moist or wet conditions, e.g., fluctuating water levels in baited manholes, steam. Little information is available about their physical stability and release rates of active ingredient when exposed to moist or wet environments (Nakagawa et al. 2015). Resistance and minimal leaching of weather-proof baits containing brodifacoum even after 500 mm of rainfall was reported by Booth et al. (2010). However, several studies reported the occurrence of anticoagulant rodenticides in sewage sludge (Gómez-Canela and Lacorte 2016), raw and treated wastewater (Gómez-Canela et al. 2014a, b), suspended particulate matter (Kotthoff et al. 2018), estuarine sediments (Cavanagh and Ward 2014), and liver tissue of freshwater fish from impacted streams (Cavanagh and Ward 2014; Kotthoff et al. 2018). These findings suggest that anticoagulant rodenticides might enter the aquatic environment via wastewater treatment plants and direct stormwater discharge into surface water bodies after baiting in and around sewer systems and drainages. Cleaning processes after indoor rodent control operation may also result in (minor) environmental exposure via the sewage system (Larsen 2003). However, in New Zealand there is increasing evidence that anticoagulant rodenticide application for both household rodent control and field pest management contributes to the contamination of aquatic wildlife, presumably through carcasses of poisoned animals entering water bodies, rather than direct contamination by bait (Cavanagh and Ward 2014). Besides biocidal use, pharmaceutical use of vitamin K antagonists should also be taken into account when assessing potential environmental exposure pathways and sources of anticoagulant rodenticides. Oral anticoagulants of the 4-hydroxycoumarin class such as warfarin (trade name Coumadin®), phenprocoumon (Marcumar®, Falithrom®), and acenocoumarol (Sintrom®) are commonly used to treat thromboembolic diseases (Lin et al. 2013).
To overcome the aforementioned knowledge gaps, we critically reviewed available information on the environmental fate and impact of anticoagulant rodenticides in the aquatic environment and direct and indirect routes of exposure. Moreover, we identified potential risks as well as further research needs. Anticoagulants entering the aquatic environment and accumulating in aquatic wildlife are likely to be transferred in the food chain, causing potentially serious consequences for the health of wildlife and humans alike. In light of recent regulatory developments in the European Union concerning rodenticides, risk mitigation measures and instructions for use of anticoagulant rodenticides are discussed with a focus on Germany. An overview of active substances and products registered worldwide for biocidal use and plant protection is provided elsewhere (Jacob and Buckle 2018).
Regulatory aspects of rodent control in the European Union and Germany
In the European Union, rodenticides need to be authorized prior to being made available on the market. European Union authorization of rodenticides distinguishes between their application as biocides for the protection of human health and manmade materials and plant protection products, respectively. Prior to European Union-wide approval of active substances, they are subject to similar but separate risk assessment processes in either sector within a review procedure involving all European Union Member States. After an active substance is approved, national product authorizations can be granted in compliance with suitable risk mitigation measures. These measures are frequently published in the best practice guidelines at national and international level (UBA 2014; CRRU 2015; EBPF 2015). Because risk mitigation measures are set by each individual member state, a single commercial product may have more than one set of measures attached to its marketing authorizations across Europe (Elliott et al. 2016). Harmonization of anticoagulant rodenticide registration and marketing by combining expertise of registration authorities and streamlining procedures would be worthwhile (Jacob and Buckle 2018). Recently, at least the majority of anticoagulant rodenticide instructions for use and risk mitigation measures were harmonized within the European Union in the context of their re-approval as biocidal active substances in 2016.
The vast majority of rodenticides are applied as biocides. The new European Union Biocidal Products Regulation No. 528/2012 (European Union 2012) regulates the sale, supply, and use of biocidal products throughout the European Union. As of 2018, second-generation anticoagulant rodenticides continue to be authorized under the Biocidal Products Regulation for use as biocides to protect public health due to the lack of safe alternatives (ECHA 2017b). Yet, their re-authorization is subject to a set of strict risk mitigation measures and restrictions regarding their marketing. For example, anticoagulant rodenticide concentrates are solely available to industrial manufacturers, but ready-to-use product formulations can be registered for use by professionals and consumers. In general, anticoagulant rodenticide bait formulations consist of a single active ingredient. Eight active substances belonging to the class of anticoagulants are currently approved in the European Union for biocidal use, thereof three first-generation with maximum permissible concentrations of 0.079% (warfarin), 0.0375% (coumatetralyl), and 0.005% (chlorophacinone), and five second-generation anticoagulant rodenticides with maximum permissible concentrations of 0.0075% (difenacoum), 0.005% (bromadiolone, brodifacoum, flocoumafen), and 0.0025% (difethialone), respectively. The first-generation anticoagulant rodenticide warfarin is concurrently authorized for pharmaceutical use.
In several European Union Member States, bromadiolone (Italy, France, Netherlands, and Romania) and difenacoum (Italy, Portugal) are also approved as active ingredients in plant protection products according to the European Union Pesticides Database (assessed on January 22, 2018). Both compounds are listed as candidates for substitution. Nevertheless, a recent trend across the European Union is to abstain from anticoagulant rodenticides for plant protection and to restrict the use of biocidal second-generation anticoagulant rodenticides to professional users because of human and environmental risks. Hence, companies increasingly tend to register biocidal anticoagulant rodenticide formulations instead of registration in the plant protection sector. In Germany, authorization of plant protection products containing anticoagulants phased out and their domestic sales and exports stopped at the end of 2013 (BVL 2012). During emergency situations in plant protection that cannot be contained by other means, chlorophacinone is still permissible for limited and controlled use, e.g., against local vole outbreaks in 2015 (BVL 2015), with a maximum duration of 120 days according to article 53 of the Plant Protection Products Regulation No. 1107/2009 (European Union 2009). Few anticoagulant rodenticide bait formulations are registered in European Union Member States that contain two active ingredients, e.g., difenacoum and bromadiolone or difenacoum and brodifacoum, to increase potency and circumvent resistance. The majority of biocidal anticoagulant rodenticide products currently authorized in Germany contain difenacoum (51), bromadiolone (41), and brodifacoum (37) followed by warfarin (7), coumatetralyl (5), difethialone (4), chlorophacinone (4), and flocoumafen (3) according to the European Chemicals Agency Biocidal Products Database (assessed on April 11, 2018).
Based on the implementation of national risk mitigation measures, second-generation anticoagulant rodenticides, as of September 2013, may no longer be used in Germany by persons other than trained pest control operators and professional users providing an appropriate proof of qualification in the context of the new European Union Biocidal Products Regulation. Yet, a loophole exists. Despite the restricted use of second-generation anticoagulant rodenticide bait formulations in Germany, consumer sales are still permissible as national legal provisions on the sale of biocides are missing. First-generation anticoagulant rodenticides may still be used by consumers against mice and rats in indoor scenarios and immediately around buildings. Few solid bait block formulations containing chlorophacinone and warfarin, respectively, are currently authorized for application in sewer systems by professional users. None of the coumatetralyl products is permitted for use in sewer systems. However, with the withdrawal of contact powder formulations from the European market due to safety concerns, an alternative water-based foam formulation containing 0.4% coumatetralyl was authorized for professional users to apply in indoor areas such as access holes, cavity walls, and pipe works.
Due to scientifically proven teratogenic effects of warfarin and an assumed analogy because of similarities in structure and mode of action (Pieper et al. 2014), all anticoagulant rodenticide containing products were recently classified as toxic for reproduction in categories 1A or 1B (European Commission 2016) by the European Chemicals Agency. The classification applies to all products with a concentration of 0.003% (30 ppm) or more of the active substance and will further restrict the range of products authorized for consumer use. Products classified and labeled as toxic for reproduction are only approved for professional users with appropriate certification (European Union 2012). This 9th adaptation of Regulation No. 1272/2008 ‘Classification, labeling, and packaging of chemical substances’ (European Commission 2016) to technical and scientific progress is expected to have consequences for the European rodenticide market. On March 1, 2018, the derogation period ended for the sale of rodenticides for which the labeling does not comply. To circumvent limitations of use in the biocidal sector, a general shift from 0.005% (50 ppm) to less than 0.003% concentration of active ingredient in second-generation anticoagulant rodenticide products is expected as soon as the amendment is taking effect. Besides difethialone, concentrations of brodifacoum and flocoumafen can be reduced to below 0.003% without reducing their effectiveness whereas efficacy of difenacoum, bromadiolone, and first-generation products with less than 0.003% active ingredient is disputed due to observed regional resistance (Buckle and Eason 2015). Along with the active ingredients, anticoagulant rodenticide bait pack sizes are also changing. All baits approved for consumer use in the European Union will have a maximum pack size (European Commission 2017c). With this, a greater distinction between professional and consumer products is intended. It is also intended to prevent consumers from buying and storing large quantities of bait which could cause environmental hazards.
European biocidal anticoagulant rodenticide market
Due to the European Chemicals Agency implemented policies restricting rodent pest management by chemical rodenticides almost entirely to anticoagulants, they account for the largest market share on the European biocidal rodenticide market in recent years. On the contrary, a wider range of non-anticoagulants is available under the United States Environmental Protection Agency on the US rodenticide market (Jacob and Buckle 2018). Nevertheless, about 95% of the chemical control of rodents in the USA is carried out using anticoagulants (Liphatech 2013). Estimates on anticoagulant rodenticide sales are in the hundreds of millions of dollars annually in the USA and European countries (Rattner et al. 2014). Market research data valued the European rodenticide market at 226 million euros in 2016 and insinuated that the strict regulation on the use of rodenticides within the European Union led to stationary market growth in the past few years. As of late 2017, market analysts projected a compound annual growth rate of 5.77% over the next 5 years, which will likely be driven by the non-anticoagulant rodenticides segment (Market Data Forecast 2017).
In contrast to active ingredients used in plant protection products within the European Union, disclosure of biocide sales and use data is not required by European law. Unfortunately, national and global rodenticide market data are mostly considered confidential business information and up-to-date, open access national or European Union-wide biocidal anticoagulant rodenticide sales data under the new Biocidal Products Regulation are scarce (Elliott et al. 2016; Jacob and Buckle 2018). Compared with other pest control and plant protection products, the market for anticoagulant rodenticides is comparatively small and actual quantities of active ingredients applied as biocides appear minor compared to major pesticides and pharmaceuticals (Endepols 2002). Anticoagulant rodenticides accounted for approximately 3% of registered biocidal products in Germany in 2014 (Schmolz et al. 2014). Based on consumer research market data from 2012 (Parker 2013), the annual national use of anticoagulant rodenticides by pest control professionals in Germany was estimated to exceed 1000 metric tons of bait material, i.e., expenses of 10 million euros on anticoagulant rodenticide containing products by professional users. This represented roughly 50 kg of active ingredients (Schmolz et al. 2014). While existing data suggest that pest control professionals are among the main users of biocidal anticoagulant rodenticides in Germany, other important user groups are agribusinesses, local authorities, and household consumers (Barten 2014). In comparison, the total amount of anticoagulant rodenticides sold in Finland in 2014 was in the range of 250 metric tons based on annual sales volumes collected by the Finnish Safety and Chemicals Agency. Notably, only 5% thereof were supposedly used by pest control professionals in 2014 according to a survey that covered approximately 75% of pest control technicians operating in Finland (Koivisto et al. 2016).
Given the non-disclosure of detailed market sales data, extensive sectoral surveys shed further light on rodenticide usage patterns within the European Union (Murphy and Oldbury 2002; Dawson and Garthwaite 2004; Krüger and Solas 2010; Hughes et al. 2013; Wardlaw et al. 2016, 2017). Krüger and Solas (2010) conducted a survey among 508 municipalities in Germany in 2010 to gain insight into rat control in and around municipal sewer systems. The participating municipalities represented a population of approximately 15.3 million residents. Person equivalents of the surveyed municipalities ranged between less than 5000 and more than 100,000. Of the municipalities surveyed, 309 provided utilizable information regarding the use of anticoagulant rodenticide containing baits in their sewer systems. The annual domestic use of anticoagulant rodenticides in sewer baiting scenarios was projected as 870.5 metric tons of bait material and 50.3 kg of active ingredients, respectively. About 88% of surveyed municipalities employed pest control in and around their sewer systems, either through contracted pest control professionals or qualified staff. If prorated irrespective of person equivalents and sewage load, each of the approximately 300 municipalities surveyed applied on average 18.4 and 8.4 g/year active ingredient of second-generation and first-generation anticoagulant rodenticides, respectively. Bromadiolone had the highest proportion of 50%, followed by 30% difenacoum and 18% brodifacoum (Krüger and Solas 2010). On the contrary, very little use of anticoagulant rodenticides in sewer baiting scenarios was encountered in a recent Scottish survey representing 81% (4.37 million) of the Scottish population. Only 20% of the respondent local authorities reported sewer baiting activities with a total combined use of 34 kg bait material, i.e., less than 0.2 g active ingredient. Bromadiolone, difenacoum, and brodifacoum were the three most commonly used anticoagulants that were reported in sectoral surveys from the UK and Finland (Dawson and Garthwaite 2004; Koivisto et al. 2016; Wardlaw et al. 2016, 2017). Among the second-generation anticoagulant rodenticides, difenacoum, bromadiolone, and brodifacoum are estimated to have the highest market shares in Germany and France (Fig. 2). Yet, establishing a relationship between anticoagulant rodenticide market shares and their environmental occurrence is not straight forward given the lack of comprehensive data. For instance, the proportion of individuals burdened with different active substances is not expected to mirror national usage patterns exactly due to differences in persistence, bioaccumulation, and elimination profiles (Hughes et al. 2013). In addition, carryover of active ingredient in the manufacture line was observed when bait formulations with different active ingredients were processed in the same facility. Tosh et al. (2012) detected brodifacoum as a contaminant in four different ready-to-use loose bait formulations of the same brand that were not supposed to contain brodifacoum as active ingredient, constituting on average 9.8% (7.7–13.2%) of the total active ingredient detected in the bait. Levels of brodifacoum contamination ranged from 63 to 197 mg/g bait. Thus, brodifacoum residues were also detected in target organisms that consumed the contaminated baits (Tosh et al. 2012).
As mentioned before, pharmaceutical use of vitamin K antagonists should also be taken into account. The global warfarin market was valued at 300 million dollars in 2008 (Lin et al. 2013). Commissioned market research data by Oktay (2015) suggest that warfarin prescriptions declined from 87.5 to 72% through 2008–2014. Pharmaceutical use of warfarin as a blood-thinning agent is still widespread in the USA and the UK, whereas phenprocoumon (Germany, Austria, Belgium, Denmark, The Netherlands, and Switzerland) and acenocoumarol (Italy and Spain) are mainly used across continental Europe. For example, warfarin prescriptions in the UK averaged 800 kg annually between 2004 and 2008 (Kasprzyk-Hordern 2010). Pharmaceutical use of phenprocoumon and warfarin in Germany in 2016 can be roughly extrapolated to a total of 1226 kg and 31 kg of active ingredient, respectively, based on healthcare membership numbers and prescription statistics (UBA 2011; Schwabe et al. 2017).
Predicted environmental emissions
Aside from primary and secondary poisoning of terrestrial nontarget organisms after bait application (Liu et al. 2015; Alomar et al. 2018), very little is known about direct and indirect routes of exposure as well as anticoagulant’s distribution and fate in the aquatic environment (Fisher et al. 2012; Masuda et al. 2015). Professional pest control companies, which are among the main users of second-generation anticoagulant rodenticides in urban settings, apply rodenticides commonly ‘in and around buildings’ (warehouses, agribusinesses), municipal sewer systems, and public open space (Schmolz et al. 2014). Several studies hypothesized that the application of anticoagulant rodenticide containing baits in municipal sewer systems is a major emission source of anticoagulants in urban areas (Gómez-Canela et al. 2014a; Kotthoff et al. 2018). From the sewers, exposure of the aquatic environment most likely occurs via wastewater treatment plant effluents if anticoagulants are not efficiently removed during conventional wastewater treatment. Moreover, stormwater overflow structures in combined sewer systems that discharge highly diluted but untreated sewage directly into receiving surface waters when precipitation causes a surcharge within the system might pose another route. As a result, poisoned rodent carcasses might be flushed from their hiding places in the sewers directly into receiving streams, bypassing mechanical removal at the wastewater treatment plant.
In Germany, all professional users (as well as consumers) are obligated to follow national best practice guidelines for the application of rodenticides which have been implemented within the national biocidal product authorization. Ideally, non-chemical methods and products containing the least potent active ingredient should be used first to control pests. Apart from exceptional cases where difenacoum or bromadiolone containing bait may be constantly applied, permanent deployment of anticoagulant rodenticide containing baits to prevent rodent infestation or to monitor rodent activities is usually not permitted (European Commission 2017a, b). This and the considerable costs associated with rodent control motivated sewer baiting regimes to switch to pulsed baiting instead of surplus baiting. Colvin et al. (1998) demonstrated that sewer baiting requires a systematic approach with close review and adjustments of the baiting strategy based on the quantities and geographic patterns of bait consumption to successfully manage a rat population. Moreover, ineffective use of rodenticides can be misdiagnosed as resistance. According to Gras et al. (2012), surface infestation is not necessarily a reliable indicator of sewer infestation. To comply with national best practice guidelines, inspection of deployed baits after 2 weeks is mandatory during sewer baiting campaigns. Likewise, the collection and appropriate disposal of remaining bait and rodent carcasses is mandated to minimize the risks of environmental exposure (UBA 2014). Yet, nationwide compliance with these guidelines is difficult to assess and control. It has been assumed that the typical use of anticoagulants commonly violates the use instructions for rodenticides (Koivisto et al. 2016). Thus, the exact whereabouts of marketed quantities of active ingredients remain unclear.
In the context of the European Union project EUBEES 2 titled ‘Gathering, review and development of environmental emission scenarios for biocides’ (Larsen 2003), a method was established to estimate the initial release of anticoagulants from biocidal products to the primary receiving environmental compartments air, soil, and water, including separate calculations for emissions under normal and realistic worstcase conditions (available at http://echa.europa.eu/en/guidance-documents/guidance-on-biocides-legislation/emission-scenario-documents). This guideline is currently under revision. Further guidance on rodenticide emission pathways and the estimation of predicted environmental concentrations in receiving environmental compartments is provided by the European Chemicals Agency (ECHA 2017a). In general, degradation and distribution processes are taken into consideration for the calculation of predicted environmental concentrations for the aquatic compartment.
Worstcase aquatic and terrestrial predicted environmental concentrations of anticoagulant rodenticides based on default values in the emission-scenario document are summarized in Table 1. Very limited information is available regarding predicted environmental concentrations in fish (oral, predator). The suggested predicted environmental concentration of difethialone in whole fish based on wet weight is 6 µg/kg (eCA2016h) and 0.245 µg/kg for difenacoum (eCA 2016g). For difenacoum, it was assumed that secondary poisoning via the aquatic food chain would not be significant due to low water solubility and high adsorption tendency (eCA 2016g).
Due to stricter regulations within the European Union, the use of sewage sludge as fertilizer in agriculture has declined over the past decade. Sewage sludge is increasingly subject to energy recovery, e.g., anaerobic digestion followed by incineration, or thermal waste treatment. Organic compounds adsorbed to the sludge will decompose during incineration, representing a possible sink. This will likely affect the potential exposure of agricultural soils with anticoagulant rodenticides via this route (Table 1). Over the last couple of years, incineration of sludge substantially increased in Germany and a ban on using sewage sludge as fertilizers in agriculture beyond January 1, 2025 (at least for municipal wastewater treatment plants with more than 50,000 person equivalents) is planned.
Physicochemical properties and environmental fate and impact of anticoagulant rodenticides
Chemical structures and selected physicochemical properties of first- and second-generation anticoagulant rodenticides discussed in this review are summarized in Fig. 3 and Table 2. All of these compounds are either derivatives of indane-1,3-dione or 4-hydroxycoumarin. Despite the similarity of their structures, differences in the physicochemical properties of these compounds exist (Table 2).
Stereochemistry
Chlorophacinone, like coumatetralyl and warfarin, contains one optically active carbon and therefore exists as two enantiomers. Furthermore, chlorophacinone has a ß-tricarbonyl system resulting in keto-enol tautomerism (Medvedovici et al. 1997). While the ratio of the enantiomers in chlorophacinone formulations is classified as proprietary information (eCA 2016d), commercially available coumatetralyl and warfarin are generally a racemic mixture of R and S enantiomers containing equal parts of each isomer (eCA 2016b, f). Studies of the metabolic fate of the R and the S isomers of warfarin revealed that the two isomers were metabolized by different routes. Furthermore, S warfarin was shown to be five times more potent than R warfarin (Lewis et al. 1974).
Second-generation anticoagulant rodenticides such as bromadiolone, difenacoum, brodifacoum, difethialone, and flocoumafen have two asymmetric carbons in their chemical structure allowing them to exist in two diastereomeric forms (cis- and trans-isomers) and thus four enantiomeric species. Commercially available second-generation anticoagulant rodenticides are generally a mixture of their cis- and trans-isomers. Trans-isomers are the major diastereomeric form (70–90%) in commercialized bromadiolone (eCA 2016e), whereas flocoumafen, difenacoum, and brodifacoum are a mixture of approximately 50–80% cis-isomers and 20–50% trans-isomers (eCA 2016a, c, g). Commercial difethialone consists of more than 70% cis-isomers (eCA 2016h). Specifics regarding diastereomer ratios in commercial products as well as reasons thereof are mostly treated as proprietary information by manufacturers. As observed for the R and S isomers of warfarin, diastereomers of second-generation anticoagulant rodenticides have slightly different chemical and physical properties and likely have different biological activities (Hauck et al. 2016; Fourel et al. 2017a, b).
Fate and behavior in the environment
Water solubility of anticoagulant rodenticides at 20 °C and neutral pH is generally low, ranging between 267 and 460 mg/L for first-generation and 0.1–18.4 mg/L for second-generation anticoagulant rodenticides, respectively. Most anticoagulants were shown to be hydrolytically stable in water under environmentally relevant conditions, i.e., half-lives exceeding 1 year, and were not expected to partition to the atmosphere due to their low vapor pressure. However, very short photolytic half-lives, i.e., less than 1 day, of most second-generation anticoagulant rodenticides in water under sunlight exposure had been predicted or observed in previous assessments (Table 2). Estimated partition coefficients indicate substantial lipophilicity and bioaccumulation potential of second-generation anticoagulant rodenticides at neutral pH (Table 2). Of the targeted active ingredients, difenacoum, chlorophacinone, difethialone, and flocoumafen exhibit the highest organic carbon adsorption coefficients, warfarin the lowest (Table 2). Notably, second-generation anticoagulant rodenticides possess at least one polar group that can potentially ionize at neutral pH. However, the lipophilic character of brodifacoum, difethialone, and flocoumafen might prevent their ionization in natural aqueous environments. Previous studies demonstrated the effect of both pH and ionic strength on the mechanism of association of anticoagulant rodenticides with humic acid, a natural organic component of soils and sediments, by use of a C18 stationary phase (Andre et al. 2004, 2005). A strong tendency of the undissociated portion of the molecule to adsorb to organic matter combined with low water solubility and a high degree of photo-instability means that second-generation anticoagulant rodenticides are unlikely to remain in the water column of surface waters. Thus, their residues are more likely to persist and accumulate in aquatic compartments such as suspended particulate matter, (organic-rich) sediments, and biological tissue of aquatic organisms.
Available information on the route and rate of degradation in aerobic natural sediment water systems is summarized in European Union Competent Authority Assessment Reports (eCA 2016a, b, c, d, e, f, g, h). For brodifacoum, immediate adsorption to the sediment was noted followed by slow transformation with low levels of degradation products, i.e., less than 10% of the applied active substance. Brodifacoum was not expected to accumulate in sediments (eCA 2016c). Bromadiolone was shown to be fairly quickly degraded in soil under aerobic conditions with an estimated dissipation half-time of several days. However, its main metabolite bromadiolone ketone, which likely has a similar level of toxicity as bromadiolone, persisted in the soil in substantial quantities (eCA 2016e). Warfarin degraded fairly quickly in soils under aerobic and ambient temperature conditions after a short lag period (Lao and Gan 2012). Information on flocoumafen degradation rates in sediment water systems is lacking. Based on its low biodegradation potential in soil, i.e., half-life of 213 days at 20 °C, and high hydrolytical stability, flocoumafen is considered to be very persistent in water and sediment (eCA 2016a).
Toxicity and bioaccumulation in aquatic organisms
Recent reviews provide a comprehensive overview on toxicity and pharmacokinetics such as absorption, distribution, metabolism/biotransformation, and excretion of anticoagulant rodenticides in target and terrestrial nontarget organisms (McLeod and Saunders 2013; Horak et al. 2018; Rattner and Mastrota 2018). Anticoagulants bind strongly to vitamin K epoxide reductase. As the liver contains high levels of this protein, it is the main organ of accumulation and storage of anticoagulants. In general, they are eliminated in a biphasic process, with the rapid initial elimination of circulating compounds, followed by slower elimination from binding sites (Huckle et al. 1988). Although anticoagulants have been in use for decades, relatively little is known about their pharmaco- and toxicokinetics in aquatic organisms as well as effects of chronic exposure with multiple active ingredients. Studies about anticoagulant rodenticide exposure in terrestrial nontarget wildlife frequently report the presence of multiple second-generation anticoagulant rodenticides in a single individual, and occasionally a combination of first and second generation. The toxicity of multiple anticoagulant rodenticides in a single organism is expected to be on principal additive. Studies showed that the efficacy to inhibit the vitamin K epoxide reductase activity was similar between most second-generation anticoagulant rodenticide diastereomers in rodents, but different half-lives and persistence behaviors in biological tissues were observed between cis- and trans-isomers (Damin-Pernik et al. 2016, 2017; Fourel et al. 2017a). According to Fourel et al. (2017b), bromadiolone cis-stereoisomer, the minor component in commercial bromadiolone, did not contribute to the toxicity of the active ingredient in red kite due to metabolic differences in rodents and raptors. Lately, hepatic anticoagulant rodenticide residues above 100–200 µg/kg wet weight have been associated with mortalities in terrestrial nontarget organisms (Fourel et al. 2017b). A study about effects of chronic low-level brodifacoum exposure on the feline immune response, however, indicated species-specific anticoagulant insensitivity. Specific pathogen-free domestic cats did not exhibit any clinical signs of brodifacoum intoxication despite elevated hepatic levels of brodifacoum in the range of 1.67–1.94 mg/kg wet weight (Kopanke et al. 2018). In toxicokinetic studies with rats, brodifacoum showed a high potential for bioaccumulation. In all studies undertaken and at all dose levels tested, the liver retained the largest percentage of the dose, even very long time after dosing. Fisher et al. (2003) showed biphasic elimination of brodifacoum from rat liver, consisting of a more rapid initial phase up to 8 days after dosing, and a slower terminal phase. The liver-elimination half-life in rat was 113.5 days for brodifacoum and 26.2 days for warfarin. Moreover, toxicokinetic data suggest that brodifacoum may be more persistent, with a longer liver retention phase than bromadiolone and difenacoum (Hughes et al. 2013). Presumably, high-single-dose-potency second-generation anticoagulant rodenticides persist in the liver for more than 1 year (Fisher et al. 2003).
Today, the embryotoxic potential of warfarin is well accepted (Weigt et al. 2012; Buckle and Eason 2015). On the contrary, other anticoagulants with similar structures and mode of action have disputed embryotoxic potential (Buckle and Eason 2015). Nevertheless, embryotoxicity induced by bromadiolone exposure at a dose of 350 µg/L was demonstrated in the amphibian model organism African clawed frog (Xenopus laevis) adhering frog embryo teratogenesis assay—Xenopus standards (Ondracek et al. 2015). Teratogenicity and embryonic lethality in zebrafish (Danio rerio) under acute warfarin exposure at elevated concentrations of greater than or equal to 400 µM (123 mg/L) were reported by Weigt et al. (2012). Fernandez et al. (2014) showed that zebrafish larvae with chronic exposure to a 25-fold lower warfarin concentration experienced significant lethal and sublethal effects, such as hemorrhages, vascular calcification, and skeletal deformities. Interestingly, warfarin demonstrated no significant, measurable metabolism in native rainbow trout liver S9 fractions at a substrate concentration of 1 µM (308 µg/L) (Connors et al. 2013). Yet, a rapid decrease in warfarin levels to below the detection limit was observed in rainbow trout (Oncorhynchus mykiss) in less than 11 days after termination of warfarin exposure in a bioconcentration test (eCA 2016f). Connors et al. (2013) pointed to the possibility for tissue-specific expression of cytochrome isozymes involved in warfarin metabolism in fish, e.g., in the gills. No effect on the tested endpoint lethality was observed for rainbow trout when exposed to coumatetralyl at the 5 µg/L level over the duration of 21 days. Furthermore, quick depuration in fish with a dissipation half-live of approximately 14.5 h and low bioaccumulation potential was reported (eCA 2016b). It is presumed that the low-single-dose-potency anticoagulant warfarin persists in the liver for up to 1 month, whereas the moderate-single-dose-potency anticoagulant rodenticide coumatetralyl persists in liver for approximately 6 months (Fisher et al. 2003).
Due to considerable acute toxicity to aquatic species (Table 3) and high mortalities during bioconcentration tests, experimentally derived bioconcentration factors in fish are not available for all anticoagulants. Flocoumafen is considered very bioaccumulative because of its high bioconcentration factor of 24,300 (Table 4). The calculated brodifacoum bioconcentration factor of 35,648 in fish is also very high. The estimated depuration time of brodifacoum in whole fish according to the Organization for Economic Co-operation and Development guideline 305 using a Log POW of 6.12 is approximately 8 days (50% dissipation) and 34 days (95% dissipation), respectively (eCA 2016c). For bromadiolone and difenacoum, experimentally derived bioconcentration factors in fish were below the threshold of 2000 which defines bioaccumulative and very bioaccumulative (greater than 5000) substances according to European Union regulation No. 253/2011 (European Commission 2011). Bromadiolone exhibited a depuration time of more than 14 days to achieve 50% dissipation. Notably, the experimental bioconcentration factor of difenacoum in fish is lower than estimations based on the n-octanol—water partition coefficient (Table 4).
Unfortunately, no detailed information is available regarding second-generation anticoagulant rodenticide uptake and metabolism in fish. In an aquarium feeding trial by Empson and Miskelly (1999), 60 individuals of the marine fish species blue cod (Parapercis colias), spotty (Notolabrus celidotus), and variable triplefin (Forsterygion varium) were exposed to brodifacoum containing bait pellets for 1 h before being transferred to clean holding tanks for 3–4 weeks. After the experiment was terminated, 5% of fish showed hepatic brodifacoum residues according to the authors but no concentrations or reporting limits were provided (Empson and Miskelly 1999). Whole-body brodifacoum residues above 200–300 µg/kg wet weight were associated with mortalities in nontarget coastal marine fish (Pitt et al. 2015).
Analytical methods for anticoagulant rodenticides in environmental samples
Extraction and cleanup
Suitable extraction methods for individual anticoagulant rodenticide residues or mixtures thereof from environmental samples include a wide range of techniques, e.g., liquid–liquid extraction, solid–liquid extraction, and solid-phase extraction, depending on analyte-specific properties (Table 2) and sample type, e.g., biological tissue, sewage, soil/sediment, and water. Optimization of important parameters like composition, type, and pH of extraction solvents, solid/liquid rate volume of extraction solvents, and number of extraction cycles is crucial for each anticoagulant rodenticide residue to facilitate efficient and exhaustive extraction, especially when covering a wide range of different compounds. Traditional high-volume solvent extraction methods, e.g., pressurized liquid extraction, microwave-assisted extraction, and Soxhlet extraction, are frequently substituted by miniaturized extraction methods aiming at minimizing costs of sample preparation while reducing consumables and waste. In particular, the emergence of rapid multi-class, multi-residue analysis methods propelled the development of efficient, rapid, and simple sample preparation techniques. However, regardless of extraction technique, environmental samples (especially biological tissues and sewage) often yield complex extract matrices requiring extensive cleanup to remove co-extracted residues, e.g., lipids and proteins that interfere with quantitative analysis (Goldade et al. 1998; Huerta et al. 2012; Morrison et al. 2016).
Imran et al. (2015) reviewed published extraction and cleanup methods for anticoagulant rodenticides from biological tissues and discussed extraction performances as well as limitations. Several recent studies utilized ultrasound-assisted extraction to extract various pharmaceutical and/or biocidal anticoagulants from aquatic organisms (Magiera et al. 2015; de Solla et al. 2016; Kotthoff et al. 2018), sludge (Gómez-Canela and Lacorte 2016), suspended particulate matter (Kotthoff et al. 2018), and soil (Hernández et al. 2013). Reversed phase solid-phase extraction on various stationary phases was carried out for enrichment and cleanup of anticoagulants in aqueous samples such as raw wastewater, treated wastewater, surface water, and groundwater (Fisher et al. 2012; Gómez-Canela et al. 2014b; Watkins et al. 2014; Wode et al. 2015). Several anticoagulants were also sufficiently extracted from spiked water samples employing liquid–liquid extraction with ethyl acetate (Hernández et al. 2013; Gómez-Canela et al. 2014b). When Gómez-Canela et al. (2014b) investigated anticoagulant rodenticides in wastewater, better performance regarding the number of detected residues, recoveries, and reproducibility was achieved using solid-phase extraction on hydrophilic–lipophilic balanced or weak anion polymeric sorbent, respectively, compared to miniaturized liquid–liquid extraction. Chen et al. (2014) proposed an ionic liquid-based ultrasonic-assisted dispersive liquid–liquid microextraction method for highly effective extraction of trace bromadiolone and brodifacoum in environmental water samples.
Approaches such as QuEChERS (quick, easy, cheap, effective, rugged, and safe) appear to be suitable for the extraction of anticoagulant rodenticides from biological tissues such as aquatic organisms (Vudathala et al. 2010; Morrison et al. 2016). This approach generally relies on dispersive solid-phase extraction as a cleanup step after extraction to remove interferences from sample extracts. Several studies demonstrated that concurrently applying C18 and primary–secondary amine removes the majority of co-extracted materials by weight from moderately fatty fish tissue (Morrison et al. 2016). Besides mixed phase dispersive solid-phase extraction (Vudathala et al. 2010; Gómez-Canela and Lacorte 2016), gel-permeation chromatography (Hunter 1983b), normal phase solid-phase extraction using alumina or florisil cartridges (Jones 1996; Gómez-Canela and Lacorte 2016), and reversed phase solid-phase extraction using aminopropyl or hydrophilic-lipophilic balance cartridges (Goldade et al. 1998; Fisher et al. 2012; de Solla et al. 2016) were among the cleanup methods applied as an additional purification step to generate sample extracts suitable for quantitative analysis of anticoagulant rodenticides.
Qualitative and quantitative analysis
While coumatetralyl (hydroxycoumarin derivative) and chlorophacinone (indane-1,3-dione derivative) are well detectable by gas chromatography–mass spectrometry, gas chromatography-based methods have not proved reliable for the analysis of other anticoagulants because of either thermal degradation of the parent compound during chromatography, i.e., by heat of injection chamber, or insufficient derivatization (Hunter 1983a; Sato 2005). Hydroxycoumarin-based anticoagulant rodenticides are nonvolatile, highly adsorptive, and possess at least one functional hydroxyl group; thus, derivatization (with the exception of coumatetralyl) is required for their gas chromatography-based analysis (Sato 2005). Recently, an in-injector pyrolysis gas chromatograph coupled with an ion trap tandem mass spectrometer was shown to be successful for the rapid analysis of bromadiolone in blood plasma and liver without need for derivatization (Doubkova et al. 2017).
In general, analysis by liquid chromatography coupled with appropriate detectors, e.g., mass spectrometer or fluorescence detector, is the method of choice for detection of anticoagulant rodenticides in environmental samples following extraction and cleanup. Given the importance of primary and secondary poisoning, most methods are tailored toward the detection of specific active ingredients in various biological tissues of rodents, humans, domestic animals, and nontarget wildlife. A comprehensive summary of analytical methods for qualitative and quantitative determination of anticoagulant rodenticides in biological samples is also included in the work by Imran et al. (2015). As some of the compounds, e.g., brodifacoum and flocoumafen, are prone to carryover due to their nonpolar and hydrophobic nature (Marek and Koskinen 2007), extensive quality control is required irrespective of the chosen method to avoid false positives. Among the established methods for anticoagulant rodenticides in biological samples are ion-chromatography coupled with fluorescence detection (Jin et al. 2007) and tandem mass spectrometry (Jin et al. 2008), two-dimensional liquid chromatography coupled with tandem mass spectrometry (Marsalek et al. 2015), liquid chromatography coupled with tandem mass spectrometry (Marek and Koskinen 2007; Chen et al. 2009; Jin et al. 2009; Bidny et al. 2015), or high resolution mass spectrometry (Schaff and Montgomery 2013; Smith et al. 2017; Kotthoff et al. 2018). While anticoagulant rodenticides are suitable for electrospray ionization in both positive and negative mode, most published tandem and high resolution mass spectrometry methods rely on negative electrospray ionization due to enhanced sensitivity. A few researchers have employed atmospheric pressure chemical ionization on a limited number of anticoagulants (Guan et al. 1999; Mandel et al. 2000). In addition, few methods employed direct injection of biological tissue extracts for the analysis of anticoagulants after solvent extraction without further cleanup (Bayen et al. 2015; Kotthoff et al. 2018). With regard to aqueous samples, sensitivity of nontarget screening methods employing minimal sample pretreatment, e.g., direct injection of aqueous samples or dilute-and-shoot approaches, might be insufficient for the detection of these compounds at very low ng/L concentrations.
Notably, none of these methods aimed at chromatographic separation of individual diastereoisomers. Core shell analytical columns or mobile phases containing acetonitrile were reported to separate anticoagulants into their different stereoisomers (Jones 1996; Fourel et al. 2017a), but chromatographic separation of all second-generation anticoagulant rodenticide stereoisomers within a single elution gradient is challenging (Damin-Pernik et al. 2016; Fourel et al. 2017a). After baseline separation of stereoisomers is achieved, peak acquisition of corresponding cis- and trans-isomers is usually carried out using identical multiple reaction monitoring settings in tandem mass spectrometry (Smith et al. 2017). A stereoselective method by Kammerer et al. (2005) determined phenprocoumon and its stereospecific metabolites based on liquid chromatography tandem mass spectrometry in positive electrospray ionization mode in human plasma and liver microsomes. In general, data on stereoisomer residues and their metabolites in rodents or nontarget wildlife after environmental exposure are limited (Damin-Pernik et al. 2016, 2017; Fourel et al. 2017a, b).
An overview of ions used for quantification and confirmation of anticoagulant rodenticides in various acquisition methods, i.e., selected ion monitoring, multiple reaction monitoring, and selected reaction monitoring, is provided elsewhere (Imran et al. 2015). Matrix- and compound-specific method detection limits (MDL) and method quantification limits (MQL) are discussed in the context of reported presence or absence of anticoagulants in the aquatic environment in subsequent sections.
Analytical challenges
The majority of studies that investigated the occurrence and fate of anticoagulant residues in the aquatic environment refrained from using adequate isotope labeled surrogates for individual compounds during analysis by liquid chromatography tandem mass spectrometry or high resolution mass spectrometry to account for incomplete extraction of bound residues, ion suppression, and matrix effects (Gómez-Canela et al. 2014a, b; Gómez-Canela and Lacorte 2016; Kotthoff et al. 2018). These days, isotopically labeled analogues of high purity can be purchased for the majority of anticoagulants. Although costly, the use of appropriate isotope labeled internal standards during analysis is highly recommended for the quantification of residues at trace levels in complex environmental samples to guarantee the required specificity and selectivity. Co-eluted matrix components can interfere and compete with ions of target analytes during ionization in samples with high protein and lipid content (Huerta et al. 2012). Internal standards can only correct for the variation in ionization efficiency if their behavior is similar to that of the target analytes. In case of warfarin, more pronounced matrix effects were observed in fish liver extracts compared to extracts derived from fillet tissue during liquid chromatography tandem mass spectrometry analysis (Ramirez et al. 2009). Moreover, findings by Ramirez et al. (2009) demonstrated that extracts derived from fish sampled at different locations exert variable influence on the analytical response of warfarin, even though extracts were derived from a single biological tissue. This is a major drawback for corrective measures such as matrix-matched external calibration or standard addition as the generation of a calibration curve for each sample is impractical and often omitted when analyzing large sample sets (Huerta et al. 2012).
Hence, reported concentrations of anticoagulant rodenticides in complex matrices should be examined carefully if their quantification was conducted by matrix-matched external standard calibration (Gómez-Canela and Lacorte 2016; Kotthoff et al. 2018) or by use of inappropriate internal standards, e.g., coumachlor as an internal standard for second-generation anticoagulant rodenticides (Gómez-Canela et al. 2014a, b). While physicochemical properties of coumachlor and the molecule’s behavior in environmental matrices are similar to those of warfarin, they differ substantially from compounds such as bromadiolone, difenacoum, brodifacoum, difethialone, and flocoumafen (Table 2). In addition, the presence of coumachlor in environmental samples was demonstrated (Gómez-Canela and Lacorte 2016), precluding its qualification as an internal standard in regional monitoring studies. The range of target analytes as well as local conditions and emission sources plays an important role in choosing appropriate internal standards if labeled analogues are not available. Difenacoum for example has been commonly used as an internal standard for the quantification of brodifacoum in environmental samples collected from islands and fenced sanctuaries after aerial brodifacoum bait application (Masuda et al. 2015).
Chirality bears another challenge during residue analysis in complex matrices. Historically, chirality as a structural characteristic of pharmaceuticals marketed as racemates, e.g., vitamin K antagonists such as phenprocoumon, warfarin, and acenocoumarol, received very little attention in the field of environmental analysis (Pérez and Barceló 2008). Despite the fact that chromatographic separation of individual stereoisomers is of great value in pharmaco- and toxicokinetic studies and offers new approaches for investigating their occurrence and fate in the environment, it adds another dimension of complexity to the screening of trace-level anticoagulant mixtures in environmental matrices. A major challenge for the quantitative assessment of individual stereoisomers in environmental samples is the lack of appropriate analytical standards and the non-disclosure of diastereoisomer ratios in biocidal products released to the environment.
Residues of anticoagulant rodenticides in terrestrial and avian nontarget species
Worldwide monitoring of anticoagulant residues focuses mainly on predators, e.g., UK (Walker et al. 2010), France (Lambert et al. 2007), Spain (López-Perea et al. 2015), USA (Murray 2011), New Zealand (Eason et al. 2002). Anticoagulant rodenticide residues are found in many birds of prey and owl species (Newton et al. 1990; Walker et al. 2008; Murray 2011; Christensen et al. 2012; Hughes et al. 2013). Here, mainly birds that prey on small mammals like common buzzards (Buteo buteo) (Berny et al. 1997; Laasko et al. 2010), red kites (Milvus milvus) (Laasko et al. 2010; Hughes et al. 2013; Coeurdassier et al. 2014), and barn owls (Tyto alba) (Hosea 2000; Lambert et al. 2007) are exposed to anticoagulant rodenticides. Furthermore, anticoagulant rodenticide residues regularly occur in mammalian predators like foxes (Berny et al. 1997; Beklova et al. 2007; Sage et al. 2010; Tosh et al. 2011; Sanchez-Barbudo et al. 2012), stoats, weasels, and polecats (McDonald et al. 1998; Shore et al. 2003). Beside ample data on the presence or absence of residues, some studies confirm (Jacquot et al. 2013) or suspect (Newton et al. 1997) population decreases in nontarget species due to anticoagulant rodenticide poisoning. Residues of mainly second-generation anticoagulant rodenticides were present in 60% of foxes (Geduhn et al. 2015) and 55% of barn owls (Geduhn et al. 2016) in Germany in 2011–2014, and density of farmland (livestock density) and urban areas was positively correlated to rodenticide exposure of foxes.
Anticoagulant rodenticide exposure of predators results from feeding on small mammals that are target or nontarget species of rodenticide applications. Few studies confirmed anticoagulant rodenticide residue occurrence in nontarget small mammals from European countries (Townsend et al. 1995; Brakes and Smith 2005; Tosh et al. 2012). In Germany, recent research demonstrated regular exposure to anticoagulant rodenticides in nontarget small mammals in a large-scale experimental study (Geduhn et al. 2014). 23% of individuals that were trapped in the surrounding of livestock farms where an anticoagulant rodenticide was applied showed anticoagulant residues in liver samples. Residues were found in all trapped small mammal species, including shrews and wood mice that are protected species in Germany. Exposure rates and residue concentrations were especially high close to the bait stations (15 m radius) and decreased with increasing distance to the baited area. Recently, the relevance of nontarget small mammals in the context of wildlife exposure to anticoagulants was demonstrated in mammals and owls (Geduhn et al. 2014, 2016). In Germany, barn owls regularly prey on nontarget small mammals and rarely on target mice or rats (Geduhn et al. 2016). Therefore, exposure of barn owls via nontarget small mammals is very likely. Furthermore, residues of anticoagulant rodenticides were detected in small mammals that were hunted by owls (Geduhn et al. 2016). The unacceptable risks of primary and secondary poisoning that has been identified within the authorization procedure of anticoagulant rodenticide under the Biocidal Product Regulation No. 528/2012 could be confirmed in different steps of the terrestrial food chain, with nontarget small mammals as a key factor in this process (Geduhn et al. 2014, 2015, 2016). Therefore, further efficient risk mitigation strategies are necessary that focus on these species to reduce overall wildlife exposure.
Occurrence and fate of anticoagulant rodenticides in the aquatic environment
Wastewater treatment plants
Despite the use of warfarin-containing baits by professional and private users in urban catchments, the presence of warfarin in raw and treated wastewater has mainly been linked to the consumption of blood-thinning medication by residents. While studies with radiolabeled warfarin in rabbits demonstrated that about 90% of the orally administered dose is recovered in urine (Wong and Solomonraj 1980), only about 2% of the typical 1–15 mg daily prescription dose is excreted as unchanged warfarin (Godfrey et al. 2007; Crouse et al. 2012). Urinary excretion of warfarin predominantly occurs in the form of metabolites as warfarin enantiomers are extensively metabolized by liver in mammals. While R warfarin is oxidized to 7-hydroxywarfarin and reduced to R,S warfarin alcohol, S warfarin (a more active enantiomer with 3–5 times higher anticoagulant potency) on the other hand is oxidized to 7-hydroxywarfarin and reduced to S,S warfarin alcohol. Both enantiomers can also be metabolized to 6-hydroxywarfarin (Kasprzyk-Hordern 2010).
The occurrence of more recalcitrant pharmaceuticals in wastewater treatment plants is often correlated with their prescription rates. The predicted national average concentration of warfarin in raw municipal wastewater, based on US marketing and pharmacological data from 2004, was estimated at 28 ng/L (Kostich and Lazorchak 2008). Very low warfarin concentrations, i.e., on average 2 ng/L, were measured in raw municipal wastewater influent at a wastewater treatment plant facility in Texas, USA (Du et al. 2014). According to the authors, elevated concentrations of warfarin were occasionally observed in treated effluent, which might be explained by cleavage of glucuronide conjugates during biotransformation. Warfarin and its monohydroxylated derivatives are potential substrates for glucuronidation during phase II metabolism in humans. As glucoronide conjugates are more water-soluble than the parent compounds, they are easily excreted via bile and urine (Zielinska et al. 2008). Due to a rather high reporting limit of 11 ng/L, warfarin was not detected in wastewater effluent samples collected from 50 large wastewater treatment plants across the USA in 2011 (Kostich et al. 2014). It was sporadically detected in treated wastewater effluent used for irrigation from a facility in Colorado at levels up to 90 ng/L (Kinney et al. 2006).
In Finland, Ajo et al. (2018) reported warfarin concentrations of 82 ng/L and 7 ng/L in raw hospital wastewater and biologically treated domestic wastewater effluent from a healthcare center, respectively. Another study from Finland indicated better removal of warfarin (initial influent concentration of 50 ng/L) during membrane bioreactor treatment (more than 60% removal) compared to conventional activated sludge process (approximately 30% removal) (Gurung et al. 2016). The results of a study by Gibs et al. (2007) indicate that warfarin reacts completely with residual chlorine within 24 h during water treatment. Ejhed et al. (2018) investigated the treatment performance of three different onsite-wastewater treatment facilities that received raw wastewater collected from a small town in Germany (2500 person equivalents). Warfarin was only detected in one raw wastewater sample at 15 ng/L. It was not detected in any of the effluent samples (Ejhed et al. 2018). On the contrary, warfarin persisted in an organic-rich anoxic septic tank environment and was frequently detected in effluents from a community septic tank serving 350 users (Godfrey et al. 2007). Gómez-Canela et al. (2014b) detected warfarin in 9 out of 9 raw wastewater samples from wastewater treatment plants with mostly urban catchments in Catalonia, Spain. Warfarin concentrations in the aqueous phase of the 24-h composite samples ranged from 8 to 156 ng/L. It was also the main anticoagulant detected in more than 80 aqueous wastewater samples retrieved from nine wastewater treatment plants in Catalonia, Spain as 24-h composite samples in 2012 by the same research group. All samples were centrifuged prior to analysis, i.e., solid-phase extraction on hydrophilic-lipophilic balanced cartridges followed by liquid chromatography tandem mass spectrometry, to remove particulate matter. Warfarin concentrations ranged from 9 to 334 ng/L in raw wastewater and 1.6–45 ng/L in biologically treated wastewater effluents, respectively. Highest warfarin concentrations were detected in facilities serving large urban catchment areas. The majority of the studied wastewater treatment plants removed warfarin to below its method detection limit (MDL) of 1.6 ng/L in treated effluents. Three other facilities achieved removal rates between 82% and 98% (Gómez-Canela et al. 2014a). Santos et al. (2013) detected warfarin in hospital and municipal effluents in Portugal in the low ng/L range, supporting the hypothesis that the presence of warfarin in wastewater is mainly caused by its use as pharmaceutical.
Gómez-Canela et al. (2014a) also reported sporadic occurrence of coumatetralyl, difenacoum, bromadiolone, flocoumafen, and brodifacoum in wastewater samples, but failed in establishing meaningful input and elimination routes. Although most of the investigated wastewater treatment plants indicated high anticoagulant rodenticide removal efficiencies from the aqueous compartment, traces of anticoagulants remained in the treated effluent and were likely discharged into receiving surface waters (Gómez-Canela et al. 2014a). In another study by the same research group, Gómez-Canela and Lacorte (2016) detected anticoagulant rodenticides in sludge intended to be used as agricultural fertilizer at 15 out of 27 investigated wastewater treatment plants across North-East Spain. Of all analyzed anticoagulant rodenticides, warfarin was detected most frequently in the low μg/kg range based on dry weight. Bromadiolone was detected in sludge samples from six treatment facilities at concentrations between 5 and 8 μg/kg. Brodifacoum occurred in two sludge samples at 15 μg/kg and 17 μg/kg levels, respectively. Difenacoum and flocoumafen were not detected in any of the sludge samples. It was concluded that anticoagulant rodenticides enter wastewater treatment plants as a result of their use as pest control in urban infrastructures, domestic applications, as pharmaceuticals, or in agriculture (Gómez-Canela et al. 2014a, b; Gómez-Canela and Lacorte 2016).
In 2008, Sweden performed a national screening program to determine concentrations of chlorophacinone, coumatetralyl, difenacoum, brodifacoum, bromadiolone, and flocoumafen in the Swedish environment. None of the analyzed anticoagulant rodenticides was detected above their respective MDL of 5 ng/L and 1 μg/kg in several raw and treated wastewater as well as sludge samples (Norström et al. 2009).
Surface water, stormwater runoff, and groundwater
A surface water monitoring campaign in Lower Saxony, Germany in 2014 included the analytes warfarin, bromadiolone, and difenacoum. None of the three were detected in aqueous samples from surface waters above their method quantification limit (MQL) of 5 ng/L (Steffen 2014). Chen et al. (2014) analyzed bromadiolone and brodifacoum in environmental water samples from streams and groundwater wells in China. With one exception, both target analytes were below their respective MQL in all analyzed samples (0.22 µm membrane filtered). Brodifacoum was detected in one surface water sample at 0.56 µg/L and was traced back to illegal untreated wastewater discharges from a production facility into the stream (Chen et al. 2014). Brodifacoum was also detected in one organic-rich freshwater sample at 0.48 µg/L several days after aerially broadcasted application of bait pellets (approximately 39 metric tons of bait with 0.975 kg active ingredient distributed across 2.5 km2) during island eradication (Pitt et al. 2015). Chlorophacinone and bromadiolone were not detectable in groundwater samples from open space in Spain after bait application to eradicate country vole (Hernández et al. 2013). Screening of water samples collected from a freshwater lake in New Zealand (approximately 0.3 km2 surface area, 10–40 m depth) after accidental discharge of 700 kg of brodifacoum containing bait pellets (14 g of active ingredient) revealed no detects of residual brodifacoum in the month after the spill (Fisher et al. 2012). None of the analyzed six anticoagulant rodenticides were detected in Swedish surface water and stormwater runoff samples above the MDL of 5 ng/L (Norström et al. 2009).
During several nationwide US groundwater and surface water monitoring campaigns, warfarin was not detected above its reporting limit of 1 ng/L in any of the analyzed samples (Kolpin et al. 2002, 2004; Barnes et al. 2008; Focazio et al. 2008). However, Watkins et al. (2014) detected warfarin in surface water samples collected from suburban streams in Houston, Texas at locations downstream of wastewater treatment plant discharges. Reported concentrations ranged between 1 and 13 ng/L. Warfarin was not detected above its MDL of 0.8 ng/L in samples collected from locations upstream of discharges. Owing to a very high MDL of 50 ng/L, warfarin was also not detected in more than 1200 groundwater samples from California (Fram and Belitz 2011). Furthermore, warfarin showed significant attenuation during soil aquifer treatment of septic tank effluents. The passage of effluent through 2 m of a partially saturated, sand-dominated vadose zone reduced warfarin concentrations to below MDL in groundwater samples collected from an adjacent well. Attenuation processes were most likely a combination of sorption to the porous media and microbial degradation (Godfrey et al. 2007).
Interestingly, only one study by Wode et al. (2015) investigated the occurrence of phenprocoumon, an anticoagulant that is predominantly administered across Europe, in surface water and groundwater samples affected by treated wastewater effluents using a liquid chromatography high resolution mass spectrometry target screening approach. Phenprocoumon was qualitatively detected in 7 out of 14 groundwater and 7 out of 11 surface water samples of a former wastewater infiltration site in Berlin, Germany. As discussed earlier, estimated prescribed doses of phenprocoumon in Germany in 2016 exceeded those of warfarin by a factor of 40. Other than warfarin, phenprocoumon is excreted almost entirely as a glucuronide conjugate, with less than 10% of the dose as unchanged drug (Kasprzyk-Hordern 2010).
Soils and sediments
Kinney et al. (2006) assessed the presence and distribution of warfarin in soil irrigated with reclaimed water derived from urban wastewater. Warfarin did not accumulate in the studied soils over time and was present in the soils as low percentage of the mass applied. Observed concentration differences within the soil profiles may indicate the potential for warfarin to be transported from the soil surface to groundwater (Kinney et al. 2006). Residual flocoumafen was confirmed in two out of 21 New Zealand estuarine sediment samples. None of the monitored anticoagulant rodenticides were detected at riverine sites (Cavanagh and Ward 2014). After the accidental spill of brodifacoum containing bait into a freshwater lake in New Zealand, surface layer sediment samples revealed no detects of residual brodifacoum (Fisher et al. 2012). Nevertheless, 32% of soil samples from areas affected by broadcasted application of pellet bait contained brodifacoum residues at levels up to 56 μg/kg (MDL 3 μg/kg) (Pitt et al. 2015). Soil and sediment samples (upper 2–3 cm layer) from urban and remote areas in Sweden contained no traces of anticoagulant rodenticide residues (Norström et al. 2009).
Suspended particulate matter
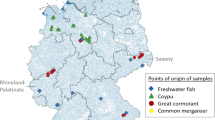
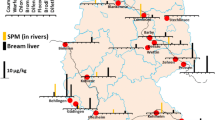
Suspended particulate matter samples from the German Specimen Bank were analyzed by Kotthoff et al. (2018) using liquid chromatography high resolution mass spectrometry to assess residue levels and distribution patterns of anticoagulant rodenticides in German surface waters. Samples, i.e., pooled samples of 12 monthly subsamples, were collected in 2015 from 16 different streams according to standardized procedures and corresponded with sampling sites of investigated limnic fish. Bromadiolone was the only anticoagulant rodenticide detected above its MQL of 1 μg/kg in nine suspended particulate matter samples and deviated from fish liver results discussed in the following. Mean concentration of bromadiolone was 4.9 μg/kg with a maximum of 9.2 μg/kg. The rather unexpected absence of other anticoagulant rodenticides in suspended particulate matter samples remained unresolved (Kotthoff et al. 2018).
Aquatic organisms
Liver samples of bream (Abramis brama) analyzed in the same study were also obtained from the German Specimen Bank (Kotthoff et al. 2018). Samples were collected in 2011 and 2015 and represented 16 river sampling sites and two lakes across Germany. In addition, decennial time series were analyzed for two sampling locations, i.e., rivers Saar and Elbe. According to their findings, five out of eight authorized anticoagulant rodenticides, namely difenacoum, brodifacoum, bromadiolone, difethialone, and flocoumafen, were detected in fish liver samples above their respective MQL of 0.2–2.0 µg/kg wet weight. In several fish liver samples, more than one residue was detected. This is in accordance with studies investigating anticoagulant rodenticide exposure in terrestrial nontarget wildlife. Different substance and concentration patterns were found between 2011 and 2015. Notably, brodifacoum was detected in 88% of the 2015 samples with an average concentration of 3.4 μg/kg (max. 12.5 μg/kg), followed by difenacoum (44%, max. 0.7 μg/kg) and bromadiolone (17%, max. 7.1 μg/kg). Metabolism and depuration of bromadiolone in fish might have caused the varying detection frequencies of bromadiolone residues in corresponding samples of fish liver (19%) and suspended particulate matter (56%) (Kotthoff et al. 2018). In a New Zealand study from 2013, a total of 49 individual freshwater fish livers, among others from brown trout (Salmo trutta) and New Zealand longfin eel (Anguilla dieffenbachii), were screened for residues of warfarin (MDL 100 µg/kg), coumatetralyl (MDL 10 µg/kg), brodifacoum, bromadiolone, and flocoumafen (all MDL 5 µg/kg). About 27% of analyzed liver samples contained bromadiolone (9–34 µg/kg wet weight) or coumatetralyl (11–24 µg/kg wet weight), respectively. Residues were not detected above their respective MDL in corresponding muscle tissue samples (Cavanagh and Ward 2014).
Warfarin was sporadically detected in tissues of wild freshwater mussels (Lasmigona costata) collected in 2012 from the Grand River, Ontario in Canada, but not in a series of corresponding surface water samples (2009–2011, n = 37). The reported maximum tissue concentration of warfarin was 1.15 µg/kg wet weight. Warfarin was not detected above its MDL in tissues of caged freshwater mussels after a 4-week deployment period in Grand River in 2010 (de Solla et al. 2016). Warfarin was also not detected in any of the fish fillet (MDL 0.9 μg/kg, n = 30) or liver (MDL 2.7 μg/kg, n = 30) composite samples from five effluent-dominated river sampling sites receiving discharge from wastewater treatment plants of major cities across the USA (Ramirez et al. 2009). The 2008 Swedish national screening program included fish muscle samples (pooled samples of hering and perch, respectively) from remote and urban surface waters to investigate the occurrence of chlorophacinone, coumatetralyl, difenacoum, brodifacoum, bromadiolone, and flocoumafen in the Swedish environment. Their concentrations were below the MDL of 1 μg/kg in all fish muscle samples (Norström et al. 2009).
Other findings in aquatic organisms include (sparse) residual concentrations of brodifacoum in coastal marine species such as sedentary mollusks and fish following island rodent eradication (Siers et al. 2016) or accidental discharge (Primus et al. 2005). Residual brodifacoum concentrations were found in liver samples, but not muscle tissue, of two blue cod (Parapercis colias) individuals at 26 µg/kg and 92 µg/kg, respectively (8% detection frequency). Brodifacoum residues were also detected in whole-body samples of four mussels (Mytilus edulis) in the range of 1–22 µg/kg and four limpets (Cellana ornata) in the range of 1–16 µg/kg (17% detection frequency) (Masuda et al. 2015). Following a hand- and aerially broadcast application of 18,000 kg of brodifacoum pellets on Wake Island Atoll in 2012, 3 out of 69 marine whole-body fish samples collected in 2012 and 5 out of 48 collected in 2015 were suspected of brodifacoum contamination (MDL 3.5 µg/kg). However, none of these whole-body samples (mostly from blacktail snappers, Lutjanus fulvus) yielded reliably quantifiable concentrations of brodifacoum above the MQL of 11.7 µg/kg (Siers et al. 2016). Pitt et al. (2015) conducted a comprehensive post-baiting monitoring for environmental brodifacoum residues after the extensive rat eradication on Palmyra Atoll. Whole-body samples of black-spot sergeants (Abudefduf sordidus) that were collected prior to baiting contained no brodifacoum residues above the MDL of 13 µg/kg, whereas average brodifacoum concentrations were in the range of 143 ± 27 µg/kg (90% detection frequency) shortly after aerially broadcasted bait application (approximately 0.39 mg active ingredient per m2 land surface). The mortality of 47 mullets (Moolgarda engeli, Liza vaigiensis) washed ashore was linked to brodifacoum bait application. Whole-body samples showed average residues of 337 ± 67 µg/kg wet weight. As mullets are common prey of many aquatic and terrestrial predatory species, the authors emphasized the likeliness of trophic transfer of brodifacoum (Pitt et al. 2015). According to a literature review by Masuda et al. (2015), detection frequencies of brodifacoum residue in coastal marine species after aerial bait application were only approximately 6% for marine invertebrates and 3% for fish. No residual brodifacoum was detected in fish liver samples (Anguilla diefenbachii) after accidental discharge of 14 g of active ingredient into a remote freshwater lake in New Zealand (Fisher et al. 2012). Green mussel (Perna viridis) samples collected in Singapore coastal waters showed no traces of warfarin above its MDL of 0.6 µg/kg wet weight (Bayen et al. 2015).
Avian and mammalian predators in the aquatic food web
Liver samples of different top-predator species with a predominantly fish-eating diet across the Loire river basin in France were screened for residues of warfarin, chlorophacinone, coumatetralyl, difenacoum, brodifacoum, bromadiolone, difethialone, and flocoumafen by Lemarchand et al. (2014). Carcasses of road-traffic killed Eurasian otter were mainly collected between 2004 and 2008 (Lemarchand et al. 2010). While samples of great cormorants (Phalacrocorax carbo carbo/sinensis) and osprey (Pandion haliaetus) revealed no traces of anticoagulants above their respective MDL of 20 µg/kg, bromadiolone was detected in 10% of the analyzed 20 Eurasian otter (Lutra lutra) liver samples at concentrations of 0.4 and 0.85 mg/kg wet weight, respectively. No clinical signs of intoxication, e.g., severe anemia or bleeding, were observed. As both individuals originated from the same riparian area that was heavily baited against proliferation of land voles (Arvicola scherman) back then, the authors deemed secondary poisoning due to predation on nontarget rodents likely (Lemarchand et al. 2010; Lemarchand et al. 2014). An earlier study by Fournier-Chambrillon et al. (2004) confirmed exposure of European otters to secondary poisoning by bromadiolone (18%) and chlorophacinone (9%) in France due to major field treatments with anticoagulants in the past. Hepatic traces of coumatetralyl (5.8–9.4 µg/kg), bromadiolone (6.2–11 µg/kg), and difenacoum (lower than 0.3–2.5 µg/kg) were also found in two roadkill European otters from Finland (Koivisto et al. 2016). No anticoagulant rodenticides were detected above their respective MQL of 0.2–2.0 µg/kg in liver samples of five European otter individuals from the river Elbe catchment in Eastern Germany (Kotthoff et al. 2018).
Following aerial rodent eradication in 2009, three out of nine little blue penguins (Eudyptula minor) found dead on island beaches showed hepatic brodifacoum residues (Fisher 2013). As a consequence, a more comprehensive screening of liver samples from 38 penguin carcasses regarding brodifacoum, bromadiolone, flocoumafen, coumatetralyl, and warfarin residues was conducted in 2010. While target analytes were absent in 50% of the penguin liver samples, 34.2% revealed the presence of one anticoagulant, 7.9% a combination of two, 5.3% of three and 2.6% of four different anticoagulants. Brodifacoum was detected in six of the little blue penguins in the range of 1–3 µg/kg (Fisher 2013).
Rating the risks of anticoagulant rodenticides in the aquatic environment
Challenges of anticoagulant rodenticide residue screening in aquatic environmental compartments
Monitoring of anticoagulant rodenticide residues in the aquatic environment involves a number of challenges, particularly with regard to establishing causative associations and robust source, pathway, and receptor relationships. Given the toxicological relevance of anticoagulants at trace concentrations and the variety of active ingredients applied worldwide, very sensitive and specific multi-methods are required that cover a wide range of different compounds and environmental matrices. As illustrated in this review, available analytical methods often suffer from elevated limits of detection caused by the complexity of environmental matrices such as sewage or biological tissues and insufficient sample pretreatment.
Another critical consideration in the context of poisoning via environmental emissions is the influence of municipal effluent discharges on in-stream hydrology when selecting sampling locations and periods for monitoring of wastewater-derived contaminant exposure (Ramirez et al. 2009). Important aspects regarding the monitoring of anticoagulant rodenticides in aquatic compartments are the frequency and amplitude of contaminant loadings. Effluent-dominated systems generally represent worstcase exposure scenarios, but it is assumed that anticoagulant rodenticide input rates are of transient character and will vary widely depending on usage patterns in urban catchments, runoff regimes, and wastewater treatment plant performance. Worstcase predicted environmental concentrations discussed earlier (Table 1) indicate that expected anticoagulant rodenticide concentrations in receiving surface waters may be out of reach for current analytical methods, even with extensive sample enrichment and cleanup. Yet, in cases where environmental dissipation rates are exceeded by prolonged input rates from effluent loadings, even at very low concentrations, effective exposure duration of organisms residing in these aquatic systems is increased, presenting particular potential for accumulation of contaminants. Thus, analysis of stationary environmental matrices such as sediments, sessile or less migratory organisms that reflect an average exposure over time can be one way to capture transient events and monitor the burden of the aquatic environment (Kotthoff et al. 2018). Bioaccumulation processes can widely differ among aquatic species due to complex interactions between various routes of uptake (aqueous uptake of water-borne chemicals, dietary uptake by ingestion of contaminated food or particles), excretion, passive release, and metabolization (Streit 1998). Therefore, determination of concomitant parameters such as trophic level, age, and lipid content is crucial to rank the exposure of aquatic organisms and link anticoagulant rodenticide residues to identified emission sources. Yet, such important parameters were often omitted in environmental monitoring studies of anticoagulants in aquatic wildlife. The occurrence and fate of anticoagulant rodenticides in fish species is likely correlated with their feeding habit and lipid metabolism, i.e., less fat after winter months. In summer and fall, lipid content in fish is usually highest and river water levels lowest, e.g., less dilution of wastewater-derived contaminants.
Emergence of anticoagulants in the aquatic environment
With the exception of warfarin, behavior and fate of anticoagulant rodenticides in the aquatic environment have received minimal attention by environmental research groups in the past. Therefore, potential risks cannot be adequately rated at this point. Several studies considered the risk of secondary poisoning via environmental emissions as marginal. The Swedish monitoring study concluded that anticoagulant rodenticides are not widely distributed in the Swedish environment, thus not posing a threat for nontarget organisms that are not affected by direct primary or secondary poisoning (Norström et al. 2009). Nonetheless, this review clearly shows that anticoagulant rodenticides can enter aquatic environmental compartments. Without question, more comprehensive monitoring data of relevant environmental matrices are needed for a thorough assessment of their emergence. Given their physicochemical properties (Table 2), environmental matrices such as sediments and suspended particulate matter might pose important exposure routes for particle-bound second-generation anticoagulant rodenticides, leading to bioaccumulation and toxicity in aquatic organisms. According to Fisher (2013), environmental spread of anticoagulant rodenticide residues in New Zealand is thought to be predominantly trophic rather than through exposure of nontarget organisms to anticoagulant rodenticide residues in water or soil/sediments. Carcasses of poisoned animals or terrestrial invertebrates that feed on bait, e.g., cockroaches, are presumed to transfer anticoagulant rodenticide residues in the aquatic environment and put predators and scavengers at risk of secondary exposure.
Warfarin is commonly monitored in environmental studies because of its substantial volume of prescriptions and sales, alongside its potential negative effects on wildlife. In developed countries, the occurrence of warfarin in the aquatic environment is mainly caused by its use as a prescription drug and incomplete removal (or reversible transformation) during conventional wastewater treatment. Low detection frequencies of warfarin in the aquatic environment are likely a combination of low consumption compared to other high-volume non-prescription drugs, high metabolic rates in the body of humans (pharmaceutical) and rodents (biocidal use), low bioaccumulation potential, and high detection limits. In surface water and groundwater, warfarin reporting limits differed by factor 50 among studies, ranging between 0.001 µg/L (Kolpin et al. 2002, 2004; Barnes et al. 2008; Focazio et al. 2008) and 0.05 µg/L (Fram and Belitz 2011; Crouse et al. 2012). The worstcase predicted environmental concentrations for surface water resulting from sewer baiting scenarios are in the range of 0.006 µg/L (Table 1). According to reviewed monitoring data, environmental levels of warfarin may not represent a high risk for aquatic species, in particular fish and mollusks. Moreover, warfarin was not ranked as an emerging contaminant in coastal and marine environments (Maruya et al. 2015). Yet, chronic exposure at low concentrations or chronic exposure with multiple active ingredients and therefore higher environmental concentrations could trigger sublethal effects (Fernandez et al. 2014).
Future research needs and risk mitigation measures
The proposed adverse outcome pathway for anticoagulant rodenticides in terrestrial nontarget wildlife by Rattner et al. (2014) reveals that the anticoagulant’s mechanism of action from the molecular through cellular levels is well understood, whereas linkages and forecasting of responses at the individual through population levels remain vague or incomplete. Among others, substantial data gaps exist regarding the understanding of exposure pathways and potential adverse effects of multiple low-level anticoagulant rodenticide exposures. For instance, the almost ubiquitous occurrence of second-generation anticoagulant rodenticides in bream liver samples from receiving surface waters throughout Germany demonstrated by Kotthoff et al. (2018) contrasts the reported (minor) quantities of active ingredients applied as biocides in German sewer systems (Krüger and Solas 2010). Notably, rodent control in sewer systems is one of the main applications of biocidal anticoagulant rodenticides in densely populated urban and peri-urban areas in Germany. At present, studies about the fate of anticoagulant rodenticide residues during wastewater treatment, e.g., conventional or advanced treatment, respectively, after confirmed bait application in sewer systems of urban catchments are lacking.
The role of invertebrates as consumers and vectors of anticoagulant poison should be another research priority in the context of anticoagulant rodenticide spread in the aquatic environment. American cockroaches (Periplaneta americana), which can be widely distributed among sewer manholes, demonstrated an ability to consume an entire anticoagulant rodenticide bait placement (Colvin et al. 1998). Although anticoagulants are unlikely to affect invertebrates in the same way as vertebrates because of fundamental differences in the blood clotting system, vertebrates that prey on invertebrates can be affected by secondary poisoning. Terrestrial invertebrates can be an important component of stream fish diets, especially during the summer months, when aquatic invertebrates are limited (Garman 1991). Cockroaches can be available as fish prey when swept into the water column. Pitt et al. (2015) demonstrated in their study that brodifacoum residue levels in cockroaches were consistently the highest among the biological samples collected. In New Zealand, residues of brodifacoum were also detected in the tissues of cave weta (Rhaphidophoridae sp.) that were found on baits (Ogilvie et al. 1997).
Moreover, the understanding of mechanistic relationships between bioaccumulation and toxicity of anticoagulant rodenticides demands further improvement, e.g., by incorporation in toxicokinetic and toxicodynamic modeling. While detection of hepatic anticoagulant rodenticide residues in aquatic organisms should not be dismissed, sole consideration of these findings and the implied potential biomagnification along the aquatic food chain is insufficient with regard to a profound anticoagulant rodenticide risk assessment. Instead, bioaccumulation of anticoagulant rodenticides should be linked to adverse effects and relative potencies on each trophic level so that risks can be evaluated for specific target species along the food web, e.g., invertebrates, fish, or top predators. In the terrestrial food chain, risk via poisoned rodents is considered significantly higher compared to risk via earthworms or other invertebrates (eCA 2016g). To mitigate the risk of secondary exposure, however, first-generation anticoagulant rodenticides and less potent second-generation anticoagulant rodenticides should always be considered as the first choice for pest control with anticoagulants.
Conclusion
Recent findings of anticoagulant rodenticides in the aquatic food web as discussed in this review demonstrate that the aquatic environment experiences a greater risk of anticoagulant exposure than previously thought. Besides the discussed analytical challenges, knowledge gaps and the lack of detailed market data clearly hamper the establishment of resilient exposure pathways with regard to the aquatic environment. Beyond doubt, more comprehensive monitoring data are required for all anticoagulants in the aquatic environment to establish robust relationships and causative associations as previously demonstrated for the unintended poisoning of terrestrial nontarget organisms. Once those are established, more effective and practical risk mitigation measures, e.g., alternatives to anticoagulant rodenticides for rodent control in sewer systems, can be proposed, implemented, and their sustained success reassessed.
References
Ajo P, Preis S, Vornamo T, Mänttäri M, Kallioinen M, Louhi-Kultanen M (2018) Hospital wastewater treatment with pilot-scale pulsed corona discharge for removal of pharmaceutical residues. J Environ Chem Eng 6:1569–1577. https://doi.org/10.1016/j.jece.2018.02.007
Alomar H, Chabert A, Coeurdassier M, Vey D, Berny P (2018) Accumulation of anticoagulant rodenticides (chlorophacinone, bromadiolone and brodifacoum) in a non-target invertebrate, the slug, Deroceras reticulatum. Sci Total Environ 610–611:576–582. https://doi.org/10.1016/j.scitotenv.2017.08.117
Andre C, Guyon C, Guillaume YC (2004) Rodenticide-humic acid adsorption mechanisms and role of humic acid on their toxicity on human keratinocytes: chromatographic approach to support the biological data. J Chromatogr, B: Anal Technol Biomed Life Sci 813:295–302. https://doi.org/10.1016/j.jchromb.2004.10.028
Andre C, Guyon C, Thomassin M, Barbier A, Richert L, Guillaume YC (2005) Association mechanism between a series of rodenticide and humic acid: a frontal analysis to support the biological data. J Chromatogr, B: Anal Technol Biomed Life Sci 820:9–14. https://doi.org/10.1016/j.jchromb.2005.02.020
Barnes KK, Kolpin DW, Furlong ET, Zaugg SD, Meyer MT, Barber LB (2008) A national reconnaissance of pharmaceuticals and other organic wastewater contaminants in the United States - I) groundwater. Sci Total Environ 402:192–200. https://doi.org/10.1016/j.scitotenv.2008.04.028
Barten R (2014) New approaches from the view of industry. In: Esther A et al (eds) Rodenticide resistance. Julius Kühn Institute, Federal Research Centre for Cultivated Plants, Braunschweig, pp 50–54
Battersby SA (2015) Rodents as carriers of disease. In: Buckle AP, Smith RH (eds) Rodent pests and their control. CABI, Oxfordshire, pp 81–100
Bayen S, Estrada ES, Juhel G, Kelly BC (2015) Direct injection of tissue extracts in liquid chromatography/tandem mass spectrometry for the determination of pharmaceuticals and other contaminants of emerging concern in mollusks. Anal Bioanal Chem 407:5553–5558. https://doi.org/10.1007/s00216-015-8760-9)
Beklova M, Krizkova S, Supalkova V et al (2007) Determination of bromadiolone in pheasants and foxes by differential pulse voltammetry. Int J Environ Anal Chem 87:459–469. https://doi.org/10.1080/03067310601170472
Berny PJ, Buronfosse T, Buronfosse F, Lamarque F, Lorgue G (1997) Field evidence of secondary poisoning of foxes (Vulpes vulpes) and buzzards (Buteo buteo) by bromadiolone, a 4-year survey. Chemosphere 35:1817–1829. https://doi.org/10.1016/S0045-6535(97)00242-7
Berny P, Velardo J, Pulce C, D’Amico A, Kammerer M, Lasseur R (2010) Prevalence of anticoagulant rodenticide poisoning in humans and animals in France and substances involved. Clin Toxicol (Phila) 48:935–941. https://doi.org/10.3109/15563650.2010.533678
Bidny S, Gago K, David M, Duong T, Albertyn D, Gunja N (2015) A validated LC-MS-MS method for simultaneous identification and quantitation of rodenticides in blood. J Anal Toxicol 39:219–224. https://doi.org/10.1093/jat/bku175
Booth LH, Ogilvie SC, Eason CT (2010) Persistence of sodium monofluoroacetate (1080), pindone, cholecalciferol, and brodifacoum in possum baits under simulated rainfall. N Z J Agric Res 42:107–112. https://doi.org/10.1080/00288233.1999.9513359
Brakes CR, Smith RH (2005) Exposure of non-target small mammals to rodenticides: short-term effects, recovery and implications for secondary poisoning. J Appl Ecol 42:118–128. https://doi.org/10.1111/j.1365-2664.2005.00997.x
Buckle AP, Eason CT (2015) Control methods: chemical. In: Buckle AP, Smith RH (eds) Rodent pests and their control. CAB International, Wallingford, pp 123–154
Buckle AP, Smith RH (eds) (2015) rodent pests and their control. CAB International, Wallingford
BVL (2012) Absatz an Pflanzenschutzmitteln in der Bundesrepublik Deutschland. Bundesamt für Verbraucherschutz und Lebensmittelsicherheit, Braunschweig
BVL (2015) Absatz an Pflanzenschutzmitteln in der Bundesrepublik Deutschland. Bundesamt für Verbraucherschutz und Lebensmittelsicherheit, Braunschweig
Cavanagh J-AE, Ward N (2014) Contaminants in estuarine and riverine sediments and biota in Southland. Environment Southland, Invercargill
Chen XH, Cai MQ, Ouyang XK, Jin MC (2009) Ion chromatography tandem mass spectrometry for simultaneous confirmation and determination of indandione rodenticides in serum. Biomed Chromatogr 23:1217–1226. https://doi.org/10.1002/bmc.1246
Chen M, Zhu G, Zhou L, Min J, Chen X, Jin M (2014) Analysis of trace bromadiolone and brodifacoum in environmental water samples by ionic liquid ultrasound-assisted dispersive liquid–liquid microextraction and LC-MS/MS. Anal Methods 6:5879–5885. https://doi.org/10.1039/c3ay42317d
Christensen TK, Lassen P, Elmeros M (2012) High exposure rates of anticoagulant rodenticides in predatory bird species in intensively managed landscapes in Denmark. Arch Environ Contam Toxicol 63:437–444. https://doi.org/10.1007/s00244-012-9771-6
Coeurdassier M, Riols R, Decors A et al (2014) Unintentional wildlife poisoning and proposals for sustainable management of rodents. Conserv Biol 28:315–321. https://doi.org/10.1111/cobi.12230
Colvin BA, Swift TB, Fothergill FE (1998) Control of Norway rats in sewer and utility systems using pulsed baiting methods. In: Proceedings of the 18th vertebrate pest conference, vol 36, pp 247–253
Connors KA, Du B, Fitzsimmons PN et al (2013) Comparative pharmaceutical metabolism by rainbow trout (Oncorhynchus mykiss) liver S9 fractions. Environ Toxicol Chem 32:1810–1818. https://doi.org/10.1002/etc.2240
Crouse BA, Ghoshdastidar AJ, Tong AZ (2012) The presence of acidic and neutral drugs in treated sewage effluents and receiving waters in the Cornwallis and Annapolis River watersheds and the Mill CoveSewage Treatment Plant in Nova Scotia, Canada. Environ Res 112:92–99. https://doi.org/10.1016/j.envres.2011.11.011
CRRU UK (2015) Campaign for responsible rodenticide Use UK code of best practice. Campaign for Responsible Rodenticide Use UK, Leeds
Damin-Pernik M, Espana B, Besse S et al (2016) Development of an ecofriendly anticoagulant rodenticide based on the stereochemistry of difenacoum. Drug Metab Dispos 44:1872–1880. https://doi.org/10.1124/dmd.116.071688
Damin-Pernik M, Espana B, Lefebvre S et al (2017) Management of rodent populations by anticoagulant rodenticides: toward third-generation anticoagulant rodenticides. Drug Metab Dispos 45:160–165. https://doi.org/10.1124/dmd.116.073791
Dawson A, Garthwaite D (2004) Rodenticide usage by local authorities in Great Britain 2001. Pesticide usage survey report 185. Department for Environment, Food and Rural Affairs, New York
de Solla SR, Gilroy EA, Klinck JS et al (2016) Bioaccumulation of pharmaceuticals and personal care products in the unionid mussel Lasmigona costata in a river receiving wastewater effluent. Chemosphere 146:486–496. https://doi.org/10.1016/j.chemosphere.2015.12.022
Doubkova V, Marsalek P, Vecerek V (2017) The rapid determination of bromadiolone in liver and blood plasma by in-injector pyrolysis gas chromatography—ion trap tandem mass spectrometry. J Chromatogr, B: Anal Technol Biomed Life Sci. https://doi.org/10.1016/j.jchromb.2017.10.027
Du B, Price AE, Scott WC et al (2014) Comparison of contaminants of emerging concern removal, discharge, and water quality hazards among centralized and on-site wastewater treatment system effluents receiving common wastewater influent. Sci Total Environ 466–467:976–984. https://doi.org/10.1016/j.scitotenv.2013.07.126
Eason CT, Murphy EC, Wright GR, Spurr EB (2002) Assessment of risks of brodifacoum to non-target birds and mammals in New Zealand. Ecotoxicol 11:35–48. https://doi.org/10.1023/A:1013793029831
EBPF (2015) Sustainable use of rodenticides as biocides in the EU. CEFIC-European Biocidal Products Forum, Brussels
eCA (2016a) Flocoumafen assessment report. Product-type 14 (Rodenticide) competent authority. European Union, The Netherlands
eCA (2016b) Coumatetralyl assessment report. Product-type 14 (Rodenticide) competent authority. European Union, Denmark
eCA (2016c) Brodifacoum assessment report. Product-type 14 (Rodenticide) competent authorities. European Union, The Netherlands and Italy
eCA (2016d) Chlorophacinone assessment report. Product-type 14 (Rodenticide) competent authority. European Union, Spain
eCA (2016e) Bromadiolone assessment report. Product-type 14 (Rodenticide) competent authority. European Union, Italy
eCA (2016f) Warfarin Assessment report. Product-type 14 (Rodenticide) competent authority. European Union, Ireland
eCA (2016g) Difenacoum assessment report. Product-type 14 (rodenticide) competent authority. European Union, Finland
eCA (2016h) Difethialone assessment report. Product-type 14 (Rodenticide) competent authority. European Union, Norway
ECHA (2017a) Guidance on the biocidal products regulation: volume IV environment—assessment and evaluation (parts B + C). European Chemicals Agency, Helsinki
ECHA (2017b) Opinion of the Biocidal Products Committee on questions related to the comparative assessment of anticoagulant rodenticides. ECHA/BPC/145/2017. European Chemicals Agency, Helsinki
Ejhed H, Fang J, Hansen K et al (2018) The effect of hydraulic retention time in onsite wastewater treatment and removal of pharmaceuticals, hormones and phenolic utility substances. Sci Total Environ 618:250–261. https://doi.org/10.1016/j.scitotenv.2017.11.011
Elliott JE, Rattner BA, Shore RF, Van Den Brink NW (2016) Paying the pipers: mitigating the impact of anticoagulant rodenticides on predators and scavengers. Biosci 66:401–407. https://doi.org/10.1093/biosci/biw028
Empson RA, Miskelly CM (1999) The risks, costs and benefits of using brodifacoum to eradicate rats from Kapiti Island, New Zealand. N Z J Ecol 23:241–254
Endepols S (2002) Rodenticides—indispensable for safe food production. Pestic Outlook 13:231–232. https://doi.org/10.1039/b211694b
European Commission (2011) Regulation (EU) No 253/2011 of 15 March 2011 amending Regulation (EC) No 1907/2006 of the European Parliament and of the Council on the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) as regards Annex XIII. Off J Eur Union L 69:7–12
European Commission (2016) Commission Regulation (EU) 2016/1179 of 19 July 2016 amending, for the purposes of its adaptation to technical and scientific progress, Regulation (EC) No 1272/2008 of the European Parliament and of the Council on classification, labelling and packaging of substances and mixtures. Off J Eur Union L 195:11–25
European Commission (2017a) Commission Implementing Regulation (EU) 2017/1380 of 25 July 2017 renewing the approval of bromadiolone as an active substance for use in biocidal products of product-type 14. Off J Eur Union L 194:33–38
European Commission (2017b) Commission Implementing Regulation (EU) 2017/1379 of 25 July 2017 renewing the approval of difenacoum as an active substance for use in biocidal products of product-type 14. Off J Eur Union L 194:27–32
European Commission (2017c) Commission Implementing Regulation (EU) 2017/1376 of 25 July 2017 renewing the approval of warfarin as an active substance for use in biocidal products of product-type 14. Off J Eur Union L 194:9–14
European Union (2009) Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. Off J Eur Union L 309:1–50
European Union (2012) Regulation (EU) No 528/2012 of the European parliament and of the council of 22 May 2012 concerning the making available on the market and use of biocidal products. Off J Eur Union L 167:1–128
Fernandez I, Santos A, Cancela ML, Laize V, Gavaia PJ (2014) Warfarin, a potential pollutant in aquatic environment acting through Pxr signaling pathway and gamma-glutamyl carboxylation of vitamin K-dependent proteins. Environ Pollut 194:86–95. https://doi.org/10.1016/j.envpol.2014.07.015
Fisher P (2013) Environmental residues of anticoagulants used for pest animal control. Landcare Research, Lincoln
Fisher P, O’Connor C, Wright GR, Eason CT (2003) Persistence of 4 anticoagulant rodenticides in the livers of laboratory rats. Department of Conservation, Wellington
Fisher P, Funnell E, Fairweather A, Brown L, Campion M (2012) Accidental discharge of brodifacoum baits into a freshwater lake: a case study. Bull Environ Contam Toxicol 88:226–228. https://doi.org/10.1007/s00128-011-0470-1
Focazio MJ, Kolpin DW, Barnes KK et al (2008) A national reconnaissance for pharmaceuticals and other organic wastewater contaminants in the United States-II) untreated drinking water sources. Sci Total Environ 402:201–216. https://doi.org/10.1016/j.scitotenv.2008.02.021
Fourel I, Damin-Pernik M, Benoit E, Lattard V (2017a) Core-shell LC–MS/MS method for quantification of second generation anticoagulant rodenticides diastereoisomers in rat liver in relationship with exposure of wild rats. J Chromatogr B 1041–1042:120–132. https://doi.org/10.1016/j.jchromb.2016.12.028
Fourel I, Damin-Pernik M, Benoit E, Lattard V (2017b) Cis-bromadiolone diastereoisomer is not involved in bromadiolone Red Kite (Milvus milvus) poisoning. Sci Total Environ 601–602:1412–1417. https://doi.org/10.1016/j.scitotenv.2017.06.011
Fournier-Chambrillon C, Berny PJ, Coiffier O et al (2004) Evidence of secondary poisoning of free-ranging riparian mustelids by anticoagulant rodenticides in France: implications for conservation of European mink (Mustela lutreola). J Wildl Dis 40:688–695. https://doi.org/10.7589/0090-3558-40.4.688
Fram MS, Belitz K (2011) Occurrence and concentrations of pharmaceutical compounds in groundwater used for public drinking-water supply in California. Sci Total Environ 409:3409–3417. https://doi.org/10.1016/j.scitotenv.2011.05.053
Garman GC (1991) Use of terrestrial arthropod prey by a stream-dwelling cyprinid fish. Environ Biol Fishes 30:325–331. https://doi.org/10.1007/BF02028848
Geduhn A (2015) Exposure of wildlife to anticoagulant rodenticides: how environmental drivers modulate the pathway of anticoagulant rodenticides from bait to predators. University of Münster, Münster
Geduhn A, Esther A, Schenke D, Mattes H, Jacob J (2014) Spatial and temporal exposure patterns in non-target small mammals during brodifacoum rat control. Sci Total Environ 496:328–338. https://doi.org/10.1016/j.scitotenv.2014.07.049
Geduhn A, Jacob J, Schenke D, Keller B, Kleinschmidt S, Esther A (2015) Relation between intensity of biocide practice and residues of anticoagulant rodenticides in Red Foxes (Vulpes vulpes). PLoS ONE 10:e0139191. https://doi.org/10.1371/journal.pone.0139191
Geduhn A, Esther A, Schenke D, Gabriel D, Jacob J (2016) Prey composition modulates exposure risk to anticoagulant rodenticides in a sentinel predator, the barn owl. Sci Total Environ 544:150–157. https://doi.org/10.1016/j.scitotenv.2015.11.117
Gibs J, Stackelberg PE, Furlong ET, Meyer M, Zaugg SD, Lippincott RL (2007) Persistence of pharmaceuticals and other organic compounds in chlorinated drinking water as a function of time. Sci Total Environ 373:240–249. https://doi.org/10.1016/j.scitotenv.2006.11.003
Godfrey E, Woessner WW, Benotti MJ (2007) Pharmaceuticals in on-site sewage effluent and ground water, Western Montana. Ground Water 45:263–271. https://doi.org/10.1111/j.1745-6584.2006.00288.x
Goldade DA, Primus TM, Johnston JJ, Zapien DC (1998) Reversed-phase ion-pair high-performance liquid chromatographic quantitation of difethialone residues in whole-body rodents with solid-phase extraction cleanup. J Agric Food Chem 46:504–508. https://doi.org/10.1021/jf970715u
Gómez-Canela C, Lacorte S (2016) Comprehensive characterization of anticoagulant rodenticides in sludge by liquid chromatography–tandem mass spectrometry. Environ Sci Pollut Res Int 23:15739–15748. https://doi.org/10.1007/s11356-016-6743-9
Gómez-Canela C, Barata C, Lacorte S (2014a) Occurrence, elimination, and risk of anticoagulant rodenticides and drugs during wastewater treatment. Environ Sci Pollut Res 21:7194–7203. https://doi.org/10.1007/s11356-014-2714-1
Gómez-Canela C, Vazquez-Chica A, Lacorte S (2014b) Comprehensive characterization of rodenticides in wastewater by liquid chromatography–tandem mass spectrometry. Anal Bioanal Chem 406:345–358. https://doi.org/10.1007/s00216-013-7449-1
Gras LM, Patergnani M, Farina M (2012) Poison-based commensal rodent control strategies in urban ecosystems: some evidence against sewer-baiting. EcoHealth 9:75–79. https://doi.org/10.1007/s10393-012-0748-8
Guan F, Ishii A, Seno H, Watanabe-Suzuki K, Kumazawa T, Suzuki O (1999) Use of an ion-pairing reagent for high-performance liquid chromatography–atmospheric pressure chemical ionization mass spectrometry determination of anionic anticoagulant rodenticides in body fluids. J Chromatogr B Biomed Sci Appl 731:155–165. https://doi.org/10.1016/S0378-4347(99)00126-7
Gurung K, Ncibi MC, Fontmorin JM (2016) Incorporating submerged MBR in conventional activated sludge process for municipal wastewater treatment: a feasibility and performance assessment. J Membr Sci Technol. https://doi.org/10.4172/2155-9589.1000158
Hauck ZZ, Feinstein DL, van Breemen RB (2016) LC-MS-MS analysis of brodifacoum isomers in rat tissue. J Anal Toxicol 40:304–309. https://doi.org/10.1093/jat/bkw008
Hernández AM, Bernal J, Bernal JL, Martín MT, Caminero C, Nozal MJ (2013) Simultaneous determination of nine anticoagulant rodenticides in soil and water by LC–ESI-MS. J Sep Sci 36:2593–2601. https://doi.org/10.1002/jssc.201300310
Horak KE, Fisher PM, Hopkins B (2018) Pharmacokinetics of anticoagulant rodenticides in target and non-target organisms. In: van den Brink NW et al (eds) Anticoagulant rodenticides and wildlife. Springer, Cham, pp 87–108
Hosea RC (2000) Exposure of non-target wildlife to anticoagulant rodenticides in California. In: Proceedings of the 19th vertebrate pest conference, pp 6–9
Huckle KR, Hutson DH, Warburton PA (1988) Elimination and accumulation of the rodenticide flocoumafen in rats following repeated oral administration. Xenobiotica 18:1465–1479. https://doi.org/10.3109/00498258809042269
Huerta B, Rodriguez-Mozaz S, Barcelo D (2012) Pharmaceuticals in biota in the aquatic environment: analytical methods and environmental implications. Anal Bioanal Chem 404:2611–2624. https://doi.org/10.1007/s00216-012-6144-y
Hughes J, Sharp E, Taylor MJ, Melton L, Hartley G (2013) Monitoring agricultural rodenticide use and secondary exposure of raptors in Scotland. Ecotoxicol 22:974–984. https://doi.org/10.1007/s10646-013-1074-9
Hunter K (1983a) Determination of coumarin anticoagulant rodenticide residues in animal tissue by high-performance liquid chromatography: II. Fluorescence detection using ion-pair chromatography. J Chromatogr A 270:277–283. https://doi.org/10.1016/S0021-9673(01)96372-2
Hunter K (1983b) Determination of coumarin anticoagulant rodenticide residues in animal tissue by high-performance liquid chromatography: I. Fluorescence detection using post-column techniques. J Chromatogr A 270:267–276. https://doi.org/10.1016/S0021-9673(01)96372-1
Imran M, Shafi H, Wattoo SA, Chaudhary MT, Usman HF (2015) Analytical methods for determination of anticoagulant rodenticides in biological samples. Forensic Sci Int 253:94–102. https://doi.org/10.1016/j.forsciint.2015.06.008
Jacob J, Buckle A (2018) Use of anticoagulant rodenticides in different applications around the world. In: van den Brink NW et al (eds) Anticoagulant rodenticides and wildlife. Springer, Cham, pp 11–43
Jacquot M, Coeurdassier M, Couval G et al (2013) Using long-term monitoring of red fox populations to assess changes in rodent control practices. J Appl Ecol 50:1406–1414. https://doi.org/10.1111/1365-2664.12151
Jin MC, Chen XH, Zhu Y (2007) Determination of five 4-hydroxycoumarin rodenticides in animal liver tissues by ion chromatography with fluorescence detection. J Chromatogr A 1155:57–61. https://doi.org/10.1016/j.chroma.2006.12.074
Jin MC, Chen XH, Ye ML, Zhu Y (2008) Analysis of indandione anticoagulant rodenticides in animal liver by eluent generator reagent free ion chromatography coupled with electrospray mass spectrometry. J Chromatogr A 1213:77–82. https://doi.org/10.1016/j.chroma.2008.08.100
Jin MC, Cai MQ, Chen XH (2009) Simultaneous measurement of indandione-type rodenticides in human serum by liquid chromatography-electrospray ionization- tandem mass spectrometry. J Anal Toxicol 33:294–300. https://doi.org/10.1093/jat/33.6.294
Jones A (1996) HPLC determination of anticoagulant rodenticide residues in animal livers. Bull Environ Contam Toxicol 56:8–15. https://doi.org/10.1007/s001289900002
Kammerer B, Kahlich R, Ufer M, Schenkel A, Laufer S, Gleiter CH (2005) Stereospecific pharmacokinetic characterisation of phenprocoumon metabolites, and mass-spectrometric identification of two novel metabolites in human plasma and liver microsomes. Anal Bioanal Chem 383:909–917. https://doi.org/10.1007/s00216-005-0113-7
Kasprzyk-Hordern B (2010) Pharmacologically active compounds in the environment and their chirality. Chem Soc Rev 39:4466–4503. https://doi.org/10.1039/c000408c
Kinney CA, Furlong ET, Werner SL, Cahill JD (2006) Presence and distribution of wastewater-derived pharmaceuticals in soil irrigated with reclaimed water. Environ Toxicol Chem 25:317–326. https://doi.org/10.1897/05-187R.1
Koivisto E, Koivisto P, Hanski IK et al (2016) Prevalence of anticoagulant rodenticides in non-target predators and scavengers in Finland. Finnish Safety and Chemicals Agency, Helsinki
Kolpin DW, Furlong ET, Meyer MT et al (2002) Pharmaceuticals, hormones, and other organic wastewater contaminants in US streams, 1999–2000: a national reconnaissance. Environ Sci Technol 36:1202–1211. https://doi.org/10.1021/es011055j
Kolpin DW, Skopec M, Meyer MT, Furlong ET, Zaugg SD (2004) Urban contribution of pharmaceuticals and other organic wastewater contaminants to streams during differing flow conditions. Sci Total Environ 328:119–130. https://doi.org/10.1016/j.scitotenv.2004.01.015
Kopanke JH, Horak KE, Musselman E et al (2018) Effects of low-level brodifacoum exposure on the feline immune response. Sci Rep 8:8168. https://doi.org/10.1038/s41598-018-26558-3
Kostich MS, Lazorchak JM (2008) Risks to aquatic organisms posed by human pharmaceutical use. Sci Total Environ 389:329–339. https://doi.org/10.1016/j.scitotenv.2007.09.008
Kostich MS, Batt AL, Lazorchak JM (2014) Concentrations of prioritized pharmaceuticals in effluents from 50 large wastewater treatment plants in the US and implications for risk estimation. Environ Pollut 184:354–359. https://doi.org/10.1016/j.envpol.2013.09.013
Kotthoff M, Rüdel H, Jürling H et al (2018) First evidence of anticoagulant rodenticides in fish and suspended particulate matter: spatial and temporal distribution in German freshwater aquatic systems. Environ Sci Pollut Res Int. https://doi.org/10.1007/s11356-018-1385-8
Krüger G, Solas H (2010) Nachbarn im Kanalnetz - Ergebnisse einer Fragebogenaktion zur Rattenbekämpfung. Korrespondenz Abwasser, Abfall 57:430–435
Laasko S, Suomalainen K, Koivisto S (2010) Literature review on residues of anticoagulant rodenticides in non-target animals. Nordic Council of Ministers, Copenhagen
Lambert O, Pouliquen H, Larhantec M, Thorin C, L’Hostis M (2007) Exposure of raptors and waterbirds to anticoagulant rodenticides (difenacoum, bromadiolone, coumatetralyl, coumafen, brodifacoum): epidemiological survey in Loire Atlantique (France). Bull Environ Contam Toxicol 79:91–94. https://doi.org/10.1007/s00128-007-9134-6
Lao W, Gan J (2012) Enantioselective degradation of warfarin in soils. Chirality 24:54–59. https://doi.org/10.1002/chir.21023
Larsen J (2003) Emission scenario document for biocides used as rodenticides. Danish EPA, Copenhagen
Lemarchand C, Rosoux R, Berny P (2010) Organochlorine pesticides, PCBs, heavy metals and anticoagulant rodenticides in tissues of Eurasian otters (Lutra lutra) from upper Loire River catchment (France). Chemosphere 80:1120–1124. https://doi.org/10.1016/j.chemosphere.2010.06.026
Lemarchand C, Rosoux R, Talon C, Berny P (2014) Flagship species conservation and introduced species invasion: toxic aspects along Loire River (France). In: pesticides—toxic aspects, pp 53–79. InTech
Lewis RJ, Trager WF, Chan KK et al (1974) Warfarin stereochemical aspects of its metabolism and the interaction with phenylbutazone. J Clin Investig 53:1607–1617. https://doi.org/10.1172/jci107711
Lin Y, Shen X, Yuan Q, Yan Y (2013) Microbial biosynthesis of the anticoagulant precursor 4-hydroxycoumarin. Nat Commun 4:2603. https://doi.org/10.1038/ncomms3603
Liphatech (2013) The veterinarian’s guide to accidental rodenticide ingestion by dogs & cats. Liphatech Inc., Milwaukee
Liu J, Xiong K, Ye X, Zhang J, Yang Y, Ji L (2015) Toxicity and bioaccumulation of bromadiolone to earthworm Eisenia fetida. Chemosphere 135:250–256. https://doi.org/10.1016/j.chemosphere.2015.04.058
López-Perea JJ, Camarero PR, Molina-López RA et al (2015) Interspecific and geographical differences in anticoagulant rodenticide residues of predatory wildlife from the Mediterranean region of Spain. Sci Total Environ 511:259–267. https://doi.org/10.1016/j.scitotenv.2014.12.042
Lund M (2015) Commensal rodents. In: Buckle AP, Smith RH (eds) Rodent pests and their control. CABI, Oxfordshire, pp 19–32
Magiera S, Pardylla A, Baranowska I (2015) Effects of various factors of ultrasonic treatment on the extraction recovery of drugs from fish tissues. Ultrason Sonochem 26:388–398. https://doi.org/10.1016/j.ultsonch.2015.03.005
Mandel F, Wendt J, Vistocco R, Bachmann C (2000) Development of an LC/MS method for the analysis of rodenticides. Application note 5989-8440EN. Agilent Technologies, Waldbronn
Marek LJ, Koskinen WC (2007) Multiresidue analysis of seven anticoagulant rodenticides by high-performance liquid chromatography/electrospray/mass spectrometry. J Agric Food Chem 55:571–576. https://doi.org/10.1021/jf061440y
Market Data Forecast (2017) Europe rodenticides market by type (non-anticoagulant, anticoagulant), by mode of application (pellets, powders, sprays), by end user (agricultural fields, urban centres, warehouses, pest control companies, household consumers), and by region-Industry analysis, size, share, growth, trends, and forecasts (2016–2021). Market Data Forecast Inc., Hyderabad
Marsalek P, Modra H, Doubkova V, Vecerek V (2015) Simultaneous determination of ten anticoagulant rodenticides in tissues by column-switching UHPLC-ESI-MS/MS. Anal Bioanal Chem. https://doi.org/10.1007/s00216-015-8954-1
Maruya KA, Dodder NG, Tang C, Lao W, Tsukada D (2015) Which coastal and marine environmental contaminants are truly emerging? Environ Sci Pollut Res 22:1644–1652. https://doi.org/10.1007/s11356-014-2856-1
Masuda BM, Fisher P, Beaven B (2015) Residue profiles of brodifacoum in coastal marine species following an island rodent eradication. Ecotoxicol Environ Saf 113:1–8. https://doi.org/10.1016/j.ecoenv.2014.11.013
McDonald RA, Harris S, Turnbull G, Brown P, Fletcher M (1998) Anticoagulant rodenticides in stoats (Mustela erminea) and weasels (Mustela nivalis) in England. Environ Pollut 103:17–23. https://doi.org/10.1016/S0269-7491(98)00141-9
McLeod L, Saunders G (2013) Pesticides used in the management of vertebrate pests in australia: a review. NSW Department of Primary Industries, New South Wales
Medvedovici A, David F, Sandra P (1997) Determination of the rodenticides warfarin, diphenadione and chlorophacinone in soil samples by HPLC-DAD. Talanta 44:1633–1640. https://doi.org/10.1016/S0039-9140(97)00068-4
Morrison SA, Sieve KK, Ratajczak RE, Bringolf RB, Belden JB (2016) Simultaneous extraction and cleanup of high-lipid organs from white sturgeon (Acipenser transmontanus) for multiple legacy and emerging organic contaminants using QuEChERS sample preparation. Talanta 146:16–22. https://doi.org/10.1016/j.talanta.2015.08.021
Murphy G, Oldbury DJ (2002) Rat control by local authorities within the United Kingdom. In: Proceedings of the 4th international conference on urban pests,vol 246, pp 413–420
Murray M (2011) Anticoagulant rodenticide exposure and toxicosis in four species of birds of prey presented to a wildlife clinic in Massachusetts, 2006–2010. J Zoo Wildl Med 42:88–97
Nakagawa L, de Masi E, Narciso E, Neto HM, Papini S (2015) Palatability and efficacy of bromadiolone rodenticide block bait previously exposed to environmental conditions. Pest Manag Sci 71:1414–1418. https://doi.org/10.1002/ps.3944
Newton I, Wyllie I, Freestone P (1990) Rodenticides in British barn owls. Environ Pollut 68:101–117. https://doi.org/10.1016/0269-7491(90)90015-5
Newton I, Wyllie I, Dale L (1997) Mortality causes in British Barn Owls (Tyto alba), based on 1,101 carcasses examined during 1963–1996. United States Department of Agriculture Forest Service, Winnipeg, pp 299–307
Norström K, Remberger M, Kaj L et al (2009) Results from the Swedish National Screening Programme 2008. Subreport 3. Biocides: difenacoum. IVL Swedish Environmental Research Institute, Stockholm
Ogilvie SC, Pierce RJ, Wright GRG, Booth LH, Eason CT (1997) Brodifacoum residue analysis in water, soil, invertebrates, and birds after rat eradication on Lady Alice Island. N Z J Ecol 21:195–197
Oktay E (2015) Will NOACs become the new standard of care in anticoagulation therapy? Int J Cardiovasc Acad 1:1–4. https://doi.org/10.1016/j.ijcac.2015.06.007
Ondracek K, Bandouchova H, Hilscherova K et al (2015) Mixture toxicity of microcystin-LR, paraoxon and bromadiolone in Xenopus laevis embryos. Neuro Endocrinol Lett 36:114–119
Parker R (2013) Gute Aussichten für die Zukunft. DpS 2:13–15
Pérez S, Barceló D (2008) Applications of LC-MS to quantitation and evaluation of the environmental fate of chiral drugs and their metabolites. TrAC Trends Anal Chem 27:836–846. https://doi.org/10.1016/j.trac.2008.09.003
Pieper C, Holthenrich D, Schneider H (2014) Health risks from pest control products. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 57:574–584. https://doi.org/10.1007/s00103-013-1920-1
Pitt WC, Berentsen AR, Shiels AB et al (2015) Non-target species mortality and the measurement of brodifacoum rodenticide residues after a rat (Rattus rattus) eradication on Palmyra Atoll, tropical Pacific. Biol Conserv 185:36–46. https://doi.org/10.1016/j.biocon.2015.01.008
Primus TM, Wright G, Fisher P (2005) Accidental discharge of brodifacoum baits in a tidal marine environment: a case study. Bull Environ Contam Toxicol 74:913–919. https://doi.org/10.1007/s00128-005-0668-1
Ramirez AJ, Brain RA, Usenko S et al (2009) Occurrence of pharmaceuticals and personal care products in fish: results of a national pilot study in the United States. Environ Toxicol Chem 28:2587–2597. https://doi.org/10.1897/08-561.1
Rattner BA, Mastrota FN (2018) Anticoagulant rodenticide toxicity to non-target wildlife under controlled exposure conditions. In: van den Brink NW et al (eds) Anticoagulant rodenticides and wildlife. Springer, Cham, pp 45–86
Rattner BA, Lazarus RS, Elliott JE, Shore RF, van den Brink N (2014) Adverse outcome pathway and risks of anticoagulant rodenticides to predatory wildlife. Environ Sci Technol 48:8433–8445. https://doi.org/10.1021/es501740n
Sage M, Fourel I, Coeurdassier M, Barrat J, Berny P, Giraudoux P (2010) Determination of bromadiolone residues in fox faeces by LC/ESI-MS in relationship with toxicological data and clinical signs after repeated exposure. Environ Res 110:664–674. https://doi.org/10.1016/j.envres.2010.07.009
Sanchez-Barbudo IS, Camarero PR, Mateo R (2012) Primary and secondary poisoning by anticoagulant rodenticides of non-target animals in Spain. Sci Total Environ 420:280–288. https://doi.org/10.1016/j.scitotenv.2012.01.028
Santos LH, Gros M, Rodriguez-Mozaz S et al (2013) Contribution of hospital effluents to the load of pharmaceuticals in urban wastewaters: identification of ecologically relevant pharmaceuticals. Sci Total Environ 461:302–316. https://doi.org/10.1016/j.scitotenv.2013.04.077
Sato S (2005) Coumarin rodenticides. In: Suzuki O, Watanabe K (eds) Drugs and poisons in humans. Springer, Heidelberg, pp 599–608
Schaff JE, Montgomery MA (2013) An HPLC-HR-MS-MS method for identification of anticoagulant rodenticides in blood. J Anal Toxicol 37:321–325. https://doi.org/10.1093/jat/bkt036
Schmolz E, Wieck S, Friesen A (2014) Nagetierbekämpfung mit Antikoagulanzien—was ändert sich durch die Biozid-Zulassung für die Praxis? UMID 2:79–86
Schwabe U, Paffrath D, Ludwig W-D, Klauber J (eds) (2017) Arzneiverordnungs-report. Springer, Heidelberg
Shore RF, Birks JDS, Afsar A, Wienburg CL, Kitchener AC (2003) Spatial and temporal analysis of second-generation anticoagulant rodenticide residues in polecats (Mustela putorius) from throughout their range in Britain, 1992–1999. Environ Pollut 122:183–193. https://doi.org/10.1016/S0269-7491(02)00297-X
Siers SR, Shiels AB, Goldade DA et al (2016) Wake atoll fish tissue sampling and analysis three years after an island wide rodenticide application. Unpublished Report QA 2241, USDA, APHIS, WS, National Wildlife Research Center, Hilo, HI, USA
Smith LL, Liang B, Booth MC, Filigenzi MS, Tkachenko A, Gaskill CL (2017) Development and validation of quantitative ultraperformance liquid chromatography-tandem mass spectrometry assay for anticoagulant rodenticides in liver. J Agric Food Chem 65:6682–6691. https://doi.org/10.1021/acs.jafc.7b02280
Steffen D (2014) Orientierende Untersuchungen niedersächsischer Oberflächengewässer auf aktuell in Deutschland zugelassener Pflanzenschutzmittel und auf Stoffe der sog. Metaboliten-Liste. Niedersächsischer Landesbetrieb für Wasserwirtschaft, Küsten-und Naturschutz (NLWKN), Hannover-Hildesheim, Germany
Streit B (1998) Bioaccumulation of contaminants in fish. In: Braunbeck T, Hinton DE, Striet B (eds) Fish ecotoxicology. Birkhäuser, Basel
Tosh DG, McDonald RA, Bearhop S et al (2011) Does small mammal prey guild affect the exposure of predators to anticoagulant rodenticides? Environ Pollut 159:3106–3112. https://doi.org/10.1016/j.envpol.2011.03.028
Tosh DG, McDonald RA, Bearhop S, Llewellyn NR, Montgomery WI, Shore RF (2012) Rodenticide exposure in wood mouse and house mouse populations on farms and potential secondary risk to predators. Ecotoxicology 21:1325–1332. https://doi.org/10.1007/s10646-012-0886-3
Townsend MG, Entwisle P, Hart ADM (1995) Use of two halogenated biphenyls as indicators of non-target exposure during rodenticides treatments. Bull Environ Contam Toxicol 54:526–533. https://doi.org/10.1007/BF00192595
UBA (2011) Identifizierung und Bewertung ausgewählter Arzneimittel und ihrer Metaboliten (Ab- und Umbauprodukte) im Wasserkreislauf. Texte 46/2011, Umweltbundesamt, Dessau-Roßlau
UBA (2014) Authorisation of anticoagulant rodenticides in Germany—risk mitigation measures, best practice code and FAQs. Umweltbundesamt, Dessau-Roßlau
van den Brink NW, Elliott JE, Shore RF, Rattner BA (eds) (2018) Anticoagulant rodenticides and wildlife. Springer, Cham
Vudathala D, Cummings M, Murphy L (2010) Analysis of multiple anticoagulant rodenticides in animal blood and liver tissue using principles of QuEChERS Method. J Anal Toxicol 34:273–279. https://doi.org/10.1093/jat/34.5.273
Walker LA, Turk A, Long SM, Wienburg CL, Best J, Shore RF (2008) Second generation anticoagulant rodenticides in tawny owls (Strix aluco) from Great Britain. Sci Total Environ 392:93–98. https://doi.org/10.1016/j.scitotenv.2007.10.061
Walker LA, Llewellyn NR, Pereira MG et al (2010) Anticoagulant rodenticides in predatory birds 2007 and 2008: a Predatory Bird Monitoring Scheme (PBMS) report. Center for Ecology and Hydrology, Lancaster
Wardlaw J, Hughes J, Monie C, Reay G (2016) Pesticide usage in Scotland—rodenticide use by local authorities 2015. Science and Advice for Scottish Agriculture, Edinburgh
Wardlaw J, Hughes J, Monie C, Reay G (2017) Pesticide usage in Scotland—rodenticides on arable farms 2016. Science and Advice for Scottish Agriculture, Edinburgh
Watkins CD, Winemiller KO, Mora MA et al (2014) Assessment of mosquitofish (Gambusia affinis) health indicators in relation to domestic wastewater discharges in suburbs of Houston, USA. Bull Environ Contam Toxicol 93:13–18. https://doi.org/10.1007/s00128-014-1248-z
Weigt S, Huebler N, Strecker R, Braunbeck T, Broschard TH (2012) Developmental effects of coumarin and the anticoagulant coumarin derivative warfarin on zebrafish (Danio rerio) embryos. Reprod Toxicol 33:133–141. https://doi.org/10.1016/j.reprotox.2011.07.001
Wode F, van Baar P, Dunnbier U et al (2015) Search for over 2000 current and legacy micropollutants on a wastewater infiltration site with a UPLC-high resolution MS target screening method. Water Res 69:274–283. https://doi.org/10.1016/j.watres.2014.11.034
Wong LT, Solomonraj G (1980) Biliary and urinary excretion of [14C]warfarin in rabbits. Xenobiotica 10:201–210. https://doi.org/10.3109/00498258009033746
Zielinska A, Lichti CF, Bratton S et al (2008) Glucuronidation of monohydroxylated warfarin metabolites by human liver microsomes and human recombinant UDP glucuronosyltransferases. J Pharmacol Exp Ther 324:139–148. https://doi.org/10.1124/jpet.107.129858
Acknowledgements
Support of this study was provided by the German Environment Agency through Grant FKZ 3716 67 403 0.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Regnery, J., Friesen, A., Geduhn, A. et al. Rating the risks of anticoagulant rodenticides in the aquatic environment: a review. Environ Chem Lett 17, 215–240 (2019). https://doi.org/10.1007/s10311-018-0788-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-018-0788-6