Abstract
As a potential feedstock for biofuel production, a high-cell-density continuous culture for the lipid production by Cryptococcus albidus was investigated in this study. The influences of dilution rates in the single-stage continuous cultures were explored first. To reach a high-cell-density culture, a single-stage continuous culture coupled with a membrane cell recycling system was carried out at a constant dilution rate of 0.36/h with varied bleeding ratios. The maximum lipid productivity of 0.69 g/L/h was achieved with the highest bleeding ratio of 0.4. To reach a better lipid yield and content, a two-stage continuous cultivation was performed by adjusting the C/N ratio in two different stages. Finally, a lipid yield of 0.32 g/g and lipid content of 56.4% were obtained. This two-stage continuous cultivation, which provided a higher lipid production performance, shows a great potential for an industrial-scale biotechnological production of microbial lipids and biofuel production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Decreasing petroleum resources and increasing environmental consequences from extracting and burning petroleum have gained tremendous attention to the exposing of sustainable renewable fuels. Major efforts on this matter have focused on the production of high-energy-density biofuels. During recent years, microbially produced lipid has been proved to be a promising alternative feedstock for the production of biodiesel, which has advantages in terms of fuel economy, compatibility with engines and current fuel delivery infrastructure and motor engine, and lower greenhouse gas emissions [33]. Oleaginous yeasts (e.g., Cryptococcus albidus) have been shown to accumulate significant amounts of lipids [9]. Compared to traditional agricultural feedstocks (oilseed plants), the production of microbial lipids offers many benefits, such as shorter life cycle, less labor demand, flexibility with location, season and climate as well as easier to scale-up [20].
With the advent of biotechnology, bioprocesses have been used to produce various value-added commodities. One of the objectives of biotechnology research is to improve productivity. For increasing volumetric productivity, design and operation of bioprocesses should be optimized. Increasing the concentration of lipids for biofuel production will be one of the most obvious methods of increasing productivity, which has been the objective of such techniques as two-stage cultivation, cell recycling, and novel bioreactor design [3, 8]. With regard to the production of the lipid, the ideal process would be the continuous culture process, which is the most efficient and cheapest means of microbial cultures for industrial applications [4]. The relatively low cell density in the culture system can limit the productivity of conventional bioconversion processes. High-cell-density continuous culture can provide much higher volumetric productivity than other suspension systems, such as batch and fed-batch cultivation [3]. To significantly improve the lipid productivity, it is necessary to boost enough cell density and control the concentration of nitrogen source to achieve a nutrient-limited condition [6].
Most studies of microbial lipid production were conducted in batch and fed-batch cultures that are able to provide a relatively higher cell density, but the lipid productivity is relatively low [10, 29, 31]. Although conventional continuous culture could provide a higher productivity, it is limited by the relatively low dilution rate causing a low cell density. Therefore, a membrane cell recycling (MCR) system has been developed to improve both cell density and productivity. Compared with sedimentation and filtration, this MCR bioprocess allows total recycling of cells. Hollow micro-channel filter membranes were first used for the ethanol production by recycling Zymomonas mobilis [17]. Since then, MCR has become an efficient technique for high-cell-density culture (HCDC) in general laboratory and pilot plant. HCDC always relies on the presence of sufficient oxygen supply in the aerobic cultivation by providing pure oxygen. Nevertheless, HCDC has been achieved in bioreactors with recycling cells using air only [16, 30, 32]. In addition to improving productivity, the MCR-based HCDC has the benefit of improving the average cell age in the bioreactors and being useful in the biosynthesis of desired products. However, to date, a very limited research has been reported on the effect of dilution rate and bleeding rate on lipid production under total cell recycling and partial cell recycling modes in high-cell-density cultivation.
Lipid production by oleaginous microorganisms starts when the cells exhaust the nitrogen source in the culture medium, but the excess of the carbon source is still supplied to be converted into lipids [22, 26]. In our previous report, a lipid content of 55% (w/w) was obtained in a two-stage fed-batch culture of C. albidus, presenting a 120% enhancement in lipid content [8]. Therefore, a two-stage continuous culture can ensure higher lipid content and productivity: in the first stage, cells grow in the nitrogen-excess medium for boosting cell mass with few lipids accumulated; in the second stage, however, the supply of nitrogen is restricted to ensure that all the carbon flux towards to lipid production.
In this study, various continuous cultivation systems for lipid production were developed and explored to accomplish a better lipid production performance in terms of lipid content, yield, and productivity. The aim of this study is also to evaluate the effects of operational variables, such as dilution rates and bleeding ratios. For higher cell density and lipid productivity, the membrane cell recycling approach was used and investigated in this high-cell-density culture. A two-stage continuous culture was also developed to achieve higher lipid content and productivity.
Materials and methods
Microorganism, media and chemicals
Cryptococcus albidus (ATCC 10,672) obtained from the Korea Biological Resource Center (Republic of Korea) was used in this study. The seed culture was performed using the minimal medium (pH 6.0) containing the following per liter distilled water: KH2PO4 3 g, MgSO4.7H2O 1 g, FeCl3.6H2O 15 mg and ZnSO4.7H2O 7.5 mg. The concentrations of glucose and ammonium are specified in the following section. Unless otherwise stated, all chemicals were purchased from Sigma-Aldrich Co. LLC (St. Louis, MO).
Single-stage continuous cultivation
A 2.5-L fermentor, fitted with pH and dissolved oxygen (DO) electrodes, was used. All continuous cultures were carried out in duplicates. The pH was controlled at pH 6.0 using 4 M NaOH automatically. DO concentration was maintained above 20% by increasing the agitation rate up to 1200 rpm and/or supplying pure oxygen automatically via the fermentor control system. The details of the setup for single-stage fermentor have been described in our previous work [16]. The fermentor was sterilized by autoclaving at 121 °C for 30 min. A 10% inoculum was added and grown as a batch culture for 24 h before commencing continuous culture. Cultures were grown at 25 °C with the air flowrate of 1 vvm (volume of air/volume of medium per min). The steady-stage conditions were obtained after at least five changes of the medium occurred before samples were taken on two consecutive days. It was considered that steady states were reached when the changes of DCW/OD and glucose concentrations were less than 5% in 12 h.
Membrane cell recycling (MCR) continuous cultivation
Figure 1 shows a schematic diagram of the MCR system used in this study. This process was set up as described previously [16]. Briefly, a hollow fiber membrane was coupled to a 1-L working volume fermentor (Bioflo model C30, New Brunswick Scientific Co.). Cell broth was pumped back to the fermentor by a peristaltic pump (Cole-Parmer Co, IL, USA). Fresh culture medium was continuously pumped into the fermentor. Meanwhile, the cell-free broth was removed through the hollow-fiber membrane system. Part of the cell broth was continuously removed through the effluent port to control cell density in the fermentor. Both the cell broth bled from the bleed effluent and the cell-free broth from the hollow-fiber membrane system were transferred into a harvesting container. The total influent flow rate was controlled to be exactly equal to the total effluent flow rate to achieve and maintain a steady state in continuous cultivation. When the steady state in MCR is maintained by controlling the bleed ratio, the material balances in MCR can be expressed by following equations [18]:
The hollow-fiber membrane used in this study was obtained from Bergh and Co. (Tübingen, Germany) and was asymmetric, with outer and inner diameters of 1.5 and 1.3 mm, respectively. The hollow-fiber membrane system was prepared in the authors’ laboratory by potting hollow-fiber bundles with polyurethane as described in previous studies [16, 21]. Before connecting the hollow-fiber membrane with the fermentor, the hollow-fiber membrane was first sterilized with 75% ethanol and then washed with sterilized distilled water. A batch culture was carried out before the MCR continuous culture with an initial glucose concentration of 35 g/L. The MCR continuous culture was performance at 40 h and culture medium was modified with glucose at 75 g/L and NH4Cl at 15 g/L to give a C/N ratio of 8 mol/mol.
Two-stage continuous cultivation
For growth in two-stage continuous cultures, the first and second fermentors were as previously specified in single-stage culture and contained 1 L of medium. The units were connected so that the entire output of fermentor one was pumped directly into fermentor two. The holdup time of yeast cells in the connecting tubing was less than 1 min. The composition of the medium for the first stage was nitrogen excess, i.e., with the above medium modified with NH4Cl at 4 g/L and glucose at 20 g/L (C/N ratio of 8 mol/mol). Glucose of 35 g/L along with NH4Cl of 1 g/L (C/N ratio of 61 mol/mol) was fed into the second stage which then caused nitrogen limited. Temperature, initial pH, and aeration rates for both fermentors were as above except when otherwise stated. The dilution rate was 0.05/h for this two-stage continuous culture.
Analyses
The cell density was determined with dry cell weight (DCW) as described before [8] The concentrations of glucose were analyzed by high-performance liquid chromatography [19]. Lipid extraction was performed according to the Soxhlet extractor method [9]. All the analytic measurements were performed in duplicates and data are expressed as a mean ± standard deviation. Significance level P was carried out using SPSS package (SPSS Inc., Chicago, IL, USA).
Results and discussion
The influence of dilution rates on lipid production in single-stage continuous culture
The effect of dilution rates on cell growth and lipid production was investigated by increasing the dilution rate from 0.02 to 0.13/h. In Fig. 2, the relation between the dilution rate and lipid accumulation is shown. The DCW gradually reduced with increasing dilution rate and at the dilution rate 0.13/h, the lipid titer reduced to 0.87 g/L from 7.95 g/L (at a dilution rate of 0.02/h). The effects of dilution rates on lipid productivity and yield in the continuous cultivation of C. albidus are summarized in Table 1. The lipid yield decreased with increasing the dilution rate, which may be due to the inefficient sugar utilization caused by the reduced retention time. It is interesting that the lipid productivity was increased with increasing the dilution rate from 0.01 to 0.05/h. However, the productivity started decreasing with increasing the dilution rate further. Similar findings were reported in the continuous cultures of R. toruloides [28] and C. curvata [2], indicating the dilution rate affected the lipid production in terms of lipid content, yield, and productivity. Finally, the highest productivity of 0.19 g/L/h was achieved in the intermediate dilution rate of 0.05/h, which was 1.7-folds higher than that at 0.13/h.
Enhanced lipid production in high-cell-density continuous culture with MCR
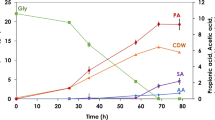
After a 40-h batch culture, a total cell recycling continuous culture was performed first without bleeding any cells. As shown in Fig. 3, the dilution rate was increased from 0.1/h to the highest 0.36/h in 88 h. By adding culture medium with 75 g/L glucose and 15 g/L NH4Cl during this period, both cell density (DCW) and lipid production increased concomitantly. Finally, a lipid content of 55% and high cell density of 70 g/L were achieved at the end of this total cell recycling culture. Figure 3 shows the effects of dilution rate on DCW and lipid content in the total cell recycle culture. It was calculated that the enhanced productivities of biomass and lipid were directly proportional to the increase of the dilution rate during total cell recycling continuous culture. The highest DCW of 68.6 g/L along with a lipid titer of 38.5 g/L was achieved with at the dilution rate of 0.36/h, which was determined by calculating lipid titer increased over the time interval from 72 to 84 h (Fig. 3). This lipid titer is nearly fivefold higher than that in single-stage continuous cultures (Table 1). However, a bleed was finally applied to achieve a steady state for maintaining a cell density suitable for operation especially when the fermentor becomes inoperable due to the high cell density [16].
Time course of membrane cell recycling continuous culture of C. albidus. Culture condition: total cell recycling continuous culture was carried out from 40 to 88 h under non-steady state by increasing the dilution rate without bleeding cells; perfusion cell recycling continuous culture was performed from 88 to 192 h by adjusting the bleeding ratio. During perfusion cell recycling continuous culture, DCW, lipid titer, and residual glucose were measured after reaching a steady state. Data points are the average of duplicate cultures. There was less than 5% variation between each data point
The bleeding started at 90 h and continued to about 100 h of culture time, with a stepwise increase of the bleed ratio from 0.05 to 0.4. A steady state was established after 12 h when the DCW and residual glucose were kept constant. It can be observed that a steady state was reached at 120 h, before increasing the bleeding ratio (Fig. 3). It was found that the concentrations of both cell density and lipid content decreased significantly with the increase of bleed ratio until the end of this culture. Accordingly, the residual glucose kept increasing to 40 g/L with a bleeding ratio of 0.4 at 192 h. As shown in Table 2, when the bleeding ratio was 0.4, the lipid productivity reached the maximum of 0.69 g/L/h. It is clear that lower bleeding ratio results in higher lipid titer and lipid yield indicating that the bleed ratio is critical in maintaining the desired cell density for lipid production. Our results suggest that high cell density culture can be achieved by regulating both dilution rate and bleed ratio together. The lipid productivity can also be improved dramatically by increasing the dilution rate without cell washout as long as the dilution rate × bleeding ratio does not exceed the maximum specific cell growth rate (Eq. 1). These characteristics of MCR continuous culture would be advantageous for high cell density and lipid production. Limitations of the performance of MCR culture, however, are only system problems, such as the filter capacity and sufficient oxygen supply. Although aforementioned problems can be resolved in pilot-scale demonstrations, some technical and economic issues observed in industrial scale in terms of membrane life, long-term operation, the final selling price of desired products are still the obstacles for the application of MCR system economically [5, 23]. It is clear that these issues still have impacts on both capital cost and operating cost in large-scale. Nevertheless, this MCR–HCDC system can be beneficial for the production of organic acids in the intracellular-based products [3, 11, 14, 15]. It has been reported that a multi-stage continuous culture system is able to provide better cell density and productivity in the manner of industrial applications [3, 25]. Therefore, a two-stage continuous culture system was developed and explored for better fermentation performance in lipid production in the following study.
Enhanced lipid production in two-stage continuous culture under nutrient limitation
Although a high lipid productivity was achieved in MCR continuous cultures using a high level bleeding ratio of 0.4 (Table 2), the lipid content and titer reduced significantly at 192 h (Fig. 3). It has been known that a nutrient-imbalance culture condition (e.g., nitrogen depletion) would result in higher lipid content because the excess carbon source is assimilated continuously to produce storage lipids [27]. To obtain both high lipid productivity and content, a two-stage continuous culture was carried out by adjusting the concentration of glucose and ammonia in different stages. The C/N ratio in the second stage was seven times higher than that in the first stage, whereas the nitrogen concentration was reduced by 75% in the second stage. As shown in Table 3, the lipid titer increased significantly up to 11.3 g/L in the second stage with a threefold enhancement from 2.8 g/L in the first stage. However, the non-lipid cell mass obtained in the first stage (6.7 g/L) and the second stage (8.8 g/L) only showed slight difference and the increased amount of cells in the second stage may account for the increased non-lipid cell mass. It is obvious that the carbon source was mainly used for cell proliferation rather than lipid production with the excess nitrogen source in the first stage. These results suggest that the growth of cells was fully achieved with supplying sufficient both carbon and nitrogen sources in the first stage and the increase of cell mass (DCW) in the second stage was primarily due to the lipid accumulation by limiting the nitrogen source. This result is in agreement with our previous study, in which a two-stage fed-batch cultivation improved the lipid production significantly by limiting the nitrogen supply [8].Therefore, by developing this two-stage culture system, both high lipid productivity (0.57 g/L/h) and high lipid content (57%) were achieved in our continuous cultivation (Table 3).
In conclusion, the better performance of lipid production in terms of lipid content and productivity as well as the sugar utilization efficiency makes this two-stage continuous culture system sufficiently attractive for an industrial evaluation, compared with other different continuous cultures (Table 4). The two-stage continuous cultivation that provided a similar productivity with the MCR continuous culture (bleeding ratio of 0.2 in Table 2) has advantages in terms of lipid content (57%) and an overall better fermentation system practice on design, operation and industrial scale-up [3, 24]. Moreover, the highest lipid yield (0.32 g/g) achieved from the two-stage continuous culture provides nearly 90% of the theoretical lipid yield (0.36 g/g [13]). From the standpoint of lipid production and the consideration of commercialization, therefore, a two-stage continuous culture is a promising approach for lipid production in industrial scale. Moreover, microbial lipid-derived biofuel produced from a two-stage HCDC using low-cost substrates derived from food waste or lignocellulosic feedstock also has the potential to become competitive with fossil-based diesel fuels [25].
Conclusion
As demonstrated in this work, MCR continuous culture has shown to be an effective culture mode for high cell density and lipid titer, even though it was relatively hard to operate under total cell recycling. For the routine industrial application of this technique, the introduction of cell perfusion is necessary to practically maintain HCDC lipid production. By controlling the C/N ratio in a two-stage continuous cultivation, the maximum lipid content (56.4%), yield (0.32 g/g) and productivity (0.57 g/L/h) were obtained. In this regard, a two-stage continuous cultivation was of great potential for the industrial-scale biotechnological production of microbial lipids and biofuel production.
References
Aggelis G, Komaitis M (1999) Enhancement of single cell oil production by Yarrowia lipolytica growing in the presence of Teucrium polium L. aqueous extract. Biotechnol Lett 21:747–749
Brown BD, Hsu KH, Hammond EG, Glatz BA (1989) A relationship between growth and lipid accumulation in Candida curvata D. J Ferment Bioeng 68:344–352
Chang HN, Jung K, Lee JC, Woo H-C (2014) Multi-stage continuous high cell density culture systems: a review. Biotechnol Adv 32:514–525
Chang HN, Kim N-J, Kang J, Jeong CM, Fei Q, Kim BJ, Kwon S, Lee SY, Kim J (2011) Multi-stage high cell continuous fermentation for high productivity and titer. Bioprocess Biosyst Eng 34:419–431
Chang HN, Yoo I-K, Kim BS (1994) High density cell culture by membrane-based cell recycle. Biotechnol Adv 12:467–487
Cheirsilp B, Kitcha S, Torpee S (2012) Co-culture of an oleaginous yeast Rhodotorula glutinis and a microalga Chlorella vulgaris for biomass and lipid production using pure and crude glycerol as a sole carbon source. Ann Microbiol 62:987–993
Evans CT, Ratledge C (1983) A comparison of the oleaginous yeast, candida curvata, grown on different carbon sources in continuous and batch culture. Lipids 18:623–629
Fei Q, Chang HN, Shang L (2011) Exploring low-cost carbon sources for microbial lipids production by fed-batch cultivation of Cryptococcus albidus. Biotechnol Bioprocess Eng 16:482–487
Fei Q, Chang HN, Shang L, Kim N, Kang J (2011) The effect of volatile fatty acids as a sole carbon source on lipid accumulation by Cryptococcus albidus for biodiesel production. Bioresour Technol 102:2695–2701
Galafassi S, Cucchetti D, Pizza F, Franzosi G, Bianchi D, Compagno C (2012) Lipid production for second generation biodiesel by the oleaginous yeast Rhodotorula graminis. Bioresour Technol 111:398–403
Haas C, El-Najjar T, Virgolini N, Smerilli M, Neureiter M (2017) High cell-density production of poly (3-hydroxybutyrate) in a membrane bioreactor. New Biotechnol 37:117–122
Hassan M, Blanc PJ, Granger L-M, Pareilleux A, Goma G (1993) Lipid production by an unsaturated fatty acid auxotroph of the oleaginous yeast Apiotrichum curvatum grown in single-stage continuous culture. Appl Microbiol Biotechnol 40:483–488
Huang W-D, Zhang Y-HP (2011) Analysis of biofuels production from sugar based on three criteria: thermodynamics, bioenergetics, and product separation. Energy Environ Sci 4:784–792
Ienczak JL, Schmidell W, de Aragão GMF (2013) High-cell-density culture strategies for polyhydroxyalkanoate production: a review. J Ind Microbiol Biotechnol 40:275–286. https://doi.org/10.1007/s10295-013-1236-z
Jansen ML, van Gulik WM (2014) Towards large scale fermentative production of succinic acid. Curr Opin Biotechnol 30:190–197
Lee CW, Chang HN (1987) Kinetics of ethanol fermentations in membrane cell recycle fermentors. Biotechnol Bioeng 29:1105–1112
Lee K, Lefebvre M, Tribe D, Rogers P (1980) High productivity ethanol fermentations with Zymomonas mobilis using continuous cell recycle. Biotechnol Lett 2:487–492
Lee YL, Chang HN (1990) High cell density culture of a recombinant Escherichia coli producing penicillin acylase in a membrane cell recycle fermentor. Biotechnol Bioeng 36:330–337
Li H, Kim N-J, Jiang M, Kang JW, Chang HN (2009) Simultaneous saccharification and fermentation of lignocellulosic residues pretreated with phosphoric acid–acetone for bioethanol production. Bioresour Technol 100:3245–3251
Liang Y, Cui Y, Trushenski J, Blackburn JW (2010) Converting crude glycerol derived from yellow grease to lipids through yeast fermentation. Bioresour Technol 101:7581–7586
Mahendran M, Pedersen S (2004) Method of potting hollow fiber membranes. US6685832B2
Meesters PAEP, Huijberts GNM, Eggink G (1996) High-cell-density cultivation of the lipid accumulating yeast Cryptococcus curvatus using glycerol as a carbon source. Appl Microbiol Biotechnol 45:575–579. https://doi.org/10.1007/s002530050731
O’Brien DJ, Roth LH, McAloon AJ (2000) Ethanol production by continuous fermentation–pervaporation: a preliminary economic analysis. J Membr Sci 166:105–111
Papanikolaou S, Aggelis G (2002) Lipid production by Yarrowia lipolytica growing on industrial glycerol in a single-stage continuous culture. Bioresour Technol 82:43–49
Park GW, Fei Q, Jung K, Chang HN, Kim Y-C, N-j Kim, J-d-r Choi, Kim S, Cho J (2014) Volatile fatty acids derived from waste organics provide an economical carbon source for microbial lipids/biodiesel production. Biotechnol J 9:1536–1546
Ratledge C (2002) Regulation of lipid accumulation in oleaginous micro-organisms. Biochem Soc Trans 30:1047–1050. https://doi.org/10.1042/bst0301047
Ratledge C (2014) The role of malic enzyme as the provider of NADPH in oleaginous microorganisms: a reappraisal and unsolved problems. Biotechnol Lett 36:1557–1568
Shen H, Gong Z, Yang X, Jin G, Bai F, Zhao ZK (2013) Kinetics of continuous cultivation of the oleaginous yeast Rhodosporidium toruloides. J Biotechnol 168:85–89
Shields-Menard SA, Amirsadeghi M, Sukhbaatar B, Revellame E, Hernandez R, Donaldson JR, French WT (2015) Lipid accumulation by Rhodococcus rhodochrous grown on glucose. J Ind Microbiol Biotechnol 42:693–699
Wen Z-Y, Chen F (2001) A perfusion–cell bleeding culture strategy for enhancing the productivity of eicosapentaenoic acid by Nitzschia laevis. Appl Microbiol Biotechnol 57:316–322
Wiebe MG, Koivuranta K, Penttilä M, Ruohonen L (2012) Lipid production in batch and fed-batch cultures of Rhodosporidium toruloides from 5 and 6 carbon carbohydrates. BMC Biotechnol 12:26
Ykema A, Verbree EC, Kater MM, Smit H (1988) Optimization of lipid production in the oleaginous yeast Apiotrichum curvatum in wheypermeate. Appl Microbiol Biotechnol 29:211–218. https://doi.org/10.1007/bf00939309
Yusuf N, Kamarudin S, Yaakub Z (2011) Overview on the current trends in biodiesel production. Energy Convers Manage 52:2741–2751
Acknowledgments
This work was supported by the Key Research and Development Program of Shaanxi Province (2017GY-146), the China postdoctoral science foundation (2017M623206), and the National Research Foundation of Korea Grant (NRF-2011-0009582).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fu, R., Fei, Q., Shang, L. et al. Enhanced microbial lipid production by Cryptococcus albidus in the high-cell-density continuous cultivation with membrane cell recycling and two-stage nutrient limitation. J Ind Microbiol Biotechnol 45, 1045–1051 (2018). https://doi.org/10.1007/s10295-018-2081-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-018-2081-x