Abstract
Oral health is maintained by the coordinated function of many organs including the teeth and salivary glands. Dysfunction of these organs causes many problems, such as dental caries, swallowing dysfunction and periodontal disease. Regenerative therapy for salivary gland tissue repair and whole-salivary gland replacement is currently considered a novel therapeutic concept that may have potential for the full recovery of salivary gland function. Salivary gland tissue stem cells are thought to be candidate cell sources for salivary gland tissue repair therapies. In addition, whole-salivary gland replacement therapy may become a novel next-generation organ regenerative therapy. Almost all organs arise from reciprocal epithelial and mesenchymal interactions of the germ layers. We developed a novel bioengineering method, an organ germ method that can reproduce organogenesis through the epithelial–mesenchymal interaction. A bioengineered salivary gland germ can regenerate a structurally correct salivary gland in vitro, and bioengineered salivary glands successfully secrete saliva into the oral cavity from ducts in the recipient through the reestablishment of the afferent–efferent neural network. The bioengineered salivary gland can also improve the symptoms of xerostomia, such as bacterial infection and swallowing dysfunction. In this review, we describe recent findings and technological developments of salivary gland regenerative therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Teeth and saliva play an important role in maintaining the health and homeostasis of the oral cavity by participating in functions such as chewing, digestion, cleaning and swallowing. Moreover, 95 % of the saliva that is secreted into the oral cavity is secreted from the main three pairs of salivary glands, which include the submandibular gland (SMG), sublingual gland (SLG) and parotid gland (PG), and the remaining 5 % is secreted from minor salivary glands [1–4]. The salivary gland is an ectodermal organ that arises from the salivary gland germ under the regulation of reciprocal epithelial–mesenchymal interactions and is composed of acinar, myoepithelial and duct cells. Serous saliva, which is rich in amylase protein, is secreted from the PG and contributes mainly to the digestion of food. In contrast, mucous saliva is secreted from the SLG, mainly contains glycoproteins such as mucin proteins and protects the oral cavity from drying. SMG secretes the seromucous saliva, which has both the mucous and serous character. Therefore, salivary gland dysfunction results in xerostomia and influences bodily health [5, 6].
Xerostomia is caused by autoimmune diseases such as Sjogren’s syndrome, radiation therapy for head and neck cancer, aging and side effects of various drugs [5–10]. As a result, clinical problems in oral health, such as dental decay, bacterial infection, mastication dysfunction and swallowing, are induced and result in a general reduction in quality of life [8]. Current therapies for xerostomia include administration of artificial saliva or saliva substitutes and parasympathetic stimulation drugs that promote salivation [9]. However, these therapies provide temporary effects and cannot restore salivary gland function. Therefore, the development of novel treatments for restoration of salivary gland function is needed [11].
Salivary gland regeneration therapy involving stem cell transplantation or gene modification may eventually be used to restore damaged tissue and recover the flow of saliva [12–16]. In addition, organ replacement therapy of ectodermal organs such as the teeth and hair follicles, which can be achieved by transplantation of bioengineered organ germs that have been reconstituted by organ culture methods, has been reported [17–20]. Recently, we induced salivary gland and the lacrimal gland regeneration using this method [21, 22]. In this review, we will discuss the novel findings and technologies for salivary gland regeneration and the possibility and feasibility of organ regenerative medicine for the salivary glands.
Salivary grand development
Ectodermal organs such as the teeth, hair follicles, salivary glands and lacrimal glands are generated from the organ germ induced by epithelial and mesenchymal stem cell interactions in the developing embryo (Fig. 1) [23, 24]. The development of the submandibular gland in mice is produced by the invagination of the oral epithelium into the mesenchymal region derived from the neural crest cells at the base of the tongue on mice embryonic day (ED) 11 (prebud) [2, 3, 25–27]. Invaginated epithelial tissue proliferates to form an epithelial stalk and a terminal bud at the tip (initial bud). During development, the epithelial stalk differentiates into duct cells and forms the excretory, striated and intercalated ducts that close the opening. The terminal bulb forms the branched structure of the gland by repeating the elongation and branching process during ED 12.5–13.5 (pseudoglandular) [28–30]. From ED 15.0 onward, the terminal bulbs differentiate into the acinar cells and mature to allow the synthesis of secretary proteins [31]. The types of secretory proteins differ depending on the type of salivary glands that produce them. The parotid and submandibular glands secrete serous saliva, which contains a large amount of digestive enzymes such as amylase, and the sublingual gland secretes mucous saliva, which, in mice, contains rich mucin protein. In humans, the submandibular gland is known as a seromucous gland that secretes both serous and mucous saliva [32–34]. After salivary gland development is complete, adult tissue stem cells are maintained in the excretory duct and contribute to the repair of injured tissue [35–37].
Schematic representation of the developmental stages of ectodermal organs. The ectodermal organs, including the teeth, hair follicles and salivary glands, are produced from organ germs induced by the interaction of the epithelial tissue and the condensed mesenchymal tissue derived from neural crest cells (ED11–12). The epithelial tissue invaginates into the mesenchymal tissue and forms a certain morphology according to the development of each organ. Salivary gland epithelial tissue is formed by the epithelial stalk and terminal bulb (ED12–E13), which will form the duct and acinar cells (E14-). The acinar cells mature and begin to synthesize and secrete secretory proteins (adult)
Salivary gland disease and treatment
Dysfunction of the salivary glands has been shown to cause atrophy of acinar cells and saliva reduction, resulting in xerostomia (dry mouth). In Europe, approximately 20 % of the population is said to suffer from dry mouth, and this disease has been estimated to occur in approximately 800 million people in Japan. Dry mouth can be caused by Sjögren’s syndrome (SS), radiation therapy for head and neck cancer, aging and side effects of various drugs [10]. The annual number of SS patients has been reported to be approximately 15,000–20,000 [38]. SS is an autoimmune disease that occurs frequently in middle-aged and elderly women, and it affects the salivary glands as well as other glands such as the lacrimal glands, resulting in dry eyes. Of all SS patients, approximately 70 % are positive for the SS antibody SSA (anti-Rho), and 40 % are positive for the SS antibody SSB (anti-La) [39–41]. However, these antibodies are not common to all patients, and the details of the pathogenic mechanism are not clear. Current therapies for dry mouth is administration of artificial saliva or saliva substitutes for increasing the moisture retention of the oral cavity [9, 40] and biologicals such as rituximab [41], abatacept [42] and belimumab [43] for suppressing the function of T cells and B cells. In addition, parasympathomimetic drugs, such as pilocarpine and cevimeline, have been used to stimulate residual functional salivary gland tissues. These drugs act on the M3 receptor and induce salivary flow.
Salivary gland regeneration using tissue repair
Transplantation of tissue stem cells has provided a method for regenerative therapy to restore damaged tissues and organs in diverse diseases [44, 45]. For salivary gland regeneration, tissue stem cell transplantation will be useful for partial acinar tissue repair, and gene therapy will affect the recovery of the amount of saliva produced [10, 46–55].
Tissue repair using adult tissue stem cells
Current studies of stem/progenitor cell studies indicate that tissue stem cells have the capacity to repair tissues in the intercalated duct of adult salivary glands. Atrophy of acinar cells induced by salivary gland duct obstruction can be repaired by tissue stem cells that are c-kit and sca-1 positive [12, 56]. Furthermore, stem cells that exhibit pluripotency can differentiate into liver or pancreas tissue [57, 58].
The acinar cells of the salivary gland are very susceptible to radiation; therefore, radiation therapy for head and neck cancer can induce atrophy in acinar cells and a reduction in saliva secretion. It is possible to culture c-kit positive salivary gland stem cells while maintaining the tissue repair capacity in vitro, and these cells can restore the amount of saliva produced by transplantation to the atrophied acinar cell region [12, 15, 59–61]. In addition, bone marrow-derived mesenchymal stem cells can increase the regenerative capacity of the salivary gland stem cells that remain in damaged salivary glands after irradiation [62]. Stem cell transplantation is expected to be an effective means for salivary gland regeneration.
Gene therapy for salivary gland regeneration
Genes can be introduced into salivary glands directly through a conduit from the opening of the oral cavity. Therefore, functional regeneration of salivary glands by gene therapy for the purpose of increasing the amount of saliva secreted into the oral cavity has been attempted. After infecting the dysfunctional adult salivary gland with irradiated adenovirus expressing the water channel aquaporin-1 (AQP1), the secretion of saliva, which was reduced by irradiation, was reported to be significantly restored [10, 16, 63]. The salivary gland not only functions as an exocrine gland to secrete saliva into the oral cavity, but also secretes material into the bloodstream that is circulated throughout the body. Gene transfer to salivary glands has also been performed as a treatment for other diseases [64–67]. It has been reported that some material, such as IL-17 receptor antibodies, growth hormones and erythropoietin, has been expressed in adult salivary glands by gene transfer and circulated throughout the body by the bloodstream [68–70]. Gene therapy, in addition to stem cell transfer therapy, is expected to be a new treatment strategy for salivary gland disorders and other diseases.
Whole-salivary gland regeneration by organ replacement
The possibility of partial tissue repair and recovery of the amount of secreted saliva has been demonstrated by stem cell transfer therapy and gene therapy. However, the final goal of regeneration of the salivary glands is the replacement of fully functional salivary glands in injured organs [54]. A recent study of salivary gland regeneration demonstrated that the only epithelial cell aggregation is produced by self-organization and branching in vitro. Moreover, the addition of mesenchymal cells to epithelial cells in this aggregate has been reported to increase the number and rate of formation of branches [71].
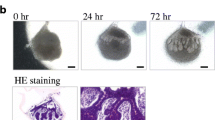
Recently, we showed the possibility of fully functional regeneration of ectodermal organs such as the teeth, hair follicles, salivary glands and lacrimal glands using “organ germ methods” that involved epithelial and mesenchymal stem cell manipulation techniques to induce the organ germ formation [17–20]. With this technique, it is possible to control the size, number and morphology of the regenerated organ; therefore, we expected to be able to control the invagination direction of the salivary gland [72]. The submandibular, sublingual and parotid gland germs were isolated from embryonic day (ED) 13.5–14.5 mice. The bioengineered salivary gland germ cells were acquired from single epithelial stem cells and mesenchymal stem cells by enzymatic treatment. The bioengineered salivary gland germ showed epithelial–mesenchymal interactions after 1 day and epithelial bud formation after 2 days in organ culture. (Fig. 2a, b) The regenerated sublingual and parotid glands also showed patterns similar to that of the submandibular gland.
Reconstitution of salivary gland using organ germ methods. a Schematic representation of the bioengineered submandibular gland germ reconstituted from epithelial and mesenchymal cells using the organ germ methods. The epithelial tissue invaginates into the mesenchymal tissue and forms the epithelial stalk and terminal bulb. b Phase-contrast images of the bioengineered submandibular gland germ at 0, 24 and 72 h of organ culture. The bioengineered submandibular gland was observed for invagination of epithelial tissue following the interaction between epithelial and mesenchymal cells. E epithelial stem cells, M mesenchymal stem cells
Regeneration of functional salivary glands in vivo
Oral functions are achieved using the acini, ducts and muscles under the control of the central nervous system. For successful salivary gland replacement therapy, bioengineered germ cells must be capable of connecting to ducts to secrete saliva into the oral cavity and achieve full functionality, including responsivity to afferent and efferent nervous stimulation from the oral cavity and regulation of water and protein secretion in response to sympathetic and parasympathetic stimulation.
Salivary gland regeneration by transplantation of the bioengineered germ
Saliva must be secreted into the oral cavity for functions such as food digestion, oral health, swallowing, pronunciation and the maintenance of tooth hard tissues [1–3]. In the clinical application of humans, by transfer of the submandibular gland to the submental space, salivary gland function may be successfully maintained and radiation-induced xerostomia prevented [73]. Therefore, for transplantation, it is important that the regenerated salivary glands secrete saliva into the oral cavity. Salivary gland defect model mice, which have excised submandibular, sublingual and parotid glands, show little secretion of saliva. A bioengineered salivary gland, transplanted into adult mice using an interepithelial tissue-connecting plastic method [20], was engrafted and connected to the parotid gland duct and bioengineered salivary gland epithelium (Fig. 3a) [21]. The tissue structure of the bioengineered salivary gland, including the localization of myoepithelial cells (Fig. 3b), the water channel aquaporin5 (AQP5) and neuronal connections (Fig. 3b), was similar to that of the natural tissue [21].
In vivo transplantation of the bioengineered salivary gland germ. a Photographs of the bioengineered submandibular gland at day 30 after transplantation. The bioengineered submandibular gland and parotid gland duct connection was observed. b Histological analysis of the submandibular gland (upper columns) and the sublingual gland (lower columns). Images of HE staining (left) and periodic acid and Schiff (PAS) staining (second from the left). The bioengineered submandibular gland was a mucous gland that showed a strongly positive PAS staining. Immunohistochemical images of calponin (red) and E-cadherin (green; third from the left) and calponin (red) and NF-H (green; right) are shown
Saliva secretion from the bioengineered salivary gland
Saliva secretion from the salivary glands is controlled by a balance between protein secretion by the sympathetic nervous system and water secretion by the parasympathetic nervous system [74–77]. The treatment for dry mouth includes pilocarpine acid and cevimeline, which is known to promote the secretion of saliva by stimulating muscarinic M3 receptors of the parasympathetic nervous system (Fig. 4a) [36]. Saliva secretion from the bioengineered submandibular gland is similar to that of the natural salivary gland and shows reactivity, which is induced by pilocarpine acid stimulation and inhibited by atropine, an antagonist of pilocarpine acid (Fig. 4b) [21]. In addition, although natural saliva contains amylase protein, which facilitates digestive functions, the secreted saliva from the bioengineered salivary gland contains amylase protein that also degrades starch [21].
Assessment of saliva secretion from the bioengineered salivary gland. a Schematic representation of the mechanism of saliva secretion. Saliva secretion including water and protein is controlled by a balance between the sympathetic and parasympathetic stimulation. b Assessment of the amount of saliva secretion associated with normal mice (light bars) and bioengineered submandibular gland-engrafted mice (dark bars) before and after the administration of pilocarpine, without or with atropine. The amount of secreted saliva had no significant difference between the natural mice and the bioengineered submandibular gland-engrafted mice
Control of saliva secretion by the central nervous system
Stimulation, such as food, heat and pain, induces saliva secretion via the afferent and efferent neural networks (Fig. 5a). In addition, saliva plays an important role in taste perception [78–80]. The saliva secretion from the bioengineered salivary gland via the central nervous system was analyzed using five tastes that are used in gustatory tests, including sour (citrate), bitter (quinine hydrochloride), salty (NaCl), sweet (sucrose) and umami (glutamate) [81, 82]. Gustatory stimulation with citrate induced significant quantities of saliva secretion compared to the control (Fig. 5b) [82]. Saliva secretion was induced in response to all tastes, not only the sour stimulus, and the secretion amount depended on the type of stimulus and exhibited the following order: sour > bitter > umami > salty = sweet (Fig. 5c) [82]. Analysis of saliva secretion by gustatory stimuli from bioengineered salivary glands demonstrates the potential for these glands to be controlled through the afferent–efferent neural network.
Saliva secretion controlled by the central nervous system. a Schematic representation of saliva secretion via the central nervous system. Gustatory stimulation induces saliva secretion. b Assessment of the amount of saliva secretion associated with natural mice (light bar) and bioengineered submandibular gland-engrafted mice (Bio; dark bar) after gustatory stimulation by citrate. The amount of secreted saliva had no significant difference. c The time course of the amount of saliva secretion associated with water stimulation (black dot) and gustatory stimulation (sour; green, bitter; yellow, umami; purple, sweet; red and salty; blue)
Functional recovery of dry mouth symptoms by saliva secretion
Saliva contains many proteins and cytokines, such as amylase, lysozyme, IgA, lactoferrin, myeloperoxidase, NGF, EGF and parotin, which are essential to the maintenance of oral health and homeostasis [83, 84]. Dysfunction of the salivary glands causes various problems such as dental caries, bacterial infection, sleep disorders and swallowing dysfunction [34]. The oral epithelium was protected from dryness and bacterial growth by secreted saliva from bioengineered salivary glands [21].
Among salivary gland functions, the swallowing function, which promotes the formation of a bolus of food or water, is critical for nutrition and reducing the risk of aspiration, which can cause chronic lung disease as well as affect the survival, quality of life, health and aging of an animal [85]. In salivary gland defect model mice, the body weight was abnormally decreased and all mice died within 5 days despite having free access to food and water (Fig. 6) [21]. The loss of body weight was rapid under conditions that did not provide water compared with those that did not provide food in natural mice, and the rate of decrease was consistent with the salivary gland defect model mice. Because dry mouth patients cannot swallow water, they often drink high-viscosity water. The salivary gland defect model mice exhibited a recovery of body weight and an increased survival rate by drinking high-viscosity water, which raises the possibility that dysphagia may occur in these animals. In contrast, all of the bioengineered salivary gland-engrafted mice survived, and their body weight increased 4 days after transplantation (Fig. 6) [21]. These findings indicate that the bioengineered salivary gland can improve the swallowing function associated with the maintenance of oral health.
Analysis of body weight and survival rate. Measurement of body weight (left graphs) and survival rate (right graphs) every 0.5 days after transplantation in normal mice (gray dots), salivary gland defect mice (black dots), salivary gland-engrafted mice (red dots) and salivary gland defect mice given high-viscosity water (green dots). All salivary gland defect mice died within 5 days (dragger) after the removal of all the major salivary glands
Future perspectives for salivary gland regenerative therapy
The progress that has been made in regenerative technology is remarkable, and many patients may be treated with salivary gland regenerative therapy. To achieve future clinical applications of salivary gland replacement therapy, suitable cell sources must be identified. Recent investigations of stem cell biology have led to the identification of candidate cell sources for salivary gland tissue regeneration and salivary gland replacement therapy [35–37]. Salivary gland adult tissue-derived stem cells, such as c-kit- and sca-1-positive cells and mesenchymal stem cells, can repair the injured acinar cells and partially recover the function of saliva secretion [12, 59–62]. Salivary gland adult stem cells would be valuable cell sources for stem cell transplantation therapy aimed toward salivary gland tissue regeneration. However, the potential of these stem cells to induce a salivary gland similar to that induced by an epithelial–mesenchymal interaction has not been reported. Pluripotent stem cells, such as embryonic stem (ES) cells and induced pluripotent stem (iPS) cells, are capable of differentiating into endodermal, ectodermal and mesodermal cells [86–88] and are also candidate cell sources for salivary gland regeneration. These pluripotent stem cells may be used to establish methods to induce salivary gland formation.
However, dry mouth due to SS is an autoimmune disease, and salivary gland damage such as atrophy of the acinar cells is caused by autoantigens [39–44]. Therefore, the possibility exists that acinar cells may again become atrophied due to autoimmune responses in the transplanted regenerated salivary glands in patients. Use of biologicals, which is one of the recent therapies for SS, can also aim at reducing disease activity and even regeneration of diseased tissue [42, 43, 89–91]. To achieve future clinical applications of salivary gland replacement therapy, it is necessary to perform a genetic modification that decreases the expression of autoantigens against patient-derived stem cells used for salivary gland regeneration in combination with the biologicals.
In this study, we have described the feasibility of salivary gland regenerative therapy. Recently, a novel treatment method for dry eye demonstrated the possibility of functional lacrimal gland regeneration by transplantation of bioengineered lacrimal gland germs [22]. Furthermore, by promoting fundamental technology development and clinical application of research for the regeneration of exocrine glands, organ replacement and the regeneration of exocrine glands may be realized.
References
Edgar M, Dawes C, Mullane OD. Saliva and oral health. 3rd ed. UK: British Dental Association; 2004.
Tucker AS. Salivary gland development. Semin Cell Dev Biol. 2007;18:237–44.
Tucker AS, Miletich I. Salivary glands, development, adaptations, and disease. London: Karger; 2010.
Ekstro MJ, Khosravani N, Castagnola M, Messana I. Saliva and the Control of Its Secretion. Berlin: Springer; 2012.
Vissink A, Burlage FR, Spijkervet FK, Jansma J, Coppes RP. Prevention and treatment of the consequences of head and neck radiotherapy. Crit Rev Oral Biol Med. 2003;14:213–25.
Vissink A, Jansma J, Spijkervet FK, Burlage FR, Coppes RP. Oral sequelae of head and neck radiotherapy. Crit Rev Oral Biol Med. 2003;14:199–212.
Ship JA, Pillemer SR, Baum BJ. Xerostomia and the geriatric patient. J Am Geriatr Soc. 2002;50:535–43.
Atkinson JC, Grisius M, Massey W. Salivary hypofunction and xerostomia: diagnosis and treatment. Dent Clin North Am. 2005;49:309–26.
Fox PC. Salivary enhancement therapies. Caries Res. 2004;38:241–6.
Aliko A, Wolff A, Dawes C, Aframian D, Proctor G, Ekström J, Narayana N, Villa A, Sia YW, Joshi RK, Mcgowan R, Beier Jensen S, Kerr AR, Lynge Pedersen AM, Vissink A. World workshop on oral medicine VI: clinical implications of medication-induced salivary gland dysfunction. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;. doi:10.1016/j.oooo.2014.10.027.
Kagami H, Wang S, Hai B. Restoring the function of salivary glands. Oral Dis. 2008;14:15–24.
Lombaert IM, Brunsting JF, Wierenga PK, Faber H, Stokman MA, Kok T, Visser WH, Kampinga HH, de Haan G, Coppes RP. Rescue of salivary gland function after stem cell transplantation in irradiated glands. PLoS One. 2008;3:e2063.
Feng J, Van der Zwaag M, Stokman MA, Van Os R, Coppes RP. Isolation and characterization of human salivary gland cells for stem cell transplantation to reduce radiation-induced hyposalivation. Radiother Oncol. 2009;92:466–71.
Baum BJ, Alevizos I, Zheng C, Cotrim AP, Liu S, McCullagh L, Goldsmith CM, Burbelo PD, Citrin DE, Mitchell JB, Nottingham LK, Rudy SF, Van Waes C, Whatley MA, Brahim JS, Chiorini JA, Danielides S, Turner RJ, Patronas NJ, Chen CC, Nikolov NP, Illei GG. Early responses to adenoviral-mediated transfer of the aquaporin-1 cDNA for radiation-induced salivary hypofunction. Proc Natl Acad Sci USA. 2012;109(47):19403–7.
Vissink A, van Luijk P, Langendijk JA, Coppes RP. Current ideas to reduce or salvage radiation damage to salivary glands. Oral Dis. 2015;21(1):e1–10.
O’Connell AC, Baccaglini L, Fox PC, O’Connell BC, Kenshalo D, Oweisy H, Hoque AT, Sun D, Herscher LL, Braddon VR, Delporte C, Baum BJ. Safety and efficacy of adenovirus-mediated transfer of the human aquaporin-1 cDNA to irradiated parotid glands of non-human primates. Cancer Gene Ther. 1999;6(6):505–13.
Nakao K, Morita R, Saji Y, Ishida K, Tomita Y, Ogawa M, Saitoh M, Tomooka Y, Tsuji T. The development of a bioengineered organ germ method. Nat Methods. 2007;4(3):227–30.
Ikeda E, Morita R, Nakao K, Ishida K, Nakamura T, Takano-Yamamoto T, Ogawa M, Mizuno M, Kasugai S, Tsuji T. Fully functional bioengineered tooth replacement as an organ replacement therapy. Proc Natl Acad Sci USA. 2009;106(32):13475–80.
Oshima M, Mizuno M, Imamura A, Ogawa M, Yasukawa M, Yamazaki H, Morita R, Ikeda E, Nakao K, Takano-Yamamoto T, Kasugai S, Saito M, Tsuji T. Functional tooth regeneration using a bioengineered tooth unit as a mature organ replacement regenerative therapy. PLoS One. 2011;6(7):e21531.
Toyoshima KE, Asakawa K, Ishibashi N, Toki H, Ogawa M, Hasegawa T, Irié T, Tachikawa T, Sato A, Takeda A, Tsuji T. Fully functional hair follicle regeneration through the rearrangement of stem cells and their niches. Nat Commun. 2012;3:784.
Ogawa M, Oshima M, Imamura A, Sekine Y, Ishida K, Yamashita K, Nakajima K, Hirayama M, Tachikawa T, Tsuji T. Functional salivary gland regeneration by transplantation of a bioengineered organ germ. Nat Commun. 2013;4:2498.
Hirayama M, Ogawa M, Oshima M, Sekine Y, Ishida K, Yamashita K, Ikeda K, Shimmura S, Kawakita T, Tsubota K, Tsuji T. Functional lacrimal gland regeneration by transplantation of a bioengineered organ germ. Nat Commun. 2013;4:2497.
Jiménez-Rojo L, Granchi Z, Graf D, Mitsiadis TA. Stem cell fate determination during development and regeneration of ectodermal organs. Front Physiol. 2012;3:107.
Pispa J, Thesleff I. Mechanisms of ectodermal organogenesis. Dev Biol. 2003;262(2):195–205.
Avery JK. Oral development and histology. New York: Thieme Press; 2002. p. 292–330.
Jaskoll T, Melnick M. Embryonic salivary gland branching morphogenesis. In: Madame Curie bioscience database 13–14. Austin: Landes Bioscience.
Knosp WM, Knox SM, Hoffman MP. Salivary gland organogenesis. Wiley Interdiscip Rev Dev Biol. 2012;1(1):69–82.
Sakai T. Epithelial branching morphogenesis of salivary gland: exploration of new functional regulators. J Med Invest. 2009;56(Suppl):234–8.
Hsu JC, Yamada KM. Salivary gland branching morphogenesis—recent progress and future opportunities. Int J Oral Sci. 2010;2(3):117–26.
Harunaga J, Hsu JC, Yamada KM. Dynamics of salivary gland morphogenesis. J Dent Res. 2011;90(9):1070–7.
Denny PC, Denny PA. Dynamics of parenchymal cell division, differentiation, and apoptosis in the young adult female mouse submandibular gland. Anat Rec. 1999;254:408–17.
Som PM, Brandwein MS. Salivary glands: anatomy and pathology. St. Louis: Head Neck Imaging; 2003. p. 2009–11.
Scott J. The proportional volume of mucous acinar cells in normal human submandibular salivary glands. Arch Oral Biol. 1979;24(6):479–81.
Dawes C, Pedersen AM, Villa A, Ekström J, Proctor GB, Vissink A, Aframian D, McGowan R, Aliko A, Narayana N, Sia YW, Joshi RK, Jensen SB, Kerr AR, Wolff A. The functions of human saliva: a review sponsored by the World Workshop on Oral Medicine VI. Arch Oral Biol. 2015;60(6):863–74.
Man YG, Ball WD, Marchetti L, Hand AR. Contributions of intercalated duct cells to the normal parenchyma of submandibular glands of adult rats. Anat Rec. 2011;263(2):202–14.
Ihrler S, Zietz C, Sendelhofert A, Lang S, Blasenbreu-Vogt S, Löhrs U. A morphogenetic concept of salivary duct regeneration and metaplasia. Virchows Arch. 2002;440(5):519–26.
Lombaert IM, Hoffman MP. Stem cells in salivary gland development and regeneration. Stem cells in craniofacial development and regeneration. Hoboken: Wiley-Blackwell; 2013. p. 271–84.
Hayashi Y, Arakaki R, Ishimaru N. Salivary gland and autoimmunity. J Med Invest. 2009;56:185–91.
Fox RI, Stern M, Michelson P. Update in Sjögren syndrome. Curr Opin Rheumatol. 2000;12(5):391–8.
Nakamura T, Matsui M, Uchida K, Futatsugi A, Kusakawa S, Matsumoto N, Nakamura K, Manabe T, Taketo MM, Mikoshiba K. M3 muscarinic acetylcholine receptor plays a critical role in parasympathetic control of salivation in mice. J Physiol. 2004;558:561–75.
Pijpe J, Meijer JM, Bootsma H, van der Wal JE, Spijkervet FK, Kallenberg CG, Vissink A, Ihrler S. Clinical and histologic evidence of salivary gland restoration supports the efficacy of rituximab treatment in Sjögren’s syndrome. Arthritis Rheum. 2009;60(11):3251–6.
Adler S, Körner M, Förger F, Huscher D, Caversaccio MD, Villiger PM. Evaluation of histologic, serologic, and clinical changes in response to abatacept treatment of primary Sjögren’s syndrome: a pilot study. Arthritis Care Res (Hoboken). 2013;65(11):1862–8.
Mariette X, Seror R, Quartuccio L, Baron G, Salvin S, Fabris M, Desmoulins F, Nocturne G, Ravaud P, De Vita S. Efficacy and safety of belimumab in primary Sjögren’s syndrome: results of the BELISS open-label phase II study. Ann Rheum Dis. 2015;74(3):526–31.
Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354:1813–26.
Segers VFM, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008;451:937–42.
Pringle S, Van Os R, Coppes RP. Concise review: adult salivary gland stem cells and a potential therapy for xerostomia. Stem Cells. 2013;31(4):613–9.
Nanduri LS, Baanstra M, Faber H, Rocchi C, Zwart E, de Haan G, van Os R, Coppes RP. Purification and ex vivo expansion of fully functional salivary gland stem cells. Stem Cell Rep. 2014;3(6):957–64.
Rotter N, Oder J, Schlenke P, Lindner U, Böhrnsen F, Kramer J, Rohwedel J, Huss R, Brandau S, Wollenberg B, Lang S. Isolation and characterization of adult stem cells from human salivary glands. Stem Cells Dev. 2008;17(3):509–18.
Denny PC, Denny PA. Dynamics of parenchymal cell division, differentiation, and apoptosis in the young adult female mouse submandibular gland. Anat Rec. 1999;254(3):408–17.
Horie K, Kagami H, Hiramatsu Y, Hata K, Shigetomi T, Ueda M. Selected salivary-gland cell culture and the effects of isoproterenol, vasoactive intestinal polypeptide and substance P. Arch Oral Biol. 1996;41(3):243–52.
Sugito T, Kagami H, Hata K, Nishiguchi H, Ueda M. Transplantation of cultured salivary gland cells into an atrophic salivary gland. Cell Transplant. 2004;13(6):691–9.
Bücheler M, Wirz C, Schütz A, Bootz F. Tissue engineering of human salivary gland organoids. Acta Otolaryngol. 2002;122(5):541–5.
Tran SD, Wang J, Bandyopadhyay BC, Redman RS, Dutra A, Pak E, Swaim WD, Gerstenhaber JA, Bryant JM, Zheng C, Goldsmith CM, Kok MR, Wellner RB, Baum BJ. Primary culture of polarized human salivary epithelial cells for use in developing an artificial salivary gland. Tissue Eng. 2005;11(1–2):172–81.
Sun T, Zhu J, Yang X, Wang S. Growth of miniature pig parotid cells on biomaterials in vitro. Arch Oral Biol. 2006;51(5):351–8.
Kishi T, Takao T, Fujita K, Taniguchi H. Clonal proliferation of multipotent stem/progenitor cells in the neonatal and adult salivary glands. Biochem Biophys Res Commun. 2006;340(2):544–52.
Takahashi S, Schoch E, Walker NI. Origin of acinar cell regeneration after atrophy of the rat parotid induced by duct obstruction. Int J Exp Pathol. 1998;79:293–301.
Hisatomi Y, Okumura K, Nakamura K, Matsumoto S, Satoh A, Nagano K, Yamamoto T, Endo F. Flow cytometric isolation of endodermal progenitors from mouse salivary gland differentiate into hepatic and pancreatic lineages. Hepatology. 2004;39(3):667–75.
Nanduri LS, Lombaert IM, van der Zwaag M, Faber H, Brunsting JF, van Os RP, Coppes RP. Salisphere derived c-Kit + cell transplantation restores tissue homeostasis in irradiated salivary gland. Radiother Oncol. 2013;108(3):458–63.
Okumura K, Nakamura K, Hisatomi Y, Nagano K, Tanaka Y, Terada K, Sugiyama T, Umeyama K, Matsumoto K, Yamamoto T, Endo F. Salivary gland progenitor cells induced by duct ligation differentiate into hepatic and pancreatic lineages. Hepatology. 2003;38(1):104–13.
Okumura K, Shinohara M, Endo F. Capability of tissue stem cells to organize into salivary rudiments. Stem Cells Int. 2012;2012:502136.
Nanduri LS, Maimets M, Pringle SA, van der Zwaag M, van Os RP, Coppes RP. Regeneration of irradiated salivary glands with stem cell marker expressing cells. Radiother Oncol. 2011;99(3):367–72.
Sumita Y, et al. Bone marrow-derived cells rescue salivary gland function in mice with head and neck irradiation. Int J Biochem Cell Biol. 2011;43:80–7.
Delporte C, O’Connell BC, He X, Lancaster HE, O’Connell AC, Agre P, Baum BJ. Increased fluid secretion after adenoviral-mediated transfer of the aquaporin-1 cDNA to irradiated rat salivary glands. Proc Natl Acad Sci USA. 1997;94(7):3268–73.
Palomino A, Hernández-Bernal F, Haedo W, Franco S, Más JA, Fernández JA, Soto G, Alonso A, González T, López-Saura P. A multicenter, randomized, double-blind clinical trial examining the effect of oral human recombinant epidermal growth factor on the healing of duodenal ulcers. Scand J Gastroenterol. 2000;35(10):1016–22.
Sonis ST, Peterson RL, Edwards LJ, Lucey CA, Wang L, Mason L, Login G, Ymamkawa M, Moses G, Bouchard P, Hayes LL, Bedrosian C, Dorner AJ. Defining mechanisms of action of interleukin-11 on the progression of radiation-induced oral mucositis in hamsters. Oral Oncol. 2000;36(4):373–81.
Dörr W, Noack R, Spekl K, Farrell CL. Modification of oral mucositis by keratinocyte growth factor: single radiation exposure. Int J Radiat Biol. 2001;77(3):341–7.
Baum BJ, Voutetakis A, Wang J. Salivary glands: novel target sites for gene therapeutics. Trends Mol Med. 2004;10(12):585–90.
Kagami H, O’Connell BC, Baum BJ. Evidence for the systemic delivery of a transgene product from salivary glands. Hum Gene Ther. 1996;7(17):2177–84.
He X, Goldsmith CM, Marmary Y, Wellner RB, Parlow AF, Nieman LK, Baum BJ. Systemic action of human growth hormone following adenovirus-mediated gene transfer to rat submandibular glands. Gene Ther. 1998;5(4):537–41.
Voutetakis A, Bossis I, Kok MR, Zhang W, Wang J, Cotrim AP, Zheng C, Chiorini JA, Nieman LK, Baum BJ. Salivary glands as a potential gene transfer target for gene therapeutics of some monogenetic endocrine disorders. J Endocrinol. 2005;185(3):363–72.
Wei CL, Larsen M, Hoffman MP, Yamada KM. Self-organization and branching morphogenesis of primary salivary epithelial cells. Tissue Eng. 2007;13(4):721–35.
Ishida K, Murofushi M, Nakao K, Morita R, Ogawa M, Tsuji T. The regulation of tooth morphogenesis is associated with epithelial cell proliferation and the expression of Sonic hedgehog through epithelial–mesenchymal interactions. Biochem Biophys Res Commun. 2011;405(3):455–61.
Zhang Y, Guo CB, Zhang L, Wang Y, Peng X, Mao C, Yu GY. Prevention of radiation-induced xerostomia by submandibular gland transfer. Head Neck. 2012;34(7):937–42.
Matsuo R, Yamamoto T, Yoshitaka K, Morimoto T. Neural substrates for reflex salivation induced by taste, mechanical, and thermal stimulation of the oral region in decerebrate rats. Jpn J Physiol. 1989;39:349–57.
Matsuo R, Garrett JR, Proctor GB, Carpenter GH. Reflex secretion of proteins into submandibular saliva in conscious rats, before and after preganglionic sympathectomy. J Physiol. 2000;527:175–84.
Proctor GB, Carpenter GH. Regulation of salivary gland function by autonomic nerves. Auton Neurosci. 2007;133:3–18.
Turner RJ, Sugiya H. Understanding salivary fluid and protein secretion. Oral Dis. 2002;8(1):3–11.
Matsuo R. Role of saliva in the maintenance of taste sensitivity. Crit Rev Oral Biol Med. 2000;11:216–29.
Froehlich DA, Pangborn RM, Whitaker JR. The effect of oral stimulation on human parotid salivary flow rate and alpha-amylase secretion. Physiol Behav. 1987;41(3):209–17.
Sasano T, Satoh-Kuriwada S, Shoji N, Sekine-Hayakawa Y, Kawai M, Uneyama H. Application of umami taste stimulation to remedy hypogeusia based on reflex salivation. Biol Pharm Bull. 2010;33(11):1791–5.
Sasano T, Satoh-Kuriwada S, Shoji N, Sekine-Hayakawa Y, Kawai M, Uneyama H. Application of umami taste stimulation to remedy hypogeusia based on reflex salivation. Biol Pharm Bull. 2010;33:1791–5.
Ogawa M, Yamashita K, Niikura M, Nakajima K, Toyoshima KE, Oshima M, Tsuji T. Saliva secretion in engrafted mouse bioengineered salivary glands using taste stimulation. J Prosthodont Res. 2014;58(1):17–25.
Lamy E, Graca G, Costa GD, Franco C, Silva FC, Baptista ES, et al. Changes in mouse whole saliva soluble proteome induced by tannin-enriched diet. Proteome Sci. 2010;8:65.
Cohen S. Isolation of a mouse submaxillary gland protein accelerating incisor eruption and eyelid opening in the new-born animal. J Biol Chem. 1962;237:1555–62.
Sreebny LM, Schwartz SS. A reference guide to drugs and dry mouth—2nd edition. Gerodontology. 1997;14(1):33–47.
Wu SM, Hochedlinger K. Harnessing the potential of induced pluripotent stem cells for regenerative medicine. Nat Cell Biol. 2011;13(5):497–505.
Cohen DE, Melton D. Turning straw into gold: directing cell fate for regenerative medicine. Nat Rev Genet. 2011;12(4):243–52.
Yan X, Qin H, Qu C, Tuan RS, Shi S, Huang GT. iPS cells reprogrammed from human mesenchymal-like stem/progenitor cells of dental tissue origin. Stem Cells Dev. 2010;19(4):469–80.
Ramos-Casals M, Tzioufas AG, Stone JH, Sisó A, Bosch X. Treatment of primary Sjögren syndrome: a systematic review. JAMA. 2010;304(4):452–60.
Meiners PM, Vissink A, Kallenberg CG, Kroese FG, Bootsma H. Treatment of primary Sjögren’s syndrome with anti-CD20 therapy (rituximab). A feasible approach or just a starting point? Expert Opin Biol Ther. 2011;11(10):1381–94.
Meiners PM, Vissink A, Kroese FG, Spijkervet FK, Smitt-Kamminga NS, Abdulahad WH, Bulthuis-Kuiper J, Brouwer E, Arends S, Bootsma H. Abatacept treatment reduces disease activity in early primary Sjögren’s syndrome (open-label proof of concept ASAP study). Ann Rheum Dis. 2014;73(7):1393–6.
Acknowledgments
This work was partially supported by a Grant-in-Aid for KIBAN (A) from the Ministry of Education, Culture, Sports and Technology (no. 25242041). This work was also partially supported by Organ Technologies Inc.
Conflict of interest
M. Ogawa and T. Tsuji have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ogawa, M., Tsuji, T. Functional salivary gland regeneration as the next generation of organ replacement regenerative therapy. Odontology 103, 248–257 (2015). https://doi.org/10.1007/s10266-015-0210-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10266-015-0210-9