Abstract
Oral health and homeostasis are maintained by the functional interactions of many organs, including the salivary glands, teeth, and tongue. Salivary gland dysfunction leads to dry mouth diseases, such as dental caries, bacterial infection, swallowing dysfunction, and reduced quality of life. The current clinical therapies for dry mouth are temporary, and they cannot repair salivary gland dysfunction. Salivary gland regenerative therapy with tissue repair and whole salivary gland replacement is a novel organ regenerative therapy. To achieve the recovery of the salivary gland function, adult tissue stem cells may be used as a cell source for salivary gland tissue repair therapies. To attain the entire salivary gland replacement therapy, which represents the next-generation regenerative therapy, we developed a novel cell manipulation method that can regenerate the ectodermal organ germ. The bioengineered salivary gland germs successfully engrafted grew in the transplantation site, generating the correct structure. The bioengineered salivary glands were able to secrete saliva into the oral cavity and improve dry mouth symptoms. In this chapter, we describe the recent progress and developmental methods for salivary gland regeneration therapy.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Salivary gland regeneration
- Salivary gland replacement regenerative therapy
- Saliva
- Bioengineered salivary gland
- Organ germ method
- Transplantation
7.1 Introduction
The salivary gland is an exocrine organ that synthesizes and secretes saliva. There are three major pairs of glands: the parotids (PG), submandibular glands (SMG), and sublingual glands (SLG) (Fig. 7.1a). Additionally, there are many minor salivary glands. The SMG and PG secrete serous saliva, which mainly contains amylase proteins. SLG secrete mucous saliva, which contains glycoproteins, such as mucin proteins (Edgar et al. 2004; Tucker and Miletich 2010; Avery 2002). Saliva plays various roles, including food digestion, taste, swallowing, protection from dryness, and oral health maintenance and homeostasis. Thus, salivary gland dysfunction induces various clinical problems in oral health. Salivary gland dysfunction is attributed to acinar cell atrophy, which is caused by radiation therapy for patients with head and neck cancer, aging, and autoimmune diseases (such as Sjögren’s syndrome), and can be a side effect of various medications. Acinar cell atrophy results in xerostomia (dry mouth syndrome) (Saleh et al. 2015; Vissink et al. 2010; Ship et al. 2002; Fox 2004).
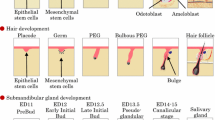
Schematic representation of the salivary glands. (a) The three major salivary glands include the submandibular glands, sublingual glands, and parotid glands. (b) Development of submandibular glands which are produced from organ germ induced by the interaction of reciprocal epithelial and mesenchymal tissue (EDs 11–12). The epithelial tissue invaginates into the mesenchymal tissue and forms the epithelial stalk and terminal bulb (EDs 12–13), which form the duct and acinar cells (ED 14). The acinar cells mature and begin to synthesize and secrete secretory proteins (adult)
Xerostomia causes various clinical oral problems, such as serious dental decay, oral bacterial infection, taste disorder, voice disorder, and swallowing disorder, which result in a general reduction in the quality of life (Atkinson et al. 2005). Current therapies for xerostomia include symptomatic treatments, the use of artificial saliva substitutes, and the administration of salivary gland stimulants and sialogogues, which enhance moisture retention in the oral cavity (Fox 2004; Nakamura et al. 2004). Parasympathetic stimulation drugs, such as pilocarpine and cevimeline, promote saliva secretion via the stimulation of residual acinar cells (Fox 2004). However, the effects of these therapies are temporary, and they cannot reproduce salivary gland dysfunction. Therefore, the development of alternative treatments that provide enduring effects or recover salivary gland function is expected (Kagami et al. 2008).
Recent regenerative therapy to restore organ function has been developed in many research fields, such as developmental biology, stem cell biology, and tissue engineering (Brockes and Kumar 2005; Langer and Vacanti 1999; Atala 2005; Madeira et al. 2015). Notably, transplantation therapy with tissue stem cells or cell sheet has been attempted for the repair of damaged tissues and organs in divergent diseases many years ago (Copelan 2006; Segers and Lee 2008). In salivary gland regeneration therapy, many research groups have reported various strategies including stem cell transplantation, gene modification, and tissue engineering to reproduce the damaged acinar tissue and restore saliva secretion (Yoo et al. 2014; Feng et al. 2009; O’Connell et al. 1999). Recently, ectodermal organ regeneration has been reported using bioengineered organ germ transplantation methods (see Chaps. 5, 6, and 8). In this chapter, we will discuss the recent findings and technologies for partial salivary gland tissue repair and whole salivary gland regeneration as a next-generation regenerative therapy that can recover function and prevent xerostomia.
7.2 Salivary Gland Development During Embryogenesis
The salivary gland is an exocrine organ arising from the salivary gland germ, which is generated by reciprocal interactions between the oral ectodermal epithelium and the neural crest-derived mesenchyme during embryogenesis (Tucker and Miletich 2010; Knosp et al. 2012; Patel et al. 2006; Knox and Hoffman 2008) (Fig. 7.1b). On embryonic day (ED) 11, the mesenchymal cells provide signals and induce oral epithelial thickening and invagination (Knosp et al .2012; Jaskoll and Melnick 2004). The expression of Fgf10, Fgfr2b, Pitx1, and p63 is essential for initial salivary gland development. The epithelial bud grows and forms terminal bulbs and a stalk (initial bud), and then branching morphogenesis occurs, including cell proliferation, cleft formation, migration, and apoptosis, which proceed during EDs 12.5–14.5 (pseudoglandular) (Sakai 2009; Hsu and Yamada 2010; Harunaga et al. 2011). After ED 15.0, the salivary gland germ begins functional differentiation. The epithelial stalk differentiates into duct cells, including the excretory, striated, and intercalated ducts, and the terminal bulbs differentiate into acinar cells and mature (Denny and Denny 1999). There are three types of acinar cells: the serous, mucous, and seromucous cells. The seromucous cells secrete both serous and mucous saliva. In the excretory duct, adult tissue stem cells are maintained and supplied to the acinar and duct cells after the salivary gland tissue is injured (Man et al. 2011; Ihrler et al. 2002; Lombaert and Hoffman 2013).
7.3 Salivary Gland Tissue Repair Using Tissue-Derived Stem Cells
Adult tissue-derived stem cells have a general capacity for self-renewal and differentiation to repair injured tissue (Fig. 7.2). Salivary gland-derived stem cells have been isolated and characterized from the exocrine ducts of PG and SMG (Rotter et al. 2008; Lombaert et al. 2008; Jeong et al. 2013; Kawakami et al. 2013). The salivary gland-derived stem cells isolated from PG express mesenchymal stem cell (MSC) markers (CD44, CD49f, CD90, and CD105). These cells have the ability to differentiate into adipocytes, osteocytes, and chondrocytes and have the capacity to recover their function in radiation-damaged salivary glands (Rotter et al. 2008). The salivary gland-derived stem cells isolated from SMG express stem cell markers (c-kit and scal-1), and these cells can induce acinar and duct cells. Furthermore, these stem cells have the potential to differentiate into liver or pancreas tissues and to form salispheres in in vitro cultures. The salisphere can repair radiation-induced atrophied acinar cells by stem cell transplantation, restoring saliva flow (Lombaert et al. 2008). Additionally, bone marrow MSCs or extracts called “soups” have the potential to repair damaged tissues, increase the tissue regeneration ability of the surviving salivary gland tissue stem cells, and promote the regeneration of damaged acinar cells after radiation (Sumita et al. 2011; Tran et al. 2013). Tissue repair by adult tissue-derived stem cell transplantation therefore has the therapeutic potential to regenerate salivary glands.
7.4 Salivary Gland Tissue Repair Using Gene Therapy
Gene therapy is also a general technique for salivary gland regeneration (Rotter et al. 2008; Denny and Denny 1999; Horie et al. 1996; Sugito et al. 2004; Bücheler et al. 2002; Tran et al. 2005; Sun et al. 2006; Kishi et al. 2006) (Fig. 7.2). Because the salivary glands have ducts that open into the oral cavity and are close to the surface, it is very convenient to directly inject into the ductal epithelium with an adenovirus or adeno-associated virus that expresses a particular gene. Gene transfer of water channel aquaporin-1 (AQP1), which is important for transcellular water transport, can significantly restore saliva secretion in irradiated salivary glands (Delporte et al .1997). Additionally, interleukin-17 (IL-17) receptor antibodies, growth hormones, and erythropoietin protein have been altered using gene transfer in salivary gland tissue. Because the salivary glands have both exocrine and endocrine functions, substances are secreted into the bloodstream and can perform systemic functions (Kagami et al. 1996; He et al. 1998; Voutetakis et al. 2005). Gene therapy has progressed to phase I and is also expected to be a new strategy for the regeneration of salivary glands and other organs.
7.5 Salivary Gland Tissue Repair Using Tissue Engineering Therapy
The important aspects involved in the tissue engineering of salivary glands are cell-cell adhesion, cell-extracellular matrix (ECM) protein adhesion, and the biocompatible and biodegradable 3D scaffold used, which can maintain the adhesion (Aframian and Palmon 2008) (Fig. 7.2). ECM proteins, such as laminin and glycosaminoglycans, are important for salivary gland epithelial cell polarity and proliferation. A combination of scaffolds, including collagen gel, Matrigel, hyaluronic acid (HA), and polyglycolic acid, causes physical changes and promotes cell migration, polarity, and cell adhesion (Peters et al. 2014; Pradhan and Farach-Carson 2010; Lombaert et al. 2016). Therefore, it is important to select the suitable cells, ECM, and scaffold and to take into consideration the molecules involved in salivary gland development and branch morphogenesis.
7.6 Whole Salivary Gland Regeneration Using Organ Germ Methods
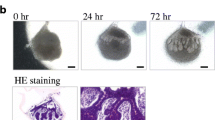
The ultimate goal of regenerative organs is the replacement of injured and dysfunctional organs with fully functional bioengineered organs. One concept for functional organ regeneration is to mimic the developmental process of organogenesis. Organ germ reconstruction using a cell aggregation method is a typical technique to reproduce self-organization and organ development. The salivary gland epithelial and mesenchymal cell mixture aggregates promote organ development and branching morphogenesis (Wei et al. 2007). Additionally, we demonstrated that a bioengineered organ germ, using organ germ methods, could regenerate ectodermal organs, such as teeth, hair follicles, and lacrimal glands (see Chaps. 5, 6, and 8). This method could be applied to achieve functional salivary gland regeneration (Ogawa et al. 2013). A bioengineered salivary gland germ was reconstructed using single epithelial and mesenchymal cells isolated from submandibular gland germs of ED 13.5 mice. The bioengineered submandibular gland germ successfully initiated salivary gland development, with branching morphogenesis followed by stalk and cleft formation in organ culture (Fig. 7.3). Bioengineered sublingual and parotid gland germs were also developed using organ germ methods, and they grew similarly to the submandibular gland.
Regeneration of salivary gland germ using organ germ methods. Phase-contrast images of the bioengineered submandibular and sublingual gland germ on 0 and 72 h of organ culture. The bioengineered salivary gland germ developed a blanching morphogenesis followed by stalk elongation and cleft formation within 72 h
7.6.1 Transplantation of the Bioengineered Salivary Gland Germs
Correct duct formation to connect the oral cavity and the bioengineered salivary gland germ is essential for correct acinar formation and saliva secretion. To achieve duct formation, bioengineered salivary gland germs were transplanted into the parotid gland ducts using an inter-epithelial tissue-connecting plastic method in a mouse model of salivary gland defects (Ogawa et al. 2013). Thirty days following transplantation, the bioengineered salivary gland and the host parotid duct were connected with nylon thread (Fig. 7.4a). The bioengineered submandibular gland regenerated serous acinar cells, and the sublingual gland regenerated mucous acinar cells. These bioengineered salivary glands had the correct organ structure, including localization of the water channel aquaporin-5 (AQP5), myoepithelial cells, and nerve fibers, which were similar to natural fibers (Fig. 7.4b).
Transplantation of bioengineered salivary gland germ. (a) Photographs of the bioengineered submandibular gland after 30-day transplantation. The bioengineered submandibular gland and parotid gland duct have established a correct connection. Arrowhead bioengineered submandibular gland. (b) Histological analysis of the natural (upper columns) and bioengineered (lower columns) submandibular gland. Images of HE staining (left) and periodic acid and Schiff (PAS) staining (second from the left). Immunohistochemical images of AQP5 (red) and E-cadherin (green; third from the left) and calponin (red) and NF-H (green; right) are shown. The bioengineered submandibular gland had a correct organ structure and regenerated serous acinar cells
7.6.2 Saliva Secretion from Bioengineered Salivary Glands
Restoration of the central nervous system is an important issue in organ regenerative therapy. Food, heat, and pain stimulation to the oral cavity induce saliva secretion via afferent and efferent nervous stimulation (Proctor and Carpenter 2014) (Fig. 7.5a). Additionally, saliva is essential for tasting; therefore, a saliva secretion was analyzed using gustatory tests, including sour (citrate), bitter (quinine hydrochloride), salty (NaCl), sweet (sucrose), and umami (glutamate) tastes (Matsuo 2000; Froehlich et al. 1987; Sasano et al. 2010; Ogawa et al. 2014). Citrate stimulation induced similar quantities of saliva secretion from the bioengineered salivary glands as natural salivary glands (Fig. 7.5b). All gustatory stimulation induced significant quantities of saliva secretion compared with non-stimulation, and the amount of saliva was dependent on the type of stimulus in the order of sour > bitter > umami > salty = sweet (Fig. 7.5c). These results indicate that saliva secretion from the bioengineered salivary glands occurred via proper nerve innervations and neurotransmission.
Assessment of saliva secretion. (a) Schematic representation of saliva secretion via the central nervous system using gustatory stimulation. (b) The time course of the amount of saliva secretion associated with normal mice (gray dots) and bioengineered submandibular gland-engrafted mice (red dots) after the gustatory stimulation by citrate. The amount of secreted saliva was not significantly different. (c) The amount of saliva secretion after 5 min of stimulation was associated with water stimulation (gray bar) and gustatory stimulation, including umami (glutamic acid), salty (NaCl), sweet (sucrose), bitter (quinine hydrochloride), and sour (citrate) (red bars)
7.6.3 Protection from Bacterial Infection and Dry Mouth
Saliva contains numerous proteins and cytokines that are essential for the maintenance of oral health and homeostasis, including amylase, lysozyme, IgA, lactoferrin, myeloperoxidase, NGF, EGF, and parotin (Lamy et al. 2010, Cohen 1962). Saliva reduction induces various clinical problems, such as bacterial infection, dental caries, sleep disorders, and swallowing dysfunction. In mouse models of salivary gland defects, the volume of oral bacteria increased compared with normal mice. In contrast, it was significantly reduced in the bioengineered salivary gland-engrafted mice compared with salivary gland defect mice (Fig. 7.6a) (Ogawa et al. 2013). These results indicate that bioengineered saliva has a cleansing function that prevents bacterial growth and dryness of the oral cavity.
Improvement of xerostomia by bioengineered salivary gland. (a) Assessment of bacterial propagation in the buccal mucosa of normal mice, salivary gland defect model mice, and bioengineered salivary gland-engrafted mice. *P < 0.05, **P < 0.001 by Student’s t-test. (b) Measurement of body weight every 0.5 days after transplantation in normal mice (gray dots), salivary gland defect mice (black dots), salivary gland-engrafted mice (red dots), and salivary gland defect mice that were given high-viscosity water (green dots). All salivary gland defect mice died within 5 days (✝) after the removal of all of the major salivary glands
7.6.4 Swallowing Function Recovery
Among the salivary gland functions, swallowing is important for the absorption of nutrition and reduces the risk of aspiration, which can cause chronic lung disease (Sreebny and Schwartz 1997). Saliva promotes the formation of a bolus of food and results in swallowing reflex. In salivary gland defect mice, body weight decreased abnormally, and all of the mice died within 5 days, despite free access to food and water. However, high-viscosity water prevented the decrease in body weight and improved the survival rate in the salivary gland defect mice (Fig. 7.6b) (Ogawa et al. 2013).
High-viscosity water is usually used to support swallowing in dry mouth patients and geriatric nursing. Thus, the salivary gland defect mice may represent a useful animal model for studying difficulties in swallowing. In the bioengineered salivary gland-engrafted mice, their body weights increased 4 days after transplantation, and they survived (Fig. 7.6b). These findings indicate that saliva secretion from bioengineered salivary glands can improve the swallowing function associated with oral health maintenance.
7.7 Future Perspectives for Salivary Gland Regenerative Therapy
Organ regenerative technology has advanced significantly, and many patients can expect to be treated with salivary gland regenerative therapy. To address the future clinical applications of salivary gland replacement therapy, it is essential to identify suitable cell sources. One candidate cell source is the patient’s own cells because there is no immunological rejection. Recent stem cell studies have revealed the presence of adult tissue stem cells in the salivary gland. These adult tissue-derived stem cells, which express stem cell markers or MSC markers, can repair injured acinar cells by stem cell transplantation. However, the possibility of utilizing these stem cells has not been studied with regard to inducing similar salivary glands to those induced by epithelial-mesenchymal interactions. In contrast, pluripotent stem cells, such as embryonic stem (ES) cells and induced pluripotent stem (iPS) cells, also have the capacity to become cell sources because these cells can differentiate into endodermal, ectodermal, and mesodermal cells (Wu and Hochedlinger 2011; Cohen and Melton 2011; Yan et al. 2010). The regeneration of some organs, such as the optic cup and pituitary gland, has been reported using ES cells or iPS cells. In the future, it is likely that methods for salivary gland regeneration using these pluripotent stem cells will be established.
In autoimmune diseases, atrophy of acinar cells and cell damage is caused by autoantigens. Because the transplanted regenerated acinar cells may also be affected by the autoimmune response, a genetic modification that decreases the expression of autoantigens against patient-derived stem cells must be performed to achieve future clinical applications of salivary gland replacement therapy in autoimmune disease. Current whole-organ regenerative therapy has the potential to become a future therapeutic technology for several diseases. Salivary gland replacement and regenerative therapy is expected to be realized by promoting fundamental technology development and the clinical application of regeneration research.
References
Aframian DJ, Palmon A (2008) Current status of the development of an artificial salivary gland. Tissue Eng Part B 14:187–198
Atala A (2005) Tissue engineering, stem cells and cloning: current concepts and changing trends. Expert Opin Biol Ther 5(7):879–892
Atkinson JC, Grisius M, Massey W (2005) Salivary hypofunction and xerostomia: diagnosis and treatment. Dent Clin N Am 49:309–326
Avery JK (2002) Oral development and histology. Thieme Press, New York, pp 292–330
Brockes JP, Kumar A (2005) Appendage regeneration in adult vertebrates and implications for regenerative medicine. Science 310(5756):1919–1923
Bücheler M, Wirz C, Schütz A, Bootz F (2002) Tissue engineering of human salivary gland organoids. Acta Otolaryngol 122(5):541–545
Cohen S (1962) Isolation of a mouse submaxillary gland protein accelerating incisor eruption and eyelid opening in the new-born animal. J Biol Chem 237:1555–1562
Cohen DE, Melton D (2011) Turning straw into gold: directing cell fate for regenerative medicine. Nat Rev Genet 12(4):243–252
Copelan EA (2006) Hematopoietic stem-cell transplantation. N Engl J Med 354:1813–1826
Delporte C, O’Connell BC, He X, Lancaster HE, O’Connell AC, Agre P, Baum BJ (1997) Increased fluid secretion after adenoviral-mediated transfer of the aquaporin-1 cDNA to irradiated rat salivary glands. Proc Natl Acad Sci U S A 94(7):3268–3273
Denny PC, Denny PA (1999) Dynamics of parenchymal cell division, differentiation, and apoptosis in the young adult female mouse submandibular gland. Anat Rec 254:408–417
Edgar M, Dawes C, Mullane OD (2004) Saliva and Oral Health, 3rd edn. British Dental Association, UK
Feng J, Van der Zwaag M, Stokman MA, Van Os R, Coppes RP (2009) Isolation and characterization of human salivary gland cells for stem cell transplantation to reduce radiation-induced hyposalivation. Radiother Oncol 92:466–471
Fox PC (2004) Salivary enhancement therapies. Caries Res 38:241–246
Froehlich DA, Pangborn RM, Whitaker JR (1987) The effect of oral stimulation on human parotid salivary flow rate and alpha-amylase secretion. Physiol Behav 41(3):209–217
Harunaga J, Hsu JC, Yamada KM (2011) Dynamics of salivary gland morphogenesis. J Dent Res 90(9):1070–1077
He X, Goldsmith CM, Marmary Y, Wellner RB, Parlow AF, Nieman LK, Baum BJ (1998) Systemic action of human growth hormone following adenovirus-mediated gene transfer to rat submandibular glands. Gene Ther 5(4):537–541
Horie K, Kagami H, Hiramatsu Y, Hata K, Shigetomi T, Ueda M (1996) Selected salivary-gland cell culture and the effects of isoproterenol, vasoactive intestinal polypeptide and substance P. Arch Oral Biol 41(3):243–252
Hsu JC, Yamada KM (2010) Salivary gland branching morphogenesis -- Recent progress and future opportunities. Int J Oral Sci 2(3):117–126
Ihrler S, Zietz C, Sendelhofert A, Lang S, Blasenbreu-Vogt S, Löhrs U (2002) A morphogenetic concept of salivary duct regeneration and metaplasia. Virchows Arch 440(5):519–526
Jaskoll T, Melnick M (2004) Embryonic salivary gland branching morphogenesis. Madame Curie:13–14
Jeong J, Baek H, Kim YJ, Choi Y, Lee H, Lee E, Kim ES, Hah JH, Kwon TK, Choi IJ, Kwon H (2013) Human salivary gland stem cells ameliorate hyposalivation of radiation-damaged rat salivary glands. Exp Mol Med 45:e58
Kagami H, O’Connell BC, Baum BJ (1996) Evidence for the systemic delivery of a transgene product from salivary glands. Hum Gene Ther 7(17):2177–2184
Kagami H, Wang S, Hai B (2008) Restoring the function of salivary glands. Oral Dis 14:15–24
Kawakami M, Ishikawa H, Tachibana T, Tanaka A, Mataga I (2013) Functional transplantation of salivary gland cells differentiated from mouse early ES cells in vitro. Hum Cell 26:80–90
Kishi T, Takao T, Fujita K, Taniguchi H (2006) Clonal proliferation of multipotent stem/progenitor cells in the neonatal and adult salivary glands. Biochem Biophys Res Commun 340(2):544–552
Knosp WM, Knox SM, Hoffman MP (2012) Salivary gland organogenesis. Wiley Interdiscip Rev Dev Biol 1(1):69–82
Knox S, Hoffman MP (2008) Salivary gland development. Blackwell Publications, Ames, IA
Lamy E, Graca G, Costa GD, Franco C, Silva FC, Baptista ES, Coelho AV (2010) Changes in mouse whole saliva soluble proteome induced by tannin-enriched diet. Proteome Sci 8:65
Langer RS, Vacanti JP (1999) Tissue engineering: the challenges ahead. Sci Am 280(4):86–89
Lombaert IM, Hoffman MP. (2013) Stem Cells in Salivary Gland Development and Regeneration. Stem Cells in Craniofacial Development and Regeneration. Hoboken, New Jersey, USA; Wiley-Blackwell. pp.271-284.
Lombaert IM, Brunsting JF, Wierenga PK, Faber H, Stokman MA, Kok T, Visser WH, Kampinga HH, de Haan G, Coppes RP (2008) Rescue of salivary gland function after stem cell transplantation in irradiated glands. PLoS One 3:e2063
Lombaert I, Movahednia MM, Adine C (2016) Ferreira JN. Therapeutic Approaches from Stem Cells to Tissue Organoids. Stem Cells, Salivary Gland Regeneration
Madeira C, Santhagunam A, Salgueiro JB, Cabral JM (2015) Advanced cell therapies for articular cartilage regeneration. Trends Biotechnol 33(1):35–42
Man YG, Ball WD, Marchetti L, Hand AR (2011) Contributions of intercalated duct cells to the normal parenchyma of submandibular glands of adult rats. Anat Rec 263(2):202–214
Matsuo R (2000) Role of saliva in the maintenance of taste sensitivity. Crit Rev Oral Biol Med 11:216–229
Nakamura T, Matsui M, Uchida K, Futatsugi A, Kusakawa S, Matsumoto N, Nakamura K, Manabe T, Taketo MM, Mikoshiba K (2004) M3 muscarinic acetylcholine receptor plays a critical role in parasympathetic control of salivation in mice. J Physiol 558:561–575
O’Connell AC, Baccaglini L, Fox PC, O’Connell BC, Kenshalo D, Oweisy H, Hoque AT, Sun D, Herscher LL, Braddon VR, Delporte C, Baum BJ (1999) Safety and efficacy of adenovirus-mediated transfer of the human aquaporin-1 cDNA to irradiated parotid glands of non-human primates. Cancer Gene Ther 6(6):505–513
Ogawa M, Oshima M, Imamura A, Sekine Y, Ishida K, Yamashita K, Nakajima K, Hirayama M, Tachikawa T, Tsuji T (2013) Functional salivary gland regeneration by transplantation of a bioengineered organ germ. Nat Commun 4:2498
Ogawa M, Yamashita K, Niikura M, Nakajima K, Toyoshima KE, Oshima M, Tsuji T (2014) Saliva secretion in engrafted mouse bioengineered salivary glands using taste stimulation. J Prosthodont Res 58(1):17–25
Patel VN, Rebustini IT, Hoffman MP (2006) Salivary gland branching morphogenesis. Differentiation 74(7):349–364
Peters SB, Naim N, Nelson DA, Mosier AP, Cady NC, Larsen M (2014) Biocompatible tissue scaffold compliance promotes salivary gland morphogenesis and differentiation. Tissue Eng Part A 20:1632–1642
Pradhan S, Farach-Carson MC (2010) Mining the extracellular matrix for tissue engineering applications. Regen Med 5:961–970
Proctor GB, Carpenter GH (2014) Salivary secretion: mechanism and neural regulation. Monogr Oral Sci 24:14–29
Rotter N, Oder J, Schlenke P, Lindner U, Bohrnsen F, Kramer J, Rohwedel J, Huss R, Brandau S, Wollenberg B, Lang S (2008) Isolation and characterization of adult stem cells from human salivary glands. Stem Cells Dev 17:509–518
Sakai T (2009) Epithelial branching morphogenesis of salivary gland: exploration of new functional regulators. J Med Investig 56(Suppl):234–238
Saleh J, Figueiredo MA, Cherubini K, Salum FG (2015) Salivary hypofunction: an update on aetiology, diagnosis and therapeutics. Arch Oral Biol 60(2):242–255
Sasano T, Satoh-Kuriwada S, Shoji N, Sekine-Hayakawa Y, Kawai M, Uneyama H (2010) Application of umami taste stimulation to remedy hypogeusia based on reflex salivation. Biol Pharm Bull 33(11):1791–1795
Segers VFM, Lee RT (2008) Stem-cell therapy for cardiac disease. Nature 451:937–942
Ship JA, Pillemer SR, Baum BJ (2002) (2002) Xerostomia and the geriatric patient. J Am Geriatr Soc 50:535–543
Sreebny LM, Schwartz SS (1997) A reference guide to drugs and dry mouth--2nd edition. Gerodontology 14(1):33–47
Sugito T, Kagami H, Hata K, Nishiguchi H, Ueda M (2004) Transplantation of cultured salivary gland cells into an atrophic salivary gland. Cell Transplant 13(6):691–699
Sumita Y, Liu Y, Khalili S, Maria OM, Xia D, Key S, Cotrim AP, Mezey E, Tran SD (2011) Bone marrow-derived cells rescue salivary gland function in mice with head and neck irradiation. Int J Biochem Cell Biol 43:80–87
Sun T, Zhu J, Yang X, Wang S (2006) Growth of miniature pig parotid cells on biomaterials in vitro. Arch Oral Biol 51(5):351–358
Tran SD, Wang J, Bandyopadhyay BC, Redman RS, Dutra A, Pak E, Swaim WD, Gerstenhaber JA, Bryant JM, Zheng C, Goldsmith CM, Kok MR, Wellner RB, Baum BJ (2005) Primary culture of polarized human salivary epithelial cells for use in developing an artificial salivary gland. Tissue Eng 11(1-2):172–181
Tran SD, Liu Y, Xia D, Maria OM, Khalili S, Wang RW-J, Quan V-H, Hu S, Seuntjens J (2013) Paracrine Effects of Bone Marrow Soup Restore Organ Function, Regeneration, and Repair in Salivary Glands Damaged by Irradiation. PLoS One 8(4):e61632
Tucker AS, Miletich I (2010) Salivary glands; Development, adaptations, and Disease. Karger, London, UK
Vissink A, Mitchell JB, Baum BJ, Limesand KH, Jensen SB, Fox PC, Elting LS, Langendijk JA, Coppes RP, Reyland ME (2010) Clinical management of salivary gland hypofunction and xerostomia in head-and-neck cancer patients: Successes and barriers. Int J Radiat Oncol Biol Phys 78:983–991
Voutetakis A, Bossis I, Kok MR, Zhang W, Wang J, Cotrim AP, Zheng C, Chiorini JA, Nieman LK, Baum BJ (2005) Salivary glands as a potential gene transfer target for gene therapeutics of some monogenetic endocrine disorders. J Endocrinol 185(3):363–372
Wei C, Larsen M, Hoffman MP, Yamada KM (2007) Self-Organization and Branching Morphogenesis of Primary Salivary Epithelial Cells. Tissue Eng 13(4):721–735
Wu SM, Hochedlinger K (2011) Harnessing the potential of induced pluripotent stem cells for regenerative medicine. Nat Cell Biol 13(5):497–505
Yan X, Qin H, Qu C, Tuan RS, Shi S, Huang GT (2010) iPS cells reprogrammed from human mesenchymal-like stem/progenitor cells of dental tissue origin. Stem Cells Dev 19(4):469–480
Yoo C, Vines JB, Alexander G, Murdock K, Hwang P, Jun HW. (2014) Adult stem cells and tissue engineering strategies for salivary gland regeneration: a review. Biomater Res 18:9.
Acknowledgments
This work was partially supported by a Grant-in-Aid for Kiban (A) from the Ministry of Education, Culture, Sports, Science and Technology (no. 25242041). `by Organ Technologies Inc.
Conflict of Interest
M. Ogawa and T. Tsuji have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Ogawa, M., Tsuji, T. (2017). Functional Salivary Gland Regeneration. In: Tsuji, T. (eds) Organ Regeneration Based on Developmental Biology. Springer, Singapore. https://doi.org/10.1007/978-981-10-3768-9_7
Download citation
DOI: https://doi.org/10.1007/978-981-10-3768-9_7
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-3766-5
Online ISBN: 978-981-10-3768-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)