Abstract
The salivary glands are exocrine organs that secrete saliva to maintain oral health and homeostasis. Dysfunctional salivary glands exhibit symptoms of dry mouth, including dental caries and dysfunction in speech and swallowing. Current clinical therapies for dry mouth disease include artificial saliva substitutes or parasympathetic stimulants, but these are transient and palliative approaches. To achieve the functional recovery of dysfunctional salivary glands, salivary gland tissue stem cells are thought to be candidate cell sources for salivary gland tissue repair therapies. In addition, whole salivary gland replacement therapy is expected to be a novel therapy resulting in the regeneration of fully functional salivary glands. The salivary glands arise from their organ germs, which are induced by epithelial-mesenchymal interactions. Recently, we developed a novel bioengineering method, i.e., the organ germ method, which can regenerate the ectodermal organs, including the teeth, hair, lacrimal glands, and salivary glands. The bioengineered salivary glands successfully secrete saliva into the oral cavity and can also improve the symptoms of dry mouth, such as bacterial infection and swallowing dysfunction. In this review, we summarize recent findings and bioengineering methods for salivary gland regeneration therapy.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Salivary gland regeneration
- Organ germ method
- Bioengineered salivary gland germ
- Stem cells
- Organ replacement regenerative therapy
- Dry mouth
1 Introduction
Exocrine glands, such as the sweat glands, lacrimal glands, and salivary glands, produce secretory fluids such as sweat, tears, and saliva. These secretory fluids have important roles in maintaining health and homeostasis. For example, saliva is secreted into the oral cavity and functions during chewing, digestion, cleaning, and swallowing [1, 2]. The salivary glands arise from the salivary gland organ germ, which is generated by the interaction of epithelial-mesenchymal stem cells during embryonic development [3, 4]. Salivary glands consist of three major glands, including the submandibular gland (SMG), sublingual gland (SLG), and parotid gland (PG), and many minor glands. Overall, 95 % of the saliva secreted per day is secreted by the SMG, SLG, and PG, and 5 % is secreted by the minor salivary glands. The SMG and PG secrete serous saliva that contributes mainly to the digestion of food. The SLG secretes mucous saliva, which protects the oral cavity from drying. Therefore, salivary gland dysfunction induces xerostomia and has an adverse effect on bodily health [5, 6].
Xerostomia induces some clinical problems, including dental decay, bacterial infection, mastication and swallowing dysfunction, and a general reduction in quality of life [5–7]. Xerostomia develops due to autoimmune diseases, such as Sjögren’s syndrome, aging, and radiation therapy for head and neck cancer. Current therapies for xerostomia rely on the use of artificial saliva substitutes or parasympathetic stimulants to promote saliva secretion and to prevent dry mouth [8, 9]. However, these therapies only provide temporary effects and cannot result in the recovery of salivary gland dysfunction, which is why the development of novel therapies for restoration of salivary gland function is necessary [10].
Regenerative therapies utilizing stem cell transplantation have been conducted in various organs, including salivary glands [11, 12]. In addition, salivary gland regeneration therapy involving gene modification and tissue engineering may eventually be used to restore damaged tissue and recover the flow of saliva [13]. Similar organ replacement therapy approaches for ectodermal organs such as the teeth and hair follicles, which can be achieved by transplantation of bioengineered organ germs that have been reconstituted using organ germ methods, have been reported [14–16]. Recently, we induced the regeneration of salivary glands and lacrimal glands using this method [17, 18]. In this book chapter, we discuss the novel findings and bioengineering methods used in salivary gland regeneration and the feasibility of these methods for future organ replacement regenerative therapy.
2 Development of Salivary Glands During Embryogenesis
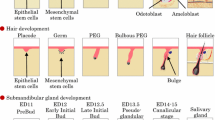
The salivary glands are generated from the organ germ, which is produced by epithelial and mesenchymal stem cell interactions during early embryonic development. The SMG, SLG, and PG are generated through similar morphogenetic events but differ in the timing and position at which generation begins [2–4, 19–22]. The development of the SMG is produced by the invagination of the oral epithelium into the mesenchymal region derived from the base of the tongue on embryonic day (ED) 11 (prebud) (Fig. 10.1). The invaginated epithelial tissue proliferates to form an epithelial stalk and a terminal bud at the tip (initial bud). The epithelial stalk differentiates into the ducts, which are called the intercalated, striated, and excretory ducts depending on their position relative to the side of the opening. The terminal bud forms the branched structure by forming a cleft and by repeating the elongation and branching process during ED 12.5–13.5 (pseudoglandular) [23–25]. From ED 15.0, the terminal bulbs differentiate into the acinar cells and begin the synthesis of secretory proteins, which differ depending on the type of salivary gland [26]. The SMG and PG secrete serous saliva, which contains a large amount of digestive enzymes such as α-amylase, which degrades starches and aids in digestion. The SLG secretes mucous saliva, which contains rich mucin protein to protect the mouth against dryness. The epithelial cells also differentiate into myoepithelial cells, and adult epithelial tissue stem cells are maintained in the excretory duct to contribute to the repair of injured tissue [27–29].
Schematic representation of the developmental stages of the salivary gland. The salivary glands, including SMG, SLG, and PG, are produced from organ germs induced by the interaction of the epithelial tissue and the mesenchymal tissue. The epithelial tissue invaginates into the mesenchymal tissue and forms a certain morphology according to the development of each organ. The salivary gland epithelial tissue is formed by the epithelial stalk and terminal bulb, which form the duct and acinar cells. The acinar cells mature and begin to synthesize and secrete secretory proteins
3 Diseases and Treatments of Salivary Glands
The various types of salivary gland diseases include salivary gland-specific diseases, such as salivary tumors, obstructive disorders, and infections, as well as the symptoms of systemic diseases, such as Sjögren’s syndrome (SS), lymphoma, and metabolic diseases [2]. Salivary dysfunction resulting from the atrophy of acinar cells and saliva reduction leads to xerostomia (dry mouth syndrome). In Europe, approximately 20 % of the population is thought to suffer from dry mouth syndrome, and this disease has been estimated to occur in approximately 800 million people in Japan [30]. The treatment of head and neck cancer, including salivary tumors, has been performed using radiation therapy. However, as the salivary glands are more sensitive to radiation, this treatment can cause atrophy of the acinar cells. Another condition that affects the salivary glands is SS, an autoimmune disease that occurs frequently in middle-aged and elderly women. It also affects the salivary glands as well as other glands such as the lacrimal glands, resulting in dry eyes. The annual number of SS patients has been reported to be approximately 15,000–20,000 [30]. Of all SS patients, approximately 70 % are positive for the SS antibody SSA (anti-Ro), and 40 % are positive for the SS antibody SSB (anti-La) [31–33]. However, these antibodies are not common to all patients, and the details of the pathogenic mechanism are not clear. Current therapies for dry mouth syndrome include symptomatic treatments such as the administration of artificial saliva and sialogogues to enhance moisture retention in the oral cavity [9]. In addition, parasympathomimetic drugs such as pilocarpine and cevimeline have been used to stimulate the muscarinic M3 receptor and induce salivary flow [32].
4 Salivary Gland Regeneration Using Stem Cells and Gene Therapy
4.1 Tissue Regeneration Using Adult Tissue Stem Cells
Transplantation of adult tissue stem cells has become a recognized method for regenerative therapy to restore damaged tissues and organs in diverse diseases [11, 34]. Regarding salivary gland regeneration, tissue stem/progenitor cell studies have reported that tissue stem cells have the capacity for tissue repair. The c-kit- and sca-1-positive tissue stem cells are localized to the intercalated duct of adult salivary glands, where these cells can induce the acinar and duct cells [35–38]. Furthermore, these stem cells are pluripotent and can differentiate into the liver or pancreas’ tissues [39, 40]. The c-kit-positive salivary gland stem cells can be cultured while maintaining the tissue repair capacity in vitro [12, 41–43]. A transplant of these cells can recover the decreased salivation resulting from irradiation-induced atrophy of the acinar cells. In addition, it has been reported that the bone marrow-derived mesenchymal stem cells have the ability to promote the regenerative capacity of the salivary gland stem cells that remain in the damaged salivary glands after irradiation [44]. Stem cell transplantation is expected to serve as an effective means of achieving salivary gland regeneration.
4.2 Gene Therapy for Salivary Gland Regeneration
Because the salivary glands are located close to the body surface, regeneration of damaged salivary glands via gene therapy has been studied. The salivary glands open via the duct into the oral cavity, and thus methods of direct gene transfection into the salivary glands via the duct have been reported. After the transfection of the water channel aquaporin-1 (AQP1) gene using adenovirus or adeno-associated virus, the saliva secretion of irradiated salivary glands was significantly recovered [45, 46]. However, salivary glands are known to function as exocrine glands that secrete saliva in the oral cavity and as endocrine glands that secrete substances into the bloodstream. Gene therapy using the salivary glands has also been performed as a treatment for other diseases, including SS and other genetic diseases [47, 48]. It has been reported that some materials, such as IL-17 receptor antibodies, growth hormones, parathyroid hormones, and erythropoietin, can be expressed in adult salivary glands via gene transfer and circulated throughout the body by the bloodstream [49–53]. Stem cell transplantation therapy and gene therapy are expected to be a new treatment strategy for salivary gland disorders and other diseases.
5 Functional Regeneration of a Bioengineered Salivary Gland
The current research for regenerating three-dimensional organs mimics organogenesis in the developing embryo. In the salivary gland regeneration field, epithelial cell aggregates are used to elucidate the mechanism of regeneration and branching morphogenesis in vitro [54]. In addition, the aggregate mix of epithelial and mesenchymal stem cells has been reported to increase the number of branches and rate of branch formation [54].
Recently, we demonstrated the possibility of full functional regeneration of the ectodermal organs, including the teeth, hair follicles, lacrimal glands, and salivary glands, using “organ germ methods” that involved epithelial and mesenchymal stem cell manipulation techniques to induce the formation of an organ germ (Fig. 10.2a) [14–18]. Using this method, it is possible to control the size, number, morphology, and invagination direction of the regenerated organ [16, 55]. For successful salivary gland replacement therapy, it is important that the invagination direction is controlled in such a way that the invaginated tissue connects to the ducts to secrete saliva into the oral cavity.
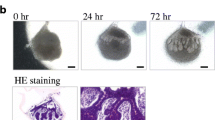
Regeneration of salivary gland germs using organ germ methods. (a) Ectodermal organs including the teeth, hair follicle, and secretory glands can be regenerated in vivo by transplanting bioengineered organ germs that are reconstituted by organ germ methods. (b) Phase-contrast images of the bioengineered submandibular gland germ at 0, 24, and 72 h of in vitro culture. The bioengineered submandibular gland showed the interaction between the epithelial and mesenchymal cells (24 h) and invagination of the epithelial tissue (72 h)
5.1 Development of a Bioengineered Salivary Gland
To reconstruct the bioengineered salivary gland, the germs including the SMG, SLG, and PG were isolated from mice at embryonic day (ED) 13.5–14.5. The bioengineered SMG germ showed epithelial-mesenchymal interactions and epithelial bud formation in organ culture (Fig. 10.2b) [17]. The regenerated SLG and PG germs also showed patterns that were similar to that of the SMG germ and structurally correct based on the natural salivary gland germ. The correct transplantation of the bioengineered salivary gland is important to achieving the secretion of saliva into the oral cavity. A bioengineered salivary gland germ was engrafted into the PG duct of the model mice with salivary gland defects using an intraepithelial tissue-connecting plastic method. In these mice, the SMG, SLG, and PG were excised. After 30 days, the growth of the bioengineered salivary gland and its connection to the PG duct was successfully achieved (Fig. 10.3a) [17]. The bioengineered salivary gland structures, including the localization of myoepithelial cells, the water channel aquaporin 5 (AQP5), and neuronal connections, were similar to those of a natural tissue (Fig. 10.3b) [17].
In vivo transplantation of a bioengineered salivary gland. (a) Photographs of the bioengineered submandibular gland at day 30 after transplantation (left). The bioengineered submandibular gland duct connected with natural PG duct (right). (b) Histological analysis of the bioengineered SMG (upper columns) and the SLG (lower columns). Images of HE staining (left) and periodic acid and Schiff (PAS) staining (second from the left). The bioengineered SLG showed a strongly positive PAS staining. Immunohistochemical images of calponin (red), E-cadherin (green, third from the left), and NF-H (green, right) are shown (Modified from Ref. Ogawa et al. [17])
5.2 Secretion of a Bioengineered Saliva
About 1–1.5 L of saliva is secreted per day from the salivary glands. This secretion is induced by eating, heat, and painful stimulation to the oral cavity. These stimulations are transmitted via the afferent and efferent neural networks from the oral cavity to the salivary glands (Fig. 10.4a) [56–60]. Moreover, because secreted saliva also plays an important role in taste perception, the hyposecretion of saliva has been known to cause taste disorders [61–63]. In the medical field, the secretion of saliva from the salivary gland has been analyzed using five tastes, including sour (citrate), bitter (quinine hydrochloride), salty (NaCl), sweet (sucrose), and umami (glutamate) [63, 64]. Compared to the control substance, citrate stimulation induced significant quantities of saliva secretion from both the natural and bioengineered salivary gland (Fig. 10.4b) [17]. Saliva secretion was induced in response to all tastes in addition to the sour stimulus. The secretion amount depended on the type of stimulus and exhibited the following order: sour > bitter > umami > salty = sweet [64]. In addition, the secreted bioengineered saliva contained the amylase protein, which has starch-degrading activity [17]. Salivation was measured about 1–3 months after transplantation and followed up for 6 months. These findings demonstrate that saliva secretion by the bioengineered salivary gland may be controlled through the afferent-efferent neural network.
Saliva secretion induced by gustatory stimulation. (a) Schematic representation of saliva secretion induced by gustatory stimulation via the central nervous system. (b) Assessment of the amount of saliva secretion from natural SMG (light bar) and bioengineered SMG (dark bar) after gustatory stimulation by citrate. The amount of secreted saliva exhibited no significant difference (b reprinted from Ref. Ogawa et al. [17])
5.3 Functional Restoration of Swallowing Dysfunction Using a Bioengineered Salivary Gland
Oral health and homeostasis are maintained by saliva and saliva proteins, such as amylase, lysozyme, IgA, lactoferrin, myeloperoxidase, NGF, EGF, and parotin. Therefore, the hyposecretion of saliva causes various problems, including dental caries, bacterial infection, sleep disorders, and swallowing dysfunction [65, 66]. The bioengineered salivary gland-engrafted mouse had fewer bacteria compared with the salivary gland defect model mouse. Among the salivary gland functions, the swallowing function is critical for reducing the risk of aspiration. The saliva promotes the formation of a bolus of food or water and triggers the swallowing reflex. Therefore, salivary gland dysfunction can cause chronic lung disease and can affect the survival, quality of life, and overall health of an individual [67]. In the salivary gland defect model mouse, the body weight was abnormally decreased, and all mice died within 5 days, despite having free access to food and water (Fig. 10.5) [17]. Dry mouth patients often drink high-viscosity water because they cannot swallow water. Similarly, the salivary gland defect model mouse exhibited a recovery of body weight and an increased survival rate when drinking high-viscosity water; this result in the model mouse raised the possibility that dysphagia may occur. In contrast, all of the bioengineered salivary gland-engrafted mice survived, and their body weight increased within 4 days after transplantation [17]. These results indicated that the bioengineered salivary gland can improve the swallowing function associated with the maintenance of oral health.
Analysis of body weight. Measurement of body weight (left graphs) every 0.5 days after transplantation in normal mice (gray dots); salivary gland defect model mice (black dots), salivary gland-engrafted mice (red dots), and salivary gland defect model mice were given high-viscosity water (green dots). All salivary gland defect model mice died within 5 days (✝) after removing all of the major salivary glands. Salivary gland-engrafted mice recovered the body weight (Reprinted from Ref. Ogawa et al. [17])
6 Future Directions of Salivary Gland Regeneration
Organ regenerative technology has advanced significantly. To achieve future clinical applications of salivary gland replacement therapy, it is important to identify suitable cell sources. The ideal cell source is the patient’s own cells because there is no immunological rejection. Recent stem cell biology studies have revealed the presence of adult tissue stem cells in the salivary gland. These adult tissue-derived stem cells, which include c-kit- and sca-1-positive cells, can repair the acinar cells injured by radiation and can partially recover the total amount of secreted saliva [12, 41–44]. The salivary gland of adult stem cells would be valuable cell sources for achieving salivary gland tissue regeneration via stem cell transplantation therapy. In contrast, pluripotent stem cells and induced pluripotent stem cells are also potential cell sources for salivary gland regeneration because these cells can differentiate into all types of cells, including endodermal, ectodermal, and mesodermal cells [68–70]. It has been reported that some organs, such as the optic cup and pituitary gland, can be derived from ES cells or iPS cells. In the future, the method of salivary gland regeneration using iPS cells is expected to be established [71–73].
Another important direction for future salivary gland regeneration therapy is to establish the mechanisms by which autoimmune diseases such as SS cause xerostomia [31–33]. In autoimmune diseases, salivary gland damage, such as the atrophy of acinar cells, is caused by self-antigens. Therefore, even if the bioengineered salivary gland is transplanted and can temporarily recover the saliva secretion, there is the possibility that the bioengineered acinar cells will again undergo atrophy. To achieve future clinical applications of salivary gland replacement therapy, genetic modifications of patient-derived stem cells will be necessary to decrease the expression of autoantigens.
Current whole organ regenerative therapy has the potential as a future therapeutic technology for several diseases. Salivary gland regenerative therapy is regarded as a model for future secretory organ replacement therapies that will substantially contribute to achieving an understanding related to organ regeneration technology.
References
Edgar M, Dawes C, Mullane OD. Saliva and oral health. 3rd ed. London: British Dental Association; 2004.
Tucker AS, Miletich I. Salivary glands; development, adaptations, and disease. London: Karger; 2010.
Avery JK. Oral development and histology. New York: Thieme Press; 2002. p. 292–330.
Tucker AS. Salivary gland development. Semin Cell Dev Biol. 2007;18:237–44.
Vissink A, Burlage FR, Spijkervet FK, Jansma J, Coppes RP. Prevention and treatment of the consequences of head and neck radiotherapy. Crit Rev Oral Biol Med. 2003;14:213–25.
Vissink A, Jansma J, Spijkervet FK, Burlage FR, Coppes RP. Oral sequelae of head and neck radiotherapy. Crit Rev Oral Biol Med. 2003;14:199–212.
Atkinson JC, Grisius M, Massey W. Salivary hypofunction and xerostomia: diagnosis and treatment. Dent Clin N Am. 2005;49:309–26.
Ship JA, Pillemer SR, Baum BJ. Xerostomia and the geriatric patient. J Am Geriatr Soc. 2002;50:535–43.
Fox PC. Salivary enhancement therapies. Caries Res. 2004;38:241–6.
Kagami H, Wang S, Hai B. Restoring the function of salivary glands. Oral Dis. 2008;14:15–24.
Körbling M, Estrov Z. Adult stem cells for tissue repair – a new therapeutic concept? N Engl J Med. 2003;349:570–82.
Lombaert IM, Brunsting JF, Wierenga PK, Faber H, Stokman MA, Kok T, Visser WH, Kampinga HH, de Haan G, Coppes RP. Rescue of salivary gland function after stem cell transplantation in irradiated glands. PLoS ONE. 2008;3:e2063.
O’Connell AC, Baccaglini L, Fox PC, O’Connell BC, Kenshalo D, Oweisy H, Hoque AT, Sun D, Herscher LL, Braddon VR, Delporte C, Baum BJ. Safety and efficacy of adenovirus-mediated transfer of the human aquaporin-1 cDNA to irradiated parotid glands of non-human primates. Cancer Gene Ther. 1999;6(6):505–13.
Nakao K, Morita R, Saji Y, Ishida K, Tomita Y, Ogawa M, Saitoh M, Tomooka Y, Tsuji T. The development of a bioengineered organ germ method. Nat Methods. 2007;4(3):227–30.
Ikeda E, Morita R, Nakao K, Ishida K, Nakamura T, Takano-Yamamoto T, Ogawa M, Mizuno M, Kasugai S, Tsuji T. Fully functional bioengineered tooth replacement as an organ replacement therapy. Proc Natl Acad Sci U S A. 2009;106(32):13475–80.
Toyoshima KE, Asakawa K, Ishibashi N, Toki H, Ogawa M, Hasegawa T, Irié T, Tachikawa T, Sato A, Takeda A, Tsuji T. Fully functional hair follicle regeneration through the rearrangement of stem cells and their niches. Nat Commun. 2012;3:784.
Ogawa M, Oshima M, Imamura A, Sekine Y, Ishida K, Yamashita K, Nakajima K, Hirayama M, Tachikawa T, Tsuji T. Functional salivary gland regeneration by transplantation of a bioengineered organ germ. Nat Commun. 2013;4:2498.
Hirayama M, Ogawa M, Oshima M, Sekine Y, Ishida K, Yamashita K, Ikeda K, Shimmura S, Kawakita T, Tsubota K, Tsuji T. Functional lacrimal gland regeneration by transplantation of a bioengineered organ germ. Nat Commun. 2013;4:2497.
Jiménez-Rojo L, Granchi Z, Graf D, Mitsiadis TA. Stem cell fate determination during development and regeneration of ectodermal organs. Front Physiol. 2012;3:107.
Pispa J, Thesleff I. Mechanisms of ectodermal organogenesis. Dev Biol. 2003;262(2):195–205.
Jaskoll T, Melnick M. Embryonic salivary gland branching morphogenesis. Austin (TX); Madame Curie Bioscience Database [Internet]; 2004.
Knosp WM, Knox SM, Hoffman MP. Salivary gland organogenesis. Wiley Interdiscip Rev Dev Biol. 2012;1(1):69–82.
Sakai T. Epithelial branching morphogenesis of salivary gland: exploration of new functional regulators. J Med Investig. 2009;56(Suppl):234–8.
Hsu JC, Yamada KM. Salivary gland branching morphogenesis – recent progress and future opportunities. Int J Oral Sci. 2010;2(3):117–26.
Harunaga J, Hsu JC, Yamada KM. Dynamics of salivary gland morphogenesis. J Dent Res. 2011;90(9):1070–7.
Denny PC, Denny PA. Dynamics of parenchymal cell division, differentiation, and apoptosis in the young adult female mouse submandibular gland. Anat Rec. 1999;254:408–17.
Man YG, Ball WD, Marchetti L, Hand AR. Contributions of intercalated duct cells to the normal parenchyma of submandibular glands of adult rats. Anat Rec. 2001;263(2):202–14.
Ihrler S, Zietz C, Sendelhofert A, Lang S, Blasenbreu-Vogt S, Löhrs U. A morphogenetic concept of salivary duct regeneration and metaplasia. Virchows Arch. 2002;440(5):519–26.
Lombaert IM, MP H. Stem cells in salivary gland development and regeneration. In: Stem cells in craniofacial development and regeneration. Hoboken: Wiley-Blackwell; 2013. p. 271–84.
Hayashi Y, Arakaki R, Ishimaru N. Salivary gland and autoimmunity. J Med Investig. 2009;56:185–91.
Fox RI, Stern M, Michelson P. Update in Sjögren syndrome. Curr Opin Rheumatol. 2000;12(5):391–8.
Nakamura T, Matsui M, Uchida K, Futatsugi A, Kusakawa S, Matsumoto N, Nakamura K, Manabe T, Taketo MM, Mikoshiba K. M3 muscarinic acetylcholine receptor plays a critical role in parasympathetic control of salivation in mice. J Physiol. 2004;558:561–75.
Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354:1813–26.
Segers VFM, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008;451:937–42.
Rotter N, Oder J, Schlenke P. Isolation and characterization of adult stem cells from human salivary glands. Stem Cells Dev. 2008;17(3):509–18.
Sugito T, Kagami H, Hata K, Nishiguchi H, Ueda M. Transplantation of cultured salivary gland cells into an atrophic salivary gland. Cell Transplant. 2004;13(6):691–9.
Kishi T, Takao T, Fujita K, Taniguchi H. Clonal proliferation of multipotent stem/progenitor cells in the neonatal and adult salivary glands. Biochem Biophys Res Commun. 2006;340(2):544–52.
Takahashi S, Schoch E, Walker NI. Origin of acinar cell regeneration after atrophy of the rat parotid induced by duct obstruction. Int J Exp Pathol. 1998;79:293–301.
Hisatomi Y, Okumura K, Nakamura K, Matsumoto S, Satoh A, Nagano K, Yamamoto T, Endo F. Flow cytometric isolation of endodermal progenitors from mouse salivary gland differentiate into hepatic and pancreatic lineages. Hepatology. 2004;39(3):667–75.
Okumura K, Nakamura K, Hisatomi Y, Nagano K, Tanaka Y, Terada K, Sugiyama T, Umeyama K, Matsumoto K, Yamamoto T, Endo F. Salivary gland progenitor cells induced by duct ligation differentiate into hepatic and pancreatic lineages. Hepatology. 2003;38(1):104–13.
Feng J, Van der Zwaag M, Stokman MA, Van Os R, Coppes RP. Isolation and characterization of human salivary gland cells for stem cell transplantation to reduce radiation-induced hyposalivation. Radiother Oncol. 2009;92:466–71.
Okumura K, Shinohara M, Endo F. Capability of tissue stem cells to organize into salivary rudiments. Stem Cells Int. 2012;2012:502136.
Nanduri LS, Maimets M, Pringle SA, van der Zwaag M, van Os RP, Coppes RP. Regeneration of irradiated salivary glands with stem cell marker expressing cells. Radiother Oncol. 2011;99(3):367–72.
Sumita Y et al. Bone marrow-derived cells rescue salivary gland function in mice with head and neck irradiation. Int J Biochem Cell Biol. 2011;43:80–7.
Shan Z, Li J, Zheng C, Liu X, Fan Z, Zhang C, Goldsmith CM, Wellner RB, Baum BJ, Wang S. Increased fluid secretion after adenoviral-mediated transfer of the human aquaporin-1 cDNA to irradiated miniature pig parotid glands. Mol Ther. 2005;11(3):444–51.
Baum BJ, Alevizos I, Zheng C, Cotrim AP, Liu S, McCullagh L, Goldsmith CM, Burbelo PD, Citrin DE, Mitchell JB, Nottingham LK, Rudy SF, Van Waes C, Whatley MA, Brahim JS, Chiorini JA, Danielides S, Turner RJ, Patronas NJ, Chen CC, Nikolov NP, Illei GG. Early responses to adenoviral-mediated transfer of the aquaporin-1 cDNA for radiation-induced salivary hypofunction. Proc Natl Acad Sci U S A. 2012;109(47):19403–7.
Yin H, Nguyen CQ, Samuni Y, Uede T, Peck AB, Chiorini JA. Local delivery of AAV2-CTLA4IgG decreases sialadenitis and improves gland function in the C57BL/6.NOD-Aec1Aec2 mouse model of Sjögren’s syndrome. Arthritis Res Ther. 2012;14(1):R40.
Wang J, Wang F, Xu J, Ding S, Guo Y. Double-strand adeno-associated virus-mediated exendin-4 expression in salivary glands is efficient in a diabetic rat model. Diabetes Res Clin Pract. 2014;103(3):466–73.
Voutetakis A, Bossis I, Kok MR, Zhang W, Wang J, Cotrim AP, Zheng C, Chiorini JA, Nieman LK, Baum BJ. Salivary glands as a potential gene transfer target for gene therapeutics of some monogenetic endocrine disorders. J Endocrinol. 2005;185(3):363–72.
Samuni Y, Zheng C, Cawley NX, Cotrim AP, Loh YP, Baum BJ. Sorting of growth hormone-erythropoietin fusion proteins in rat salivary glands. Biochem Biophys Res Commun. 2008;373(1):136–9.
Samuni Y, Cawley NX, Zheng C, Cotrim AP, Loh YP, Baum BJ. Sorting behavior of a transgenic erythropoietin-growth hormone fusion protein in murine salivary glands. Hum Gene Ther. 2008;19(3):279–86.
Voutetakis A, Zheng C, Metzger M, Cotrim AP, Donahue RE, Dunbar CE, Baum BJ. Sorting of transgenic secretory proteins in rhesus macaque parotid glands after adenovirus-mediated gene transfer. Hum Gene Ther. 2008;19(12):1401–5.
Nguyen CQ, Yin H, Lee BH, Chiorini JA, Peck AB. IL17: potential therapeutic target in Sjögren’s syndrome using adenovirus-mediated gene transfer. Lab Investig. 2011;91(1):54–62.
Wei C, Larsen M, Hoffman MP, Yamada KM. Self-organization and branching morphogenesis of primary salivary epithelial cells. Tissue Eng. 2007;13(4):721–35.
Ishida K, Murofushi M, Nakao K, Morita R, Ogawa M, Tsuji T. The regulation of tooth morphogenesis is associated with epithelial cell proliferation and the expression of Sonic hedgehog through epithelial-mesenchymal interactions. Biochem Biophys Res Commun. 2011;405(3):455–61.
Ekström J, Khosravani N, Castagnola M, Messana I. Saliva and the control of its secretion. Berlin: Springer; 2012.
Matsuo R, Yamamoto T, Yoshitaka K, Morimoto T. Neural substrates for reflex salivation induced by taste, mechanical, and thermal stimulation of the oral region in decerebrate rats. Jpn J Physiol. 1989;39:349–57.
Matsuo R, Garrett JR, Proctor GB, Carpenter GH. Reflex secretion of proteins into submandibular saliva in conscious rats, before and after preganglionic sympathectomy. J Physiol. 2000;527:175–84.
Proctor GB, Guy HC. Regulation of salivary gland function by autonomic nerves. Auton Neurosci. 2007;133:3–18.
Turner RJ, Sugiya H. Understanding salivary fluid and protein secretion. Oral Dis. 2002;8(1):3–11.
Matsuo R. Role of saliva in the maintenance of taste sensitivity. Crit Rev Oral Biol Med. 2000;11:216–29.
Froehlich DA, Pangborn RM, Whitaker JR. The effect of oral stimulation on human parotid salivary flow rate and alpha-amylase secretion. Physiol Behav. 1987;41(3):209–17.
Sasano T, Satoh-Kuriwada S, Shoji N, Sekine-Hayakawa Y, Kawai M, Uneyama H. Application of umami taste stimulation to remedy hypogeusia based on reflex salivation. Biol Pharm Bull. 2010;33(11):1791–5.
Ogawa M, Yamashita K, Niikura M, Nakajima K, Toyoshima KE, Oshima M, Tsuji T. Saliva secretion in engrafted mouse bioengineered salivary glands using taste stimulation. J Prosthodont Res. 2014;58(1):17–25.
Lamy E, Graca G, Costa GD, Franco C, Silva FC, Baptista ES, et al. Changes in mouse whole saliva soluble proteome induced by tannin-enriched diet. Proteome Sci. 2010;8:65.
Cohen S. Isolation of a mouse submaxillary gland protein accelerating incisor eruption and eyelid opening in the new-born animal. J Biol Chem. 1962;237:1555–62.
Sreebny LM, Schwartz SS. A reference guide to drugs and dry mouth – 2nd edition. Gerodontology. 1997;14(1):33–47.
Wu SM, Hochedlinger K. Harnessing the potential of induced pluripotent stem cells for regenerative medicine. Nat Cell Biol. 2011;13(5):497–505.
Cohen DE, Melton D. Turning straw into gold: directing cell fate for regenerative medicine. Nat Rev Genet. 2011;12(4):243–52.
Yan X, Qin H, Qu C, Tuan RS, Shi S, Huang GT. iPS cells reprogrammed from human mesenchymal-like stem/progenitor cells of dental tissue origin. Stem Cells Dev. 2010;19(4):469–80.
Eiraku M, Takata N, Ishibashi H, Kawada M, Sakakura E, Okuda S, Sekiguchi K, Adachi T, Sasai Y. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature. 2011;472(7341):51–6.
Suga H, Kadoshima T, Minaguchi M, Ohgushi M, Soen M, Nakano T, Takata N, Wataya T, Muguruma K, Miyoshi H, Yonemura S, Oiso Y, Sasai Y. Self-formation of functional adenohypophysis in three-dimensional culture. Nature. 2011;480(7375):57–62.
Ozone C, Suga H, Eiraku M, Kadoshima T, Yonemura S, Takata N, Oiso Y, Tsuji T, Sasai Y. Functional anterior pituitary generated in self-organizing culture of human embryonic stem cells. Nat Commun. 2016;7:10351.
Acknowledgments
This work was partially supported by a Grant-in-Aid for KIBAN (A) from the Ministry of Education, Culture, Sports, Science and Technology (no. 25242041). This work was also partially supported by Organ Technologies, Inc.
Conflict of Interest
This work was partially funded by Organ Technologies Inc. M.O. is a researcher and T.T. is a director at Organ Technologies Inc. This work was performed under an Invention Agreement between Tokyo University of Science, RIKEN and Organ Technologies Inc.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Ogawa, M., Tsuji, T. (2017). Functional Salivary Gland Regeneration by Organ Replacement Therapy. In: Cha, S. (eds) Salivary Gland Development and Regeneration. Springer, Cham. https://doi.org/10.1007/978-3-319-43513-8_10
Download citation
DOI: https://doi.org/10.1007/978-3-319-43513-8_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-43511-4
Online ISBN: 978-3-319-43513-8
eBook Packages: MedicineMedicine (R0)