Abstract
Passive treatment systems (PTSs) are frequently used to treat mine drainage that contains elevated concentrations of Fe, sulfate, and trace metals. Vertical flow bioreactors (VFBRs), often designed specifically to remove trace metals from mine drainage and retain them in an organic substrate, are a common PTS component. Many factors, including ionic strength, affect the performance of VFBR and their ability to remove trace metals. A paired-comparison study was performed in which two sets of columns were constructed, filled with an organic substrate, and fed synthetic mine water of differing ionic strengths, dominated by Na and sulfate, for 1 year. Elevated ionic strength significantly increased the rate of removal of Cd and Zn, and possibly Pb. Conversely, elevated ionic strength significantly decreased the rate of removal of Ni and Mn and even caused the eventual release of Mn from the substrate.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mine drainage can contain elevated cations (e.g., Fe2+, Na+, Ca2+, Mg2+) and sulfate (\({\text{SO}}_{4}^{2-}\)) from oxidation of pyrite and other sulfide minerals and through secondary reactions and ion exchange processes (Capo et al. 2001; Watzlaf et al. 2004; Younger et al. 2002). Base cations, sulfate, and bicarbonate (\({\text{HCO}}_{3}^{-}\)) alkalinity make up most of the inorganic contribution to total dissolved solids (TDSs) in mine drainage (Palmer et al. 2010; Skousen et al. 2000; Timpano et al. 2010). Some mine waters also contain elevated concentrations of Na and chloride due to the associated geology or connections with abandoned gas and oil wells and abandoned mine workings (Emrich and Merritt 1969; Hedin et al. 2005; Stiles et al. 2004). Water from mines flooded less than 15 years ago may exhibit elevated TDS (Stiles et al. 2004). Electrical conductivity (EC), TDS, and ionic strength are closely related, with all three indicative of the total number of dissolved ions (Cravotta 2008; Cravotta and Brady 2015; Hem 1985).
Vertical flow bioreactors (VFBRs) were originally conceived as a method to create anoxic, reducing conditions to promote alkalinity generation via bacterial sulfate reduction (BSR) and dissolution of limestone mixed with or underlying an organic substrate (Kepler and McCleary 1994). However, for hard rock mine drainage, VFBR have also been used extensively to remove trace metals through sorption to organic matter and by precipitation as sulfides, hydroxides, and carbonates (Dvorak et al. 1992; Machemer and Wildeman 1992; Neculita et al. 2008; Webb et al. 1998). In addition, VFBR can be very effective in removing sulfate, depending on the substrate used and hydraulic retention time (HRT; Cocos et al. 2002; Dvorak et al. 1992; Neculita et al. 2007; Pinto et al. 2015; Willow and Cohen 2003).
When treating net-alkaline mine drainage, VFBR will often be preceded by oxidation and settling ponds, where Fe2+ is oxidized to Fe3+, hydrolyzed, and precipitated as Fe oxyhydroxides (e.g., FeOOH) (Watzlaf et al. 2004). As a result, water entering VFBR will often exhibit near-neutral pH, but will have lost substantial amounts of alkalinity (Dvorak et al. 1992; Neculita et al. 2007). Although small amounts of trace metals may be removed via sorption and coprecipitation with Fe oxyhydroxides, the remainder may be removed in VFBR (Dzombak and Morel 1990; Kairies et al. 2005). In addition, some sulfate may be removed via sorption to Fe oxyhydroxides, but most will make its way to the VFBR (Ali and Dzombak 1996; Webster et al. 1998).
Although secondary standards for drinking water already exist, the US Environmental Protection Agency (US EPA) has proposed protocols for regulating conductivity concentrations in natural waters (US EPA 2011). However, TDS removal down to the proposed conductivity limits via conventional mine drainage treatment schemes can be inconsistent (Pinto et al. 2015). In addition, elevated TDS concentrations may affect some water treatment methods (Cravotta 2008; Hem 1985; Langmuir 1997).
Elevated ionic strength can enhance or inhibit trace metal removal in VFBR. An increase in bicarbonate concentration and pH due to BSR may provide a suitable environment for divalent trace metals to precipitate as carbonates (Dvorak et al. 1992; Sobolewski 1996). However, some studies have shown an increase in metal–carbonate solubilities at higher ionic strength, leaving trace metals to be removed by other mechanisms or left in solution (Plassard et al. 2000; Sun et al. 2009). Theoretically, sulfide mineral solubility will also increase as ionic strength increases (Lewis 2010). However, at least one study has found that the solubility of some sulfide minerals may decrease as ionic strength increases (Shpiner et al. 2009). In addition, ionic strength may impact the solubility of hydrogen sulfide, thus limiting the potential for metal–sulfide formation (Duan et al. 2007). In order to evaluate the impact of TDS/conductivity/ionic strength on trace metal removal efficiency, an examination of how they affect VFBR performance as a whole was necessary.
Experimental Design
Column Construction
To evaluate the effect of ionic strength on trace metal removal in VFBRs, a paired comparison column study was designed. Continuously fed, downward-flow columns were used to approximate field bioreactors. A HRT of 72 h was used in order to provide ample time for sulfate reduction and subsequent sulfide precipitation (Neculita et al. 2007).
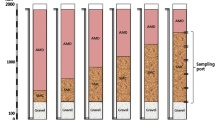
In addition to HRT, a target flow rate of approximately 3 L/day and an assumed substrate porosity of 0.5 were used to determine the dimensions of the columns. These calculations resulted in the construction of six 38 cm (15 in.) long columns constructed with 25 cm (10 in.) diameter PVC pipe, each with a total volume of 19.3 L PVC caps with 1.3 cm (0.5 in.) threaded holes were used to enclose the columns. Opaque PVC was used to better simulate field bioreactor conditions and to prevent the growth of phototrophic bacteria and algae. The effluent port was covered with 20-mesh nylon screen, which was covered with a 2.5 cm (1 in.) layer of inert pea gravel, to prevent large particulates from entering and collecting in the effluent tubing. Over the pea gravel, all columns were filled with a 2:1 mixture (by volume) of spent mushroom compost (SMC) and inert river rock (to provide structure and prevent compaction/loss of permeability). The fill mixture for each column was stirred manually for 5 min before placement in the columns.
Readily available SMC from J-M Farms, Inc., Miami, OK was used as the substrate and carbon source. This SMC consisted of a base of wheat straw amended with chicken litter, cottonseed meal, soybean meal, peat moss, sugar beet lime, and gypsum. Water elevation was controlled by maintaining the effluent tubing at an elevation ≈ 2 cm below the top of the columns (Supplemental Fig. 1).
Simulated Mine Drainage
The six columns were divided into two sets of three replicates; one set received low ionic (LI) strength simulated mine drainage (SMD) and the other received high ionic (HI) strength SMD. All SMD was intended to simulate approximate conditions in net-alkaline mine drainage after Fe has been removed by an oxidation pond. Both LI and HI SMD contained 0.5 mg/L each of Cd, Mn, Ni, Pb, and Zn. However, the LI SMD contained base cation concentrations of 10 mg/L Ca and 25 mg/L Na and anion concentrations of 15 mg/L Cl and 125 mg/L sulfate, resulting in an approximate ionic strength of 10−3 M. In contrast, the HI SMD contained base cation concentrations of 250 mg/L Ca and 1000 mg/L Na and anion concentrations of 440 mg/L Cl and 2100 mg/L sulfate, resulting in an approximate ionic strength of 10−1 M.
Each SMD was prepared in a 50 L carboy approximately every 5 days. The respective SMD solutions were fed into the top of each column with peristaltic pumps at a target flow rate of 3.1 L/day (130 mL/h) and allowed to flow naturally via gravity through the remainder of the system. This flow rate was selected to balance the size of the columns with the desired 72 h HRT; a higher flow rate would have required column sizes infeasible for the laboratory. Saturation of all material was ensured by maintaining approximately 5 cm (2 in.) of water cover over the surface of the substrate.
Methods
After an adjustment period of 4 weeks, effluent samples were collected from each column every 2 weeks for 1 year (approximately equivalent to 100 pore volumes). Temperature, pH, dissolved oxygen (DO), EC, oxidation–reduction potential (ORP), and total alkalinity were measured immediately. Samples for total and dissolved metals were collected in 60 mL HDPE bottles and acidified with trace metal grade nitric acid to pH < 2. Dissolved metals samples were filtered with 0.45 μm nylon syringe filters prior to acidification. Samples for total and dissolved carbon analyses were collected in 40 mL amber glass bottles that had been pre-baked at 400 °C for 1 h. Dissolved carbon samples were filtered with 0.45 μm nylon syringe filters; all carbon samples were stored at ≤4 °C and analyzed as soon as possible after collection. Samples for sulfate and chloride analysis were collected in 30 mL polypropylene scintillation vials, stored at ≤4 °C, and analyzed within 48 h. Sulfide samples were collected in 60 mL HDPE bottles, preserved with zinc acetate and sodium hydroxide, and analyzed within 24 h. Methods used for all sample analyses are listed in Supplemental Table 1.
In addition to samples collected for chemical analyses, effluent samples were collected quarterly for determination of SRB populations using the most probable number technique, as well as overall anaerobic bacteria populations. Bacteria culture bottles containing either the American Petroleum Institute RP-38 sulfate reducer medium (for SRB) or thioglycollate medium (for anaerobic bacteria) were obtained from VK Enterprises in Edmond, OK. Bottles were inoculated and serial diluted to 10−6 to determine populations in both media. Samples were incubated at room temperature up to 28 days before determining the populations.
Minitab version 17.2.1 was used to compute all statistics (Minitab 2010). A significance level of 0.05 was applied. All data sets were checked for normality using the Anderson–Darling statistic. The Mann–Whitney test (also known as the Wilcoxon rank sum test) was used to determine significant differences between data sets. Upward and/or downward trends were determined using the Mann–Kendall test.
Results and Discussion
Influent Characteristics
The LI and HI columns were each fed SMD by their own low-flow peristaltic pump. During the study, an average flow of 3.24 L/day (2.25 L/min) was maintained in the LI columns and an average flow of 3.11 L/day (2.16 mL/min) was maintained in the HI columns. Seventy batches (50 L carboys) each of LI and HI SMD were prepared through the study, and each column was treated with ≈1215 L of SMD, which took 375 days for the LI columns and 391 days for the HI columns.
LI and HI SMD samples were collected approximately every 10 batches during the study. Mean SMD water quality is listed in Supplemental Table 2. Sulfide data are not included in the table as all samples contained the constituent at concentrations below the detection limit of 0.1 mg/L. In addition, total carbon concentrations were all less than 1.0 mg/L and excluded from the table. Mean total and dissolved metal data are listed in Supplemental Table 3.
There were significant differences in EC, pH, and sulfate between the LI and HI SMD. The differences in conductivity and sulfate were due to the designed differences in chemical constituents and ionic strength and were expected. The pH was most affected by the different sulfate concentrations. An increased sulfate concentration was correlated with a lower pH, as indicated by the Spearman’s rho value of −0.705 (p = 0.023). Cravotta (2008) found a similar correlation between pH and sulfate in a survey of 140 coal mine drainages in Pennsylvania. Although there was a significant difference in pH in LI and HI SMD, the values were within the desirable range for treatment in a VFBR.
Total and dissolved Pb concentrations in the HI SMD were close to the target of 0.5 mg/L in the first two batches at the beginning of the study. However, a new supply of Na2SO4 was obtained and inadvertently contributed additional Pb to the HI SMD. This disparity accounts for the significant difference in LI and HI SMD Pb concentrations and larger standard deviations in Pb concentrations in both SMDs. However, there were no significant differences in mean Cd, Mn, Ni, and Zn concentrations between LI and HI SMD. A significant difference was also found between the total and dissolved Na concentrations in the HI SMD, most likely due to incomplete dissolution of the Na2SO4 salt used to prepare the solution.
Effluent Characteristics
Mean effluent water quality data are shown in Table 1. Over the course of the study, all columns sustained anoxic and reducing conditions and exhibited near-neutral pH. Concentrations of DO quickly dropped to <1 mg/L and remained so throughout the study. In addition, reducing conditions developed in all columns within 2 weeks after saturation; ORP remained below −200 mV in LI and HI column effluents for the entire study. Chloride was conservative in both sets of columns, with no significant concentration changes.
pH, Conductivity, and Alkalinity
Throughout the study, effluent pH in both sets of columns remained significantly higher than the influent pH. It also generally remained in the 5–8 range considered optimal for SRB productivity and metal removal as sulfides (Elliot et al. 1998; Postgate 1984; Tsukamoto et al. 2004; Willow and Cohen 2003), with two exceptions. In weeks 44 and 50, average effluent pH in the HI columns were slightly above 8 at 8.18 and 8.06, respectively. The pH in the HI effluent was slightly, but significantly, higher than the pH in the LI effluent throughout most of the study, with means of 7.62 and 7.37, respectively. Both sets of columns exhibited significant upward trends in pH, with pH increasing from ≈10 weeks until the end of the study.
Effluent EC and alkalinity were initially significantly greater than the influent due to flushing of dissolved organic matter, inorganic base cations and anions from the amendments (chicken litter, lime, gypsum, etc.) in the SMC (Supplemental Figs. 2, 3; Dvorak et al. 1992; Guo et al. 2001; Song et al. 2012). These parameters decreased quickly as the excess amendments were depleted, with effluent EC returning to levels similar to the influent concentrations in both sets of columns within 12 weeks. Although effluent alkalinity from both sets of columns decreased throughout the study, concentrations remained significantly greater than influent alkalinity.
Contribution of Sulfate Reduction to Alkalinity
Dissolution of CaCO3 (in the form of sugar beet lime, a by-product of sugar beet processing) contained in the SMC was likely the largest contributor to the elevated alkalinity in the beginning of the study, as evidenced by elevated Ca concentrations in both the LI and HI columns (Eq. 1; Supplemental Fig. 4; Gross et al. 1993).
Sulfate reduction may also produce bicarbonate alkalinity (Eq. 2).
Dissolved Ca concentrations remained elevated throughout the study, indicating continued dissolution of CaCO3, but BSR likely became a larger contributor to alkalinity over time. The differences in effluent and influent dissolved Ca and sulfate concentrations, as well as relationships from Eqs. 1 and 2, were used to calculate bicarbonate alkalinity. This was then converted to CaCO3 alkalinity and compared to measured alkalinity concentrations (Dvorak et al. 1992; Watzlaf et al. 2004; Eqs. 3, 4).
In the LI columns, the equivalent alkalinity from dissolved Ca was very near the measured alkalinity. However, from approximately 8 weeks into the study until the end, the alkalinity added by sulfate reduction was required to account for part of the measured alkalinity (Fig. 1).
Comparison of alkalinity contributions from CaCO3 dissolution and sulfate reduction was not so straightforward in the HI columns, as CaCO3 dissolution made a much greater contribution to alkalinity at the beginning of the study than it did in the LI columns. However, this contribution very quickly dropped near to the values in the LI columns, until approximately 20 weeks into the study, when CaCO3 alkalinity in the HI columns fell below the LI columns and remained there for the rest of the study. In addition, the calculated contribution of alkalinity from sulfate reduction was far greater in the HI columns due to the much faster sulfate removal rate.
The sulfur balance was calculated for both sets of columns to understand the discrepancy in summed and measured alkalinities (Eq. 5). Assuming that all trace metals were removed as sulfides, an effluent mass of sulfur could be estimated and compared to the influent mass of sulfur.
The results of the sulfur mass balance (Table 2) indicated that total effluent masses exceeded influent masses in the LI columns throughout the study by an average of 0.37 moles, or 30%. This may be an indication that gypsum contained in the raw SMC dissolved, raising the porewater sulfate-S concentration and making it difficult to determine how much of the influent sulfur was actually removed. The calculations revealed the opposite relationship in the HI columns, with an average of 3.05 mmol/L, or 17%, more sulfur in the influent than in the effluent. This could indicate gypsum precipitation in the pore waters of these columns, which would effectively lower the influent sulfate-S concentration, making accurate calculation of sulfate removal difficult. It is certainly clear that sulfate reduction was not the only sulfate removal mechanism in the HI columns.
Although gypsum may precipitate from mine waters high in Ca and sulfate, it was not assumed that concentrations would be great enough to create a supersaturated environment (Palmer et al. 2010). Indeed, speciation modeling using PHREEQC (Parkhurst and Appelo 2013) indicated that both the LI and HI systems were approaching saturation with respect to gypsum, but both saturation indices were still negative (SI = −1.72 and −0.34, respectively). These analyses were conducted on the bulk effluent water quality and cannot account for the potential for supersaturated conditions in porewater. Nonetheless, these results decrease the likelihood that gypsum precipitation was the sole cause of lower than anticipated effluent sulfate concentrations.
Other possible explanations for the greater sulfate removal in the HI columns are sorption of the \({{\text{CaSO}}_{4}^{0}}\) ion pair to the organic substrate, transformation of inorganic sulfate into organic sulfur, formation of elemental sulfur, or systemic error in measurements of influent sulfate concentrations (Bolan et al. 1991; Machemer et al. 1993; Sokolova and Alekseeva 2008). None of these potential mechanisms can be definitively accounted for or ruled out within the constraints of the data collected.
Sulfate Removal and Sulfide Production
Sulfate and sulfide data were highly variable in both sets of columns, but the effect was not as apparent in the HI column effluents due to the much higher sulfate concentrations (Supplemental Fig. 5). Although there was not a significant trend in effluent sulfate concentrations in the LI columns, there was a significant downward trend in effluent sulfide concentrations. This indicates that while sulfate was removed throughout the study, the dominant removal mechanism may have shifted. In the HI columns, there was a significant upward trend in effluent sulfate concentrations coupled with a significant downward trend in sulfide concentrations. The mean sulfate removal rate in LI columns was 81 mmol/m3/day, which is substantially less than the 300 mmol/m3/day expected under ideal conditions (URS 2003) and the 691 mmol/m3/day found in the HI columns. Mean SRB populations were consistently between 102 and 103 organisms/mL in the LI columns and between 103 and 104 organisms/mL in the HI columns. Influent sulfate concentrations affect SRB growth and sulfate reduction kinetics (Moosa et al. 2002), so a significant difference in LI and HI SRB populations and sulfate removal rates was anticipated. However, the calculated sulfate removal rate in the HI columns was more than likely artificially inflated due to the reasons discussed previously.
Carbon
Total, inorganic, and organic carbon data exhibited large standard deviations over the course of the study (Supplemental Table 4). However, this was due to the large impact of the initial flush of dissolved organic carbon, particularly in the first 12 weeks of operation (Supplemental Fig. 6). Statistically, total and dissolved carbon concentrations were not different. Surprisingly, despite the aforementioned potential for increased CaCO3 dissolution in the HI columns, there were no significant differences between any of the total or dissolved carbon fractions in the LI and HI columns. Comparing inorganic carbon data to the alkalinity balance (Fig. 1), 3–39% of the inorganic carbon in the LI columns came from sulfate reduction, with the smallest contributions being made at the beginning of the study when the bulk of inorganic carbon came from CaCO3 dissolution. Due to the uncertainties in the alkalinity balance for the HI columns, it was impossible to estimate the respective contributions of sulfate reduction and CaCO3 dissolution to the measured inorganic carbon.
Despite the appearance that carbon concentrations may have been different in the LI and HI columns (Supplemental Fig. 6), there were no significant differences when considering the entirety of the data. However, dissolved inorganic carbon was significantly higher in the HI columns for approximately the first 12 weeks of the study. This is most likely due to the fact that elevated ionic strength increases the solubility product of CaCO3, resulting in a greater amount of dissolution in the HI columns at the beginning of the study than in the LI columns (Stumm and Morgan 1996). There is also potential for enhanced dissolution of CaCO3 in the HI columns due to removal of free Ca2+ ions from solution due to ion pairing with sulfate, creating the \({{\text{CaSO}}_{4}^{0}}\) pair (Drever 1997).
Calcium and Sodium
Effluent calcium concentrations were significantly greater than influent concentrations in both sets of columns, even when removing the influence of the initial flush on effluent concentrations (Supplemental Fig. 4). However, total and dissolved effluent calcium concentrations exhibited significant downward trends in the LI and HI columns and nearly returned to influent concentrations in the HI columns. Calcium concentrations in the LI columns remained elevated throughout the study, presumably due to continued dissolution of CaCO3, whereas concentrations in the HI columns returned to near influent concentrations by the end of the study. This is likely due to both more rapid CaCO3 dissolution associated with elevated ionic strength and sorption of \({{\text{CaSO}}_{4}^{0}}\) ion pairs to the SMC.
Effluent sodium concentrations also exhibited a downward trend in the LI columns (Supplemental Fig. 7). In addition to calcium, a small amount of sodium appeared to leach out of the SMC for several weeks after the beginning of the study. In contrast, there were no significant trends in total or dissolved sodium concentrations in the HI columns.
Trace Metals
Mean total and dissolved concentrations of Cd, Ni, Pb, and Zn were all decreased significantly, from ≈0.50 mg/L to below 0.10 mg/L, in both sets of columns. Manganese was not completely removed in any of the columns, with effluent concentrations in LI being significantly lower than in HI (Table 3).
It is well documented that, upon startup of any VFBR, a substantial portion of dissolved metals are removed by adsorption to the organic substrate (e.g., Gibert et al. 2005; Machemer and Wildeman 1992; Neculita et al. 2008; O’Sullivan et al. 2004; Stark et al. 1994; Zagury et al. 2006). This was the most likely explanation for the initial decrease in effluent Cd, Pb, and Zn concentrations in the LI columns. Total and dissolved Cd, Pb, and Zn concentrations in the LI effluent were all at their lowest in the first 2–4 weeks of the study, after which effluent concentrations of all three metals increased slightly (Fig. 2). After sorption sites become saturated, precipitation as carbonates and sulfides becomes the dominant removal mechanism. Examples of these precipitation reactions are illustrated in Eqs. 6 and 7.
Past studies have demonstrated that most of the Cd, Ni, Pb, and Zn are removed as sulfides, but large fractions may be removed as carbonates or by complexation with humic substances in the organic substrate (Chagué-Goff 2005; Dvorak et al. 1992; Neculita et al. 2008; O’Sullivan et al. 2004). Effluent sulfide, pH, alkalinity, and dissolved carbon concentrations were all high enough in both sets of columns to support any of these mechanisms.
None of the trace metal effluent concentrations in the HI columns demonstrated the initial adsorption-associated concentration drop seen in the LI columns. This is likely due to adsorption inhibition due to the elevated ionic strength. Regardless, effluent Cd and Zn concentrations were consistently significantly less in the HI columns than in the LI columns, while effluent Ni concentrations were significantly less in the LI columns, indicating that elevated ionic strength inhibited removal. Effluent Ni concentrations in the LI columns and effluent Cd, Ni, and Zn concentrations in the HI columns all demonstrated downward trends, indicating that removal efficiency increased over time. Conversely, Mn and Pb concentrations exhibited upward trends, indicating a loss in removal efficiency.
Effluent Pb concentrations were significantly less in the LI columns than in the HI columns (Fig. 2g–h), but the influent concentrations were also significantly less. Despite the differences between the columns, it is believed that most of the Pb, as well as Cd, Ni, and Zn, were removed as sulfides and carbonates. Speciation modeling in PHREEQC indicated that effluents from both sets of columns were supersaturated with respect to Cd, Ni, Pb, and Zn sulfides and Cd and Pb carbonates, but were undersaturated with respect to hydroxides.
LI and HI effluents were not found to be supersaturated with respect to any Mn-sulfide, -carbonate, or -hydroxide minerals, indicating that most of the removal was likely due to adsorption to or complexation with the organic substrate. Bless et al. (2008) found that Mn adsorption onto organic substrates is important, but that it is pH dependent and often temporary. As pH increases, Mn may be replaced on the substrate by more readily adsorbed metals. Effluent Mn concentrations in the LI columns were significantly less than in the HI columns (Fig. 2c, d). However, a similar trend was observed in both sets of columns, with a decrease in effluent concentrations for the first 10–12 weeks, after which effluent concentrations began to increase. The increase in HI effluent Mn concentrations was much more pronounced than in the LI columns, with effluent concentrations eventually exceeding the influent concentration. The increase in effluent concentrations strongly indicates adsorption (with subsequent desorption) as the removal mechanism, rather than complexation with organic matter. Whether this behavior was due directly to the elevated ionic strength or the slightly increased pH caused by the elevated ionic strength is unknown.
Correlations
The relationships between water quality parameters and dissolved constituents of the LI and HI column effluents were evaluated using a Spearman’s rank correlation coefficient table (Minitab 2010; Table 4). Positive correlations between conductivity and sulfate, alkalinity, inorganic and organic carbon, calcium, and Na were expected simply due to the definition of conductivity, as well as the large initial flush of dissolved organic matter and base cations and anions. Positive correlations between indicators of ionic strength (conductivity, Ca, Na, and sulfate) and Mn and Ni, however, reinforce the possibility that ionic strength affects trace metal removal in VFBR. These correlations provide insight to the significant difference between the LI and HI effluent concentrations, particularly with regard to Ni.
On opening the columns at the end of the study, it was noticed that the substrate had settled approximately 5 cm (2 in.) and was covered by approximately 4 cm (1.5 in.) of influent water. The treatment volume was considered equivalent to the volume of the submerged substrate, so the treatment volume used to calculate removal rates were adjusted from 19.3 to 16.7 L (0.0167 m3). This value was used to calculate the volume adjusted removal rates (Table 5).
Overall, there were significant differences in all of the calculated removal rates. The LI columns had significantly higher Mn and Ni removal rates, mirroring the significantly lower effluent concentrations found in those columns. Conversely, the HI columns had significantly higher Cd, Pb, and Zn removal rates.
Conclusions
Elevated ionic strength appeared to significantly affect some inorganic constituents of the study, namely pH, alkalinity, and trace metal removal. The pH and alkalinity impacts were most likely due to increased solubility of the CaCO3 contained in the SMC, but may have also been affected by the greater sulfate concentrations in the HI columns. The two organic constituents evaluated, carbon concentrations and SRB populations, had no evident effect. It was not anticipated that the elevated ionic strength (TDS/conductivity) would affect the five selected trace metals differently. However, it was found that elevated ionic strength increased the rate of removal of Cd and Zn, and possibly Pb. Elevated ionic strength decreased the rate of Ni removal and greatly decreased the rate of Mn removal, and also caused the eventual release of Mn from the substrate. Manganese concentrations demonstrated by far the most difference between LI and HI columns. Removal mechanisms were not determined in this portion of the study, but a subsequent study on the spent substrates will elucidate more clearly the impact of elevated ionic strength on products of trace metal removal in VFBR.
References
Ali M, Dzombak D (1996) Interactions of copper, organic acids, and sulfate in goethite suspensions. Geochim Cosmochim Acta 60:5045–5053
Bless D, Park B, Nordwick S, Zaluski M, Joyce H, Hiebert R, Clavelot C (2008) Operational lessons learned during bioreactor demonstrations for acid rock drainage treatment. Mine Water Environ 27:241–250
Bolan N, Syers J, Sumner M (1991) Calcium-induced sulfate adsorption by soils. Soil Sci Soc Am J 57:691–696
Capo R, Winters W, Weaver T, Stafford S, Hedin R, Stewart B (2001) Hydrogeologic and geochemical evolution of deep mine discharges, Irwin Syncline, Pennsylvania. In: Proceedings of the 22nd annual West Virginia Surface Mine Drainage Task Force Symposium, Morgantown, WV, p 144–153
Chagué-Goff C (2005) Assessing the removal efficiency of Zn, Cu, Fe, and Pb in a treatment wetland using selective sequential extraction: a case study. Water Air Soil Pollut 160:161–179
Cocos I, Zagury G, Clement B, Samson R (2002) Multiple factor design for reactive mixture selection for use in reactive walls in mine drainage treatment. Water Res 36:167–177
Cravotta C (2008) Dissolved metals and associated constituents in abandoned coal-mine discharges, Pennsylvania, USA. Part 1: constituent quantities and correlations. Appl Geochem 23:166–202
Cravotta C, Brady K (2015) Priority pollutants and associate constituents in untreated and treated discharges from coal mining or processing facilities in Pennsylvania, USA. Appl Geochem 62:108–130
Drever J (1997) The geochemistry of natural waters: surface and groundwater environment, 3rd edn. Prentice Hall, Upper Saddle River
Duan Z, Sun R, Liu R, Zhu C (2007) Accurate thermodynamic model for the calculation of H2S solubility in pure water and brines. Energy Fuel 21:2056–2065
Dvorak D, Hedin R, Edenborn H, McIntire P (1992) Treatment of metal-contaminate water using bacterial sulfate reduction: results from pilot-scale reactors. Biotechnol Bioeng 40:609–616
Dzombak D, Morel F (1990) Surface complexation modeling: hydrous ferric oxide. Wiley-Interscience, New York
Elliot P, Ragusa S, Catcheside D (1998) Growth of sulfate-reducing bacteria under acidic conditions in an upflow anaerobic bioreactor as a treatment system for acidic mine drainage. Water Res 32:3724–3730
Emrich G, Merritt G (1969) Effects of mine drainage on ground water. Ground Water 7:27–32
Gibert O, de Pablo J, Cortina J, Ayora C (2005) Municipal compost-based mixture for acid mine drainage bioremediation: metal retention mechanisms. Appl Geochem 20:1648–1657
Gross M, Formica S, Gandy L, Hestir J (1993) A comparison of local waste materials for sulfate-reducing wetlands substrate. In: Moshiri G (ed) Constructed wetlands for water quality improvement. CRC Press, Boca Raton
Guo M, Chorover J, Rosario R, Richard H (2001) Leachate chemistry of field-weathered spent mushroom substrate. J Environ Qual 30:1699–1709
Hedin R, Stafford S, Weaver T (2005) Acid mine drainage flowing from abandoned gas wells. Mine Water Environ 24:104–106
Hem J (1985) Study and interpretation of the chemical characteristics of natural waters, 3rd edn. USGS Water Supply Paper 2254, Washington, DC
Kairies C, Capo R, Watzlaf G (2005) Chemical and physical properties of iron hydroxide precipitates associated with passively treated coal mine drainage in the Bituminous Region of Pennsylvania and Maryland. Appl Geochem 20:1445–1460
Kepler D, McCleary E (1994) Successive alkalinity-producing systems (SAPS) for the treatment of acidic mine drainage. In: Proceedings of the international land reclamation and mine drainage conference. US Bureau of Mines SP 06A-94, Pittsburgh, p 194–204
Langmuir D (1997) Aqueous environmental geochemistry. Prentice Hall, Upper Saddle River
Lewis A (2010) Review of metal sulphide precipitation. Hydrometallurgy 104:222–234
Machemer S, Wildeman T (1992) Adsorption compared with sulfide precipitation as metal removal processes from acid mine drainage in a constructed wetland. J Contam Hydrol 9:115–131
Machemer S, Reynolds J, Laudon L, Wildeman T (1993) Balance of S in a constructed wetland built to treat acid mine drainage, Idaho Springs, Colorado, USA. Appl Geochem 8:587–603
Minitab 17 Statistical Software (2010) Computer software. Minitab, Inc., State College. http://www.minitab.com. Accessed 15 May 2016
Moosa S, Nemati M, Harrison S (2002) A kinetic study of anaerobic reduction of sulfate: Part I. Effect of sulfate concentration. Chem Eng Sci 57:2773–2780
Neculita C, Zagury G, Bussière B (2007) Passive treatment of acid mine drainage in bioreactors using sulfate-reducing bacteria: critical review and research needs. J Environ Qual 36:1–16
Neculita C, Zagury G, Bussière B (2008) Effectiveness of sulfate-reducing passive bioreactors for treating highly contaminated acid mine drainage: II. Metal removal mechanisms and potential mobility. Appl Geochem 23:3545–3560
O’Sullivan A, Moran B, Otte M (2004) Accumulation and fate of contaminants (Zn, Pb, Fe, and S) in substrates of wetlands constructed for treating mine wastewater. Water Air Soil Pollut 157:345–364
Palmer M, Bernhardt E, Schlesinger W, Eshleman K, Foufoula-Georgiou E, Hendryx M, Lemly A, Likens G, Loucks O, Power M, White P, Wilcock P (2010) Mountaintop mining consequences. Science 327:148–149
Parkhurst D, Appelo C (2013) Description of input and examples for PHREEQC, vers. 3—a computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations. USGS Techniques and Methods, Book 6:A43. http://www.pubs.usgs.gov/tm/06/a43/. Accessed 15 Apr 2016
Pinto P, Souhail R, Balz D, Butler B, Landy R, Smith S (2015) Bench-scale and pilot-scale treatment technologies for the removal of total dissolved solids from coal mine water: a review. Mine Water Environ. doi:10.1007/s10230-015-0351-7
Plassard F, Winiarski T, Petit-Ramel M (2000) Retention and distribution of three heavy metals in a carbonated soil: comparison between batch and unsaturated column studies. J Contam Hydrol 42:99–111
Postgate J (1984) The sulfate-reducing bacteria, 2nd edn. Cambridge University Press, Cambridge
Shpiner R, Vathi S, Stuckey D (2009) Treatment of oil well “produced water” by waste stabilization ponds: removal of heavy metals. Water Res 43:4258–4268
Skousen J, Sextone A, Ziemkiewicz P (2000) Acid mine drainage control and treatment. In: Barnhisel R, Daniels W, Darmody R (eds) Reclamation of drastically disturbed lands. American Society of Agronomy monograph, p 131–168
Sobolewski A (1996) Metal species indicate the potential of constructed wetlands for long-term treatment of metal mine drainage. Ecol Eng 6:259–271
Sokolova T, Alekseeva S. (2008) Adsorption of sulfate ions by soils (a review). Eurasian Soil Sci 41:140–148
Song H, Yim G, Ji S, Neculita C, Hwang T (2012) Pilot-scale passive bioreactors for the treatment of acid mine drainage: efficiency of mushroom compost vs. mixed substrates for metal removal. J Environ Manag 111:150–158
Stark L, Wenerick W, Williams F, Stevens S, Wuest P (1994) Restoring the capacity of spent mushroom compost to treat coal mine drainage by reducing the inflow rate: a microcosm experiment. Water Air Soil Pollut 75:405–420
Stiles J, Donovan J, Dzombak D, Capo R, Cook L (2004) Geochemical cluster analysis of mine water quality within the Monongahela Basin. In: Barnhisel R (ed) Proceedings of the 2004 national meeting of the American Society of Mining and Reclamation and 25th West Virginia Surface Mine Drainage Task Force Symposium, Morgantown, p 1819–1830
Stumm W, Morgan J (1996) Aquatic chemistry: chemical equilibria and rates in natural waters, 3rd edn. Wiley, New York
Sun W, Nešić S, Woollam R (2009) The effect of temperature and ionic strength on iron carbonate (FeCO3) solubility limit. Corros Sci 51:1273–1276
Timpano A, Schoenholtz S, Zipper C, Soucek D (2010) Isolating effects of total dissolved solids on aquatic life in central Appalachian Coalfield streams. In: Barnhisel R (ed) Proceedings of the 2010 national meeting of the American Society of Mining and Reclamation, Pittsburgh, PA, p 1284–1302
Tsukamoto T, Killion H, Miller G (2004) Column experiments for microbiological treatment of acid mine drainage: low-temperature, low-pH, and matrix investigations. Water Res 38:1405–1418
URS (2003) Passive and semi-active treatment of acid rock drainage from metal mines—state of the practice. Prepared for US Army Corps of Engineers, Portland
US EPA (2011) A field-based aquatic life benchmark for conductivity in central Appalachian Streams. EPA/600/R-10/023F. Office of Research and Development, National Center for Environmental Assessment, Washington, DC
Watzlaf G, Schroeder K, Kleinmann R, Kairies C, Nairn R (2004) The passive treatment of coal mine drainage. DOE/NETL 2004/1202, Pittsburgh
Webb J, McGinness S, Lappin-Scott H (1998) Metal removal by sulphate-reducing bacteria from natural and constructed wetlands. J Appl Microbiol 84:240–248
Webster J, Swedlund P, Webster K (1998) Trace metal adsorption onto an acid mine drainage iron(III) oxy hydroxy sulfate. Environ Sci Technol 32:1361–1368
Willow M, Cohen R (2003) pH, dissolved oxygen, and adsorption effects on metal removal in anaerobic bioreactors. J Environ Qual 32:1212–1221
Younger P, Banwart S, Hedin R (2002) Mine water: hydrology, pollution, remediation. Kluwer Academic Publishers, Dordrecht
Zagury G, Kulnieks V, Neculita C (2006) Characterization and reactivity assessment of organic substrates for sulphate-reducing bacteria in acid mine drainage treatment. Chemosphere 64:944–954
Acknowledgements
Stipend and research funding were provided by American Society of Mining and Reclamation, Grand River Dam Authority (Project 100052), U.S. Geological Survey (Agreement DOI-USG 04HQAG0131), US Environmental Protection Agency (Agreement X7-97682001-0), and the Oklahoma Department of Environmental Quality (Agreement PO2929019163).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
LaBar, J.A., Nairn, R.W. Evaluation of the Impact of Na-SO4 Dominated Ionic Strength on Effluent Water Quality in Bench-Scale Vertical Flow Bioreactors Using Spent Mushroom Compost. Mine Water Environ 36, 572–582 (2017). https://doi.org/10.1007/s10230-017-0446-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10230-017-0446-4