Abstract
Mine water containing copper and zinc exceeding the permissible discharge limit has been discharged from the S mine site in South Korea. To evaluate the feasibility of applying passive treatment methods for treating water discharged from this mine, bench-scale experiments were conducted by using columns simulating successive alkalinity-producing systems (SAPS) and bioreactors. As substrate materials, limestone and spent mushroom compost (SMC) were applied, with their structures and mixing ratios varied. The efficiency of metal removal for each column was then evaluated. SAPS (column B) and bioreactors (columns C and D) exhibited Cu removal efficiencies of 99.7%, 98.0%, and 97.1%, respectively, while the limestone reactor (column A) had an average removal efficiency of 81.3%. Except for the re-dissolution events, Zn removal efficiencies were 99.5%, 97.6%, and 88.4%, respectively, while the limestone reactor had an average removal efficiency of 29.2%. Facilitated by a pH increase caused by the dissolution of limestone, the bacterial sulfate reduction (BSR) reaction was shown to be effective at removing metal in the SAPS and bioreactor columns; the process was revealed through the presence of sulfide in the effluent. When comparing bioreactors with different compost mixing ratios, columns with greater SMC ratios had higher removal efficiencies, as well as higher alkalinity, which shows the importance of SMC in metal removal. Overall, this study will be helpful in determining on-site treatment methods for Cu- and Zn-rich mine water by reusing waste materials, SMC, through bacterial metal reduction reactions, as well as considering the potential lifespan of the treatment facility.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mine water or mine drainage, produced from abandoned mines, is a worldwide concern due to its adverse impacts on the environments. This water is either acidic or alkaline and it also usually contains a high level of contaminants, such as heavy metals. When it enters water systems, it can cause biotic impacts by heavy metals as well as aesthetic impacts due to yellow or orange colored precipitates (De Nicol and Stapleton 2002; Younger et al. 2002).
To remediate mine water, various treatment methods, including active and passive treatments, have been developed. Active treatment is effective in remediating large quantities of mine water that is highly contaminant. Briefly, this process entails the use of chemicals to neutralize the water’s pH and remove toxic metals; however, it involves high cost problems in the context of long-term use (Lee et al. 2018). In contrast, passive treatment entails the use of natural materials such as limestone and organic matter to remediate mine water. It is usually cost-efficient and ecofriendly, as it does not require electricity and labor (Wu et al. 2010; Corral-Bobadilla et al. 2019; Skousen et al. 2019).
With regard to passive water remediation methods, biologically mediated treatment systems, called bioreactors, have garnered attention as promising technologies (Neculita et al. 2007; McCullough and Lund 2011; Vasquez et al. 2016; Rambabu et al. 2020). Bioreactors typically refer to anaerobic wetlands, which are constructed using organic substrates such as compost, sawdust, and manure, often mixed with limestone. As mine water flows through organic substrates, anoxic and near-neutral pH conditions are created by the oxygen demand of the organic substrate and dissolution of the calcite fraction (Klein et al. 2013). Alkalinity generation by limestone dissolution (Eq. 1) and bacterial sulfate reduction (BSR, Eq. 2) followed by metal sequestration into insoluble sulfides (Eq. 3) contribute to the removal of metals from mine water (Christensen et al. 1996).
where M2+ is a metal cation.
The BSR mediated by sulfate-reducing bacteria (SRB) is a key mechanism for mine water treatment in bioreactors. SRB growth can be activated using organic substrates, which serve as energy sources for SRB. Oxidation of organic substrate (CH2O) with BSR and precipitation of metal sulfides occur as shown in Eq. 2 (Mayes et al. 2011). Moreover, anoxic and circumneutral pH conditions are established when mine water flows through bioreactors consisting of limestone and organic substrate, contributing to BSR because these conditions are optimal for SRB growth (Cohen 2006; Carlier et al. 2020).
Vertical flow wetlands (VFWs) have also been applied as an modified form of traditional anaerobic wetlands. Mechanisms underlying this approach are the same as those of anaerobic wetlands, except that mine water travels vertically through substrates by hydraulic head difference. VFWs demand less area than that of horizontal flow wetlands, and their systems can be easily flushed when precipitates clog the pores of the substrate layer (Kepler 1988). The bioreactors consist of two types of designs: successive alkalinity-producing systems (SAPS) and SAPS with mixed substrates (Watzlaf et al. 2000; Johnson and Hallberg 2005). The former has a layered substrate, while the latter has a mixed substrate of limestone and organic matter. The SAPS is known to be effective for remediating iron-containing mine water through the reduction of Fe in the organic layer and the production of alkalinity in the limestone layer (Cheong et al. 2010; Ordonez et al. 2012). Bioreactors are generally used for treating mine water containing heavy metals (e.g., copper and zinc) with an affinity for sulfide (Oh et al. 2020). By mixing the organic substrate with limestone and occupying the organic substrate throughout the reactor, the BSR mechanism is maximized, resulting in metal precipitation through the removal of oxygen and an increase in alkalinity (Wolkersdorfer and Bowell 2005).
Many studies have investigated the efficiency of bioreactors in the remediation of mine water using various organic substrates. Field experiments using herbaceous and woody organic substrates in bioreactors to remediate coal-generated acid mine drainage (AMD) with high concentrations of Fe, Al, and other trace metals, such as Cu and Zn, have been conducted. These experiments have shown that microbially mediated reduction and precipitation of sulfides were the main mechanisms underlying the remediation of AMD (Lefticariu et al. 2015). Spent mushroom compost (SMC) has also been studied for its use as an organic-based substrate in the bioremediation of AMD (Neculita et al. 2011; Das et al. 2012; Muhammad et al. 2017). SMC, which is organic matter left after the harvesting of mushrooms, has been shown to be applicable in the remediation of industrial wastewater containing heavy metals (Cheong et al. 2010; Corral-Bobadilla et al. 2019). Specifically, the use of SMC as a substrate for bioreactors has been linked to its organic carbon-rich content and circumneutral pH (Jordan et al. 2008). Moreover, the reuse of SMC for mine water treatment is economically beneficial as SMC is considered as a waste material, which involves substantial disposal costs (Kapoor and Viraraghavan 1995). The main mechanism on metal removal by using SMC is the occurrence of BSR. As well as precipitation as a form of sulfides, adsorption onto organic matters has been known to affect removal of metal in this method. Moreover, Fe/Mn oxides on surfaces of substrates can facilitate BSR in the presence of organic matters (Muhammad et al. 2018).
Although these studies have verified the efficiency of bioreactors in mine water treatment, mine water generated from different sites has variable conditions in terms of quality and quantity. The composition of mine water can differ markedly from one site to another because of geology, climate, groundwater conditions, etc. (Simate and Ndlovu 2014). Therefore, selecting the optimal treatment methods considering the physiochemical properties of water at each site is necessary. Many studies have focused on specific sites and conducted various experiments based on bioreactors to select the most appropriate methods for treating mine water (Demchak et al. 2001; Trumm and Watts 2010; Clyde et al. 2016; Singh and Chakraborty 2020; Thisani et al. 2021).
In South Korea, there are 40 passive facilities in total, and 25 of these facilities have applied SAPS, as shown in Fig. 1. All SAPS units are used for treating AMD from abandoned coal mines, which mostly contain Fe and Al; however, none of the facilities have applied bioreactors for treating Cu and Zn in mine water. Although mine water from an abandoned metallic mine, for example, S mine in this study, has Cu exceeding the permissible discharge limit, only limestone reactors have been applied, resulting in a low treatment efficiency (Oh et al. 2020).
This study practically assessed the effectiveness of various passive treatment methods based on an organic substrate, SMC, for Cu and Zn removal from mine water. Specifically, mine water from an S mine was directly used as an influent to simulate field conditions. Reactors with limestone and SMC were used to evaluate the efficiency of removal of Cu and Zn. Then, the effect of SMC, which triggers the microbial mediated mechanisms, or BSR, along with limestone dissolution was evaluated. Overall, this study ultimately will be helpful in determining optimal on-site treatment methods.
Materials and methods
Study area
S mine, is an abandoned metallic mine in Gyeongsangnam-do, South Korea. The monsoonal and the monthly average temperatures of this area range between 1.6 and 26.8 ℃; the minimum and maximum temperature have been recorded in January and August, respectively, between 1991 and 2020. During this time, the average annual precipitation was 1470.4 mm, with most of the precipitation occurring in summer (Korea Meteorological Administration 2022). S mine has a single adit discharging mine drainage that has a neutral pH and contains dissolved Cu and Zn exceeding the permissible discharge limit in South Korea (Cu: 1 mg/L, Zn: 1 mg/L) (Ministry of Environment, Korea 2020).
Mine water as an influent
Mine water discharged from the adit of the S mine area was used directly as an influent flow for the column experiments. Based on the monitoring results acquired from 2007 to 2014 by the Korea Mine Rehabilitation and Mineral Resources Corporation (KOMIR), the mine water was circumneutral (average pH 6.5), with 6.7 and 4.1 mg/L of Cu and Zn concentrations, respectively; these concentrations exceeded the permissible discharge limits outlined by the South Korean government. The total dissolved Fe and Mn contents were negligible. The flow rate and chemical properties of the mine water that were recorded during the monitoring period are shown in Table 1. Mine water was sampled at the inlet of the pre-existing treatment facility and stored in the reservoir prior to being injected into the columns.
Column experiments
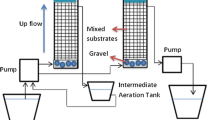
Vertical flow column experiments were conducted for 130 days with various types of reactors. Cylindrical columns were made of transparent polyacrylate cylinder with diameter of 0.2 m and height of 2 m; these columns were then filled with the substrates. Columns were wrapped in aluminum foil after the observation of algae in the column to prevent light from the effect of phototrophic bacteria. The experimental set-up is shown in Fig. 2. As substrates, limestone and SMC were applied with their ratios and structure varied across the different columns. A certain pH range and carbon are required for mine water remediation via passive methods involved with bioremediation, or BSR. This sulfate reduction has been known to occur actively in company with limestone because limestone dissolution can maintain alkaline conditions that can optimize SRB growth (Muhammad et al. 2017).
Limestone was collected from a pre-existing facility, and it was composed of more than 99% calcite (CaCO3) with few impurities. The limestone was crushed into small particles (2–3 cm) to increase the surface area. The SMC, as an organic substrate, was supplied by a local mushroom harvest farm. To produce SMC, substrates containing rice straw, chicken manure, urea fertilizer, and limestone were mixed and piled up outside for 18 days for fermentation. These substrates were then inoculated using mushroom seed and clay loam soil was applied on the top of substrates. After the mushrooms were grown for 20 days, the SMC was extracted from them and subsequently used as a substrate for the column experiments. The characterization of the SMC is shown in Table 2. The pH was measured using a pH meter (Orion 3 star, Thermo) in accordance with ASTM testing methods (ASTM 2021). The water content of SMC was measured by calculating the difference between the weight of a sample before drying and that after drying at 105 ℃ for 24 h. Total volatile solids were measured in accordance with the test methods outlined by Karam (1993). Dissolved organic carbon and elemental composition of the SMC were analyzed using total organic carbon analyzer (multi N/C 3100, Analytik Jena) and elemental analyzer (EA 2400 Series II, PerkinElmer), respectively, at KOMIR. The C/N ratio was 10, which is an adequate condition for the biological degradation of the substrate (Béchard et al. 1994; Neculita et al. 2011). The polysaccharide content in SMC is generally degraded via hydrolytic fermentative anaerobes to alcohols and fatty acids, which can support SRB growth (Chang et al. 2000). Since SMC has a slow degradation rate, it can be a suitable material as bioreactor for mine water treatment facility as well as long term operation of facility.
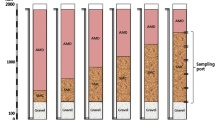
There were three types of columns as follows: column filled with only limestone (column A), layered structure of limestone and SMC (column B), and mixed structure of limestone and SMC (column C and D). The composition of the columns is described in detail in Table 3. Instead of using column with only SMC, the ratio of SMC was adjusted in mixed column, because mine water influent had difficulty to flow due to low permeability when only SMC was filled in column. Column A was the control reactor using only limestone with the height of 1 m to stimulate the pre-existing mine water treatment facility. By adding SMC with limestone in column B, C, and D, the effect of SMC on metal removal from mine water was evaluated. Column B was a layered structure, which consisted of a 1 m limestone layer at the bottom and 0.3 m of compost at the top to simulate SAPS structure. Column C (C1 and C2) and D were mixed structures which were filled with a mixture of limestone and compost at different ratios to simulate a bioreactor. The ratio of limestone and compost in column C1 was originally 3:7; however, it was changed to 5:5 and the column was named as column C2 after 8 weeks because of the permeability problem. Column D was composed of 7:3 limestone and compost and had less compost ratio than column C. The heights of columns C and D were 1 m, which were identical to that of column B. A continuous flow rate of 5–6 mL/min was maintained in the hydraulic retention time (HRT) of 1.7–1.8 days for column A, C, and D and 2.4 days for column B using peristaltic pump (Table 4).
During the experiment, Cu precipitates were formed at the bottom of the inflow reservoir, lowering the Cu concentration of the influent. Approximately, 100 mL of hydrochloric acid (HCl) was added to the 200 L of inflow reservoir on 57th and 103rd day of the experiment to recover the concentration and mobility of Cu in the influent in order to maintain a certain level of target elements.
Analysis of influent and effluent
The influent and effluent of each reactor were monitored during the experiments. The oxidation–reduction potential (ORP), pH, and concentration of dissolved oxygen (DO) were measured in the field using a portable multimeter (Orion 3 star, Thermo). Alkalinity was measured by titration using a digital titrator kit (AL-DT, Hach). After filtering with a 0.45 m filter paper and adding 5 mL of 20% nitric acid, samples were kept in 50 mL centrifuge tubes for laboratory analysis. The concentrations of Cu, Zn, Ca, and total dissolved sulfur were analyzed using inductively coupled plasma optical emission spectrometry (ICP-OES) (Varian 720-ES) and the concentrations of sulfate (SO42−) were analyzed using ion chromatography (IC) (Metrohm 850) at KOMIR. ICP-OES and IC were calibrated by using standard solutions and purified water. Determination coefficients (R2) were more than 0.999 in the calibration curves. Before sample analysis, certified reference materials were analyzed, and the calibration solutions of medium concentration were analyzed at every 20 samples for quality assurance (QA) and quality control (QC). Samples were analyzed repeatedly for three times, and less than 5% of relative standard deviation was found among the triplicates (Kim et al. 2021).
Metal removal efficiency
The metal removal efficiency of each reactor was calculated to compare its treatment ability using Eq. 4 as follows:
where Ci and Ce are the metal concentrations of the influent and effluent, respectively.
Changes in metal removal efficiency with respect to pH were also noted to evaluate the effect of pH on Cu and Zn removal. Finally, efficiency was calculated after 2nd week of the experiments, because 1st week was regarded as a stabilization period.
Results and discussion
Columns simulating SAPS and bioreactors were shown to efficiently remove Cu and Zn from mine drainage. Along with a pH increase by limestone dissolution, the BSR reaction by SMC was considered as a main factor for removal of metals in SAPS and bioreactor columns; this process was validated through the presence of sulfide in the effluent. When comparing bioreactors with different compost mixing ratios, columns with greater SMC ratios had higher removal efficiencies as well as higher alkalinity, which shows the importance of SMC in metal removal.
pH
The pH of the adit drainage from the S mine was circumneutral, ranging from 5.8 to 6.9. To prevent precipitation of Cu during the storage of adit drainage in the reservoir and maintain a certain level of target elements, HCl was added in the 7th and 14th week, resulting in an effluent that had a pH ranging from 2.6 to 7.2. Effluents from all column reactors showed a trend toward an increase in pH compared to the influent (Fig. 3). In column A, the effluent pH increased slightly compared to that of the influent, ranging from 4.8 to 8.0, with an average of 6.5. Column B, C (C1 and C2), and D consistently showed a high range of effluent pH, with averages of 7.6, 7.4, 7.7, and 8.3, respectively, regardless of acid addition.
Metal removal efficiency
The concentration and removal efficiencies of Cu and Zn for each column are shown in Fig. 3 and 4, respectively. The Cu concentration increased to 91.9 and 109.0 mg/L at the 17th and 18th week, respectively, when acid was added. After the influent passed through all the columns, the concentration of Cu was reduced, with an average removal efficiency of 94.1% for all columns. Column A showed the smallest decrease in Cu concentration as compared to other columns during the experimental period. The average Cu removal efficiency of column A was 81.3%, and the Cu concentration in the effluent exceeded Korea’s permissible discharge limit (1 mg/L) at five sampling events. In particular, the efficiency decreased sharply when the influent was rich in Cu. The Cu concentration of the influent was significantly high; specifically, it was at 91.9 and 109.0 mg/L during the 17th and 18th week, respectively. The increased pH was due to the addition of acid. When the limestone in column A reacted firstly with the Cu-rich influent at 17th week, it removed most of the Cu with a 98.0% efficiency. However, on the 18th week, the efficiency dropped to 66.7%. Results show that limestone was supposedly passivated after reacting with a large amount of Cu, which can be observed from precipitates coating the limestone surface (Offeddu et al. 2015). At the site of the pre-existing facility, Cu precipitates (i.e., bluish precipitates) were occasionally observed on the surface of the limestone in the facility (Fig. 5).
During the first 8 weeks, the effluent from column C1 showed the lowest Cu concentration, followed by column B and D in sequence. With respect to Cu removal efficiency (Fig. 4), column B and C1 consistently showed high efficiencies of 99.4% and 99.7% on average, respectively, indicating that the bulk of Cu was removed. In column D, 95.8% of Cu was removed from the influent. After 8 weeks, when C1 was changed to C2 with a lower percentage of compost, the lowest concentration was observed in column B, followed by columns D and C2 in sequence, but with subtle difference, which means these columns still exhibited a high efficiency. Column B had the highest efficiency, with an average of 99.9%. Column C2 and D also efficiently removed Cu, with an average efficiency of 96.9% and 98.1%, respectively. A high efficiency was maintained even when the Cu-rich influent flowed into the reactors after the addition of acid, which shows the ability of both SAPS and bioreactors to treat a broad range of Cu concentrations from 0.7 to 109.0 mg/L.
The Zn concentration in the influent ranged from 1.9 to 3.9 mg/L, showing no significant increase despite the addition of acid. Zn was thought to be less influenced by a decrease in pH than Cu because it hardly formed precipitates under the circumneutral pH conditions that characterized S mine water. Zn was removed and its concentration was under 0.5 mg/L in all columns’ effluent except that of column A. In column A, the average Zn concentration in the effluent was 2.7 mg/L. When acid was added to the influent at the 17th and 18th weeks, Zn was re-dissolved to 6.2 mg/L in the effluent. Excluding re-dissolution events, the average Zn removal efficiency in column A was only 29.2%. In column B, C1, C2, and D, Zn was removed with efficiencies of 99.5%, 99.4%, 96.2%, and 88.4%, respectively.
The statistical analysis on the data for concentrations and removal efficiencies of Cu and Zn was performed using a two-way ANOVA at the 95% confidence level. The elapsed time and treatment type were used as sources of variation. The results of statistical analysis indicate that there was no statistical influence of both elapsed time and treatment type on the Cu concentration (p-values: 0.38 for elapsed time and 0.15 for treatment type). However, the treatment type (p values: 0.15 for Cu and 1.1 × 10–11 for Zn) had a statistically greater effect on the Cu and Zn concentrations than the elapsed time (p values: 0.38 for Cu and 0.13 for Zn). In particular, the treatment type statistically affected the Zn concentration (p value: 1.1 × 10–11) at the 95% confidence level. On the other hand, the treatment type strongly influenced the removal efficiencies of Cu and Zn (p values: 4.6 × 10–11 for Cu and 7.0 × 10–33 for Zn).
Effect of pH increase on metal removal in bioreactor
The increase in pH in the columns due to the dissolution of limestone and BSR has been shown to be an important factor in the context of metal removal from mine water in this study. The relationship between pH and metal removal efficiency is shown in Fig. 6. In column A, which had no organic materials, only limestone dissolution could have occurred. In other columns, which had both limestone and organic materials, BSR affected pH increase as well as limestone dissolution.
When limestone is dissolved, alkalinity is increased through the resultant presence of HCO3−, which ultimately increases the pH of mine water (Eq. 1). An increase in pH results in the precipitation of insoluble hydroxides and/or carbonates. In column A, which only consisted of limestone, limestone dissolution was the main factor influencing the removal of metals from mine water. Cu can be precipitated as Cu hydroxides and/or carbonates by increasing the pH. Although the metal removal efficiency in column A exhibited an increasing trend with pH, the removal efficiency was lower than that in the other columns. In other columns, which consisted of not only limestone but also compost, the pH range of effluents and removal efficiency were higher than those in column A (Fig. 3 and Fig. 4). With only limestone dissolution, it is difficult to reach a pH value for precipitation of each metal hydroxide because the pH required for Cu and Zn precipitation as hydroxides is 7.0 and 8.5, respectively (Verburg et al. 2009). The average effluent pH in column A was only 6.5.
Meanwhile, the compost can increase the pH to a greater extent than limestone alone. The HCO3− generated by the decomposition of organic matter (compost) can be the primary factor influencing the increase in pH (Eq. 2). As the influent flows through the compost layer, O2 in the upper part of the layer is removed by oxidation, which creates a reducing condition in the lower part. In other words, aerobic and anaerobic decomposition of organic matter by microbes can occur in the upper and lower parts, respectively (Eqs. 5 and 6).
Additionally, the average Ca concentration of the influent was 55.6 mg/L and increased in column A, B, C, and D to 92.7, 107.0, 177.4, and 104.9 mg/L, respectively. The highest Ca concentration in column C indicated that calcite was dissolved most vigorously with an abundance of organic substrate. This suggests that CO2 released via the decomposition of the organic substrate (Eqs. 5 and 6) enhances the dissolution of calcite.
Moreover, column B had a pH that was remarkably higher than that of the other columns in the early stages of experiment, which may be relevant to photosynthesis by algal growth. During the early stages, DO increased to > 9 mg/L in the effluent of column B despite the negative ORP, suggesting metabolic activity of algae. As algae consume CO2, the form of alkalinity can change from bicarbonate to carbonate and hydroxide (Boyd 2015). Algae can continuously consume CO2 until the pH reaches 9.5; this pH then limits the growth of algae (Pedersen and Hensen 2003). During the night, the rate of CO2 production exceeds the rate of consumption, but limestone can buffer these effects. The pH of the effluent from column C and D did not exceed pH 9 in the early stages of the experiments, which may be due to the reducing conditions established by mixing limestone and compost. Under reducing conditions, the activity of algae can be relatively low because of oxygen deficiency, which is necessary for the respiration of algae, while CO2 can be consistently produced by reducing bacteria. Algae also have been shown to be excellent absorbents of heavy metals. However, algal adsorption of heavy metals is known to be more effective in moderate to weak acidic environments (Lin et al. 2020). Because alkaline environments were established by limestone dissolution and sulfate reduction reaction, the effect of adsorption on metal removal in columns was regarded as weak.
Effect of BSR on metal removal
The BSR influenced by SRB activity is the main factor affecting the removal of heavy metals from columns with organic substrates. It is known to be a key mechanism for metal removal in bioreactors (Rambabu et al. 2020). The presence of SRB in experimental conditions was detected using SRB test kit (BART™), which is a simple tool that detects SRB in solutions by changing the color when SRB exists. The degree of color becomes dark with an increase in the amount of SRB present, so the quantitative interpretations of SRB presence are possible. By using SRB test kits, we detected more SRB presence in the effluent from mixed reactor (limestone + SMC) than that in only limestone reactor (Fig. 7). Through this simple test, we confirmed that the experimental conditions are adequate for the presence of SRB and the occurrence of BSR. Even though the SRB test kit is only a tool for estimating the presence of SRB, and not a precise quantitative tool, it can be used to validate the occurrence of BSR.
In column B, C, and D, which simulated the SAPS and bioreactor, oxygen was consumed until anoxic conditions were established, as the influent flowed downward through the substrate. Combining these anoxic conditions and organic substrates with a sufficient carbon source, SRB can be activated then sulfate reduction occurs and H2S and HCO3− are produced (Riefler et al. 2008), as shown in Eq. 2. Dissolved heavy metals can be precipitated as sulfide H2S and alkaline conditions can be generated via increase in HCO3− levels (Benedetto et al. 2005), as shown in Eq. 3. The ideal pH for SRB growth is in the range 6.8–7.2 (Gibert et al. 2002). The effluent of columns B, C, and D showed a similar pH range, indicating that heavy metal removal is possible via SRB activities.
To verify metal removal by BSR in bioreactors and SAPS, the concentrations of total dissolved S and S existing in sulfate form (sulfate-S) in the effluents were compared, and concentrations of S existing in a sulfide form (sulfide-S) were determined from the difference between total dissolved S and sulfate-S (Fig. 8). In the case of column A, there were no significant variations in the concentrations of the total dissolved S between the influent and effluent. As the SMC contained gypsum, the effluent of column C2 showed an immediate increase in sulfate after the substitution of SMC on the 10th of September. Nevertheless, until the 4th of September, the concentrations of total dissolved S in the effluent were comparable to those in the inflow in column B, indicating the consumption of sulfate by BSR. Then, the input of acid resulted in an increase in the total dissolved S, possibly due to the re-dissolution of sulfides. Column C, bioreactor with a relatively high ratio of compost, showed especially low total dissolved S concentrations, except at the initial stages of each SMC installation. Column D, with a relatively low ratio of compost, also exhibited a decrease in sulfate until the 4th of September. Nevertheless, the decrease in sulfate diminished afterwards. As shown in Fig. 3, alkalinity decreased with time in the effluent of reactors containing organic substrates. These observations suggest a decreasing effect of BSR due to (1) decrease in bioavailable organic matter with time and (2) decrease in SRB activity as a result of decreasing water temperature during fall.
The statistical analysis on the data for total dissolved sulfur, dissolved sulfate, and dissolved sulfide contents was performed using a two-way ANOVA at the 95% confidence level. The elapsed time and treatment type were used as sources of variation. The results of statistical analysis indicated that the elapsed time and treatment type did not have an influence on the total dissolved sulfur, dissolved sulfate, and dissolved sulfide contents at the 95% confidence level (p values: 0.37, 0.32, 0.16 for elapsed time, respectively, and 0.08, 0.08, and 0.43 for treatment type, respectively). However, the treatment type had a statistically greater effect on the total dissolved sulfur and dissolved sulfate contents than the elapsed time (p-values: 0.37 and 0.32 for elapsed time, respectively and 0.08 and 0.08 for treatment type). The precipitation of metal sulfide occurs when sulfide of a sufficient concentration is produced by the BSR. Presence of dissolved sulfide in the effluent indicates that the column has the capacity to remove more metal by reacting with sulfide (Castro Neto et al. 2018). Therefore, sulfide in the effluent can be used as an indicator of the occurrence of BSR.
Evaluation of bioreactors and SAPS for water treatment of S mine
On comparing column C and D, which simulated the bioreactor, the column with a higher ratio of compost was found to have a relatively high Cu and Zn removal efficiency, as shown in Fig. 4. The exceptionally high efficiencies of column D from 4 to 17th of September may be due to the exceptionally high pH of 9.5–9.9, which possibly resulted from the algal activity occurring at that time. In particular, the efficiency in the early phase of the experiments was relatively lower in column D than in column C, indicating that the ratio of compost is an important factor in the Cu and Zn removal from mine water.
With regard to the comparison of column B and C, which simulated the SAPS and bioreactor, respectively, both showed a similarly high efficiency of 99.7% and 98.0% for Cu removal and of 99.5% and 97.6% for Zn removal, respectively, regardless of the structure of limestone and compost. SAPS showed a slightly higher efficiency, which resulted from the higher HRT of the SAPS column. Generally, it is known that bioreactors are beneficial for heavy metal treatment, such as Cu removal from mine water, because bioreactors can maximize the BSR mechanism by mixing organic substrate and limestone. In other words, the organic substrate occupies the entire area in the bioreactor, while only a few organic substrates in SAPS have a layered structure. However, Cu and Zn removal efficiencies of the SAPS and bioreactor were similar in this study. These results can be explained by the concentration of heavy metals in mine water and the duration of the experiment. Firstly, the Cu and Zn concentrations in mine water from S mine were 6.7 and 4.1 mg/L on average, which are not too high, although they exceed Korea's permissible discharge limit. Secondly, 18 weeks time could be insufficient to evaluate the long-term effect compared to the operating duration of the real system. To overcome these limitations, alkalinity in each column was compared, as shown in Fig. 3. Column C became more alkaline than the other columns. Alkalinity can be a factor that determines the treatment efficiency in passive systems or bioreactors in this study. As a result, higher alkalinity over a longer period of time in the bioreactor column may indicate the effectiveness of bioreactor application in the S mine area in terms of water treatment operation lifespan.
Conclusions
For treating mine water discharged from S mine region, treatment facilities in the form of limestone reactors have been built, but the low effectiveness of heavy metal removal has led to the investigation of novel substrate materials that are best suited for the specific site at hand. Column experiments were conducted for 18 weeks to evaluate various types of reactors, including SAPS and bioreactors. Both the SAPS and bioreactor columns showed high Cu and Zn removal efficiencies. The Cu removal efficiencies of the SAPS, bioreactor (higher SMC ratio), and another bioreactor (lower SMC ratio) were 99.7%, 98.0%, and 97.1% on average, respectively, while that of the limestone reactor was 81.3%. Zn removal efficiencies were 99.5%, 97.6%, and 88.4% on average, while that of the limestone reactor was 29.2%, except for re-dissolution events. By comparing pH and metal removal efficiencies, it was shown that an increase in pH contributed to mine water treatment in bioreactors. BSR also affected Cu and Zn treatment by precipitating metals in a sulfide form, as demonstrated by residual sulfide measured in the bioreactor and SAPS column. In addition, among the bioreactor columns, column with higher compost ratios showed higher efficiency, thus demonstrating the role of compost and BSR in removing Cu and Zn.
In this study, bioreactors and SAPS were found to effectively remediate Cu- and Zn-containing mine water with satisfying the permissible discharge limits. In addition, those methods showed a similarly high removal efficiency with slight differences. These results were inferred to be due to the relatively low concentration of heavy metals in mine water and the insufficient duration of the experiments. To supplement these results, the alkalinity of each column was analyzed because it can be a critical factor that determines the lifespan of passive treatment systems. The highest range of alkalinity was observed in the bioreactor column, indicating that bioreactor may be more beneficial in terms of long-term operation for mine water treatment facilities than SAPS in S mine site. Although more research is needed to corroborate the apparent occurrence of SRB activity, the effects of BSR on metal removal from mine water in a bioreactor was demonstrated via presence of residual sulfide in the effluent. However, in this study, only a single experiment for each column was conducted. Thus, further duplicate tests are needed to estimate errors in column experiments to evaluate the metal removal from mine water.
This study focuses on the practical evaluation of bench-scale experiments prior to full-scale treatment. It is significant from the standpoint of site-specific research, which focuses on finding appropriate treatment approaches for the S mine region in South Korea. Bioreactors have not been applied in any of South Korea's mine water treatment facilities, including that for removing Cu and Zn from abandoned metallic mines. This research will be helpful in selecting the most appropriate bioreactor for S mine water treatment facilities and is the first application of bioreactors for treating mine water from metallic mine region.
Data availibility
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
ASTM (American Society for Testing and Materials) (2021) Standard test method for pH of soils. In: Annual Book of ASTM Standards D4972–95a, Washington, DC
Béchard G, Yamazaki H, Gould WD, Bédard P (1994) Use of cellulosic substrates for the microbial treatment of acid mine drainage. J Environ Qual 23:111–116
Benedetto JdS, De Almeida SK, Gomes HA et al (2005) Monitoring of sulfate-reducing bacteria in acid water from uranium mines. Miner Eng 18:1341–1343
Boyd CE (2015) pH, carbon dioxide, and alkalinity. Water Qual Springer:153–178
Carlier JD, Luís AT, Alexandre LM, Costa MC (2020) Feasibility of co-treating olive mill wastewater and acid mine drainage. Mine Water Environ 39:859–880
Castro Neto ES, Aguiar ABS, Rodriguez RP, Sancinetti GP (2018) Acid mine drainage treatment and metal removal based on a biological sulfate-reducing process. Braz J Chem Eng 35:543–552
Chang IS, Shin PK, Kim BH (2000) Biological treatment of acid mine drainage under sulphate-reducing conditions with solid waste materials as substrate. Water Res 34(4):1269–1277
Cheong YW, Das BK, Roy A, Bhattacharya J (2010) Performance of a SAPS-based chemo-bioreactor treating acid mine drainage using low-DOC spent mushroom compost, and limestone as substrate. Mine Water Environ 29:217–224
Christensen B, Laake M, Lien T (1996) Treatment of acid mine water by sulfate-reducing bacteria: results from a bench scale experiment. Water Res 30:1617–1624
Clyde EJ, Champagne P, Jamieson HE et al (2016) The use of a passive treatment system for the mitigation of acid mine drainage at the Williams Brothers Mine (California): pilot-scale study. J Clean Prod 130:116–125
Cohen RRH (2006) Use of microbes for cost reduction of metal removal from metals and mining industry waste streams. J Clean Prod 14:1146–1157
Corral-Bobadilla M, González-Marcos A, Vergara-González EP et al (2019) Bioremediation of wastewater to remove heavy metals using the spent mushroom substrate of Agaricus bisporus. Water 11:15
Das BK, Mandal SM, Bhattacharya J (2012) Understanding of the biochemical events in a chemo-bioreactor during continuous acid mine drainage treatment. Environ Earth Sci 66:607–614
Demchak J, Morrow T, Skousen J (2001) Treatment of acid mine drainage by four vertical flow wetlands in Pennsylvania. Geochem Explor Environ Anal 1:71–80
DeNicol DM, Stapleton MG (2002) Impact of acid mine drainage on benthic communities in streams: the relative roles of substratum vs. aqueous effects. Environ Pollut 119:303–315
Gibert O, De Pablo J, Cortina JL, Ayora C (2002) Treatment of acid mine drainage by sulphate-reducing bacteria using permeable reactive barriers: a review from laboratory to full-scale experiments. Rev Environ Sci Biotechnol 1:327–333
Johnson DB, Hallberg KB (2005) Acid mine drainage remediation options: a review. Sci Total Environ 338:3–14
Jordan SN, Mullen GJ, Murphy MC (2008) Composition variability of spent mushroom compost in Ireland. Bioresour Techno 99:411–418
Kapoor A, Viraraghavan T (1995) Fungal biosorption. An alternative treatment option for heavy metal bearing wastewaters: a review. Bioresour Techno 53:195–206
Karam A (1993) Chemical properties of organic soils. In: Carter MR (ed) Soil Sampling and methods of analysis, Ch. 44. Lewis Publ., Chelsea, pp 459–471
Kepler D (1988) An overview of the role of algae in the treatment of acid mine drainage. ASMR 1988:286–290
Kim DM, Kwon OH, Oh YS et al (2021) Determination of soil contamination sources in mining area using Zn/Cd ratios with mobile Cd. Environ Geochem Health 43:4061–4074
Klein R, Tischler JS, Mühling M, Schlömann M (2013) Bioremediation of mine water. Geobiotechnology I:109–172
Korea Meteorological Administration (2022) Normal climate in Korea. https://data.kma.go.kr/climate/average30Years/selectAverage30YearsKoreaList.do?pgmNo=188. Accessed December 13, 2022
Lee DK, Shin SW, Cheong YW (2018) Characterization of iron precipitates in a SAPS limestone layer for flushing system design. Mine Water Environ 37:796–806
Lefticariu L, Walters ER, Pugh CW, Bender KS (2015) Sulfate reducing bioreactor dependence on organic substrates for remediation of coal-generated acid mine drainage: field experiments. J Appl Geochem 63:70–82
Lin Z, Li J, Luan Y (2020) Application of algae for heavy metal adsorption: a 20-year meta-analysis. Ecotoxicol Environ Saf 190:110089
Mayes WM, Davis J, Silva V, Jarvis AP (2011) Treatment of zinc-rich acid mine water in low residence time bioreactors incorporating waste shells and methanol dosing. J Hazard Mater 193:279–287
McCullough CD, Lund MA (2011) Bioremediation of acidic and metalliferous drainage (AMD) through organic carbon amendment by municipal sewage and green waste. J Environ Manage 92:2419–2426
Ministry of Environment, Korea (2020) Water Environment Conservation Act (No. 17326). https://elaw.klri.re.kr/kor_service/lawView.do?hseq=54838&lang=ENG. Accessed December 13, 2022
Muhammad SN, Kusin FM, Md Zahar MS et al (2017) Passive bioremediation technology incorporating lignocellulosic spent mushroom compost and limestone for metal- and sulfate-rich acid mine drainage. Environ Technol 38:2003–2012
Muhammad SN, Kusin FM, Madzin Z (2018) Coupled physicochemical and bacterial reduction mechanisms for passive remediation of sulfate- and metal-rich acid mine drainage. Int J Environ Sci Technol 15:2325–2336
Neculita CM, Zagury GJ, Bussière B (2007) Passive treatment of acid mine drainage in bioreactors using sulfate-reducing bacteria: critical review and research needs. J Environ Qual 36:1–16
Neculita CM, Yim GJ, Lee G et al (2011) Comparative effectiveness of mixed organic substrates to mushroom compost for treatment of mine drainage in passive bioreactors. Chemosphere 83:76–82
Offeddu FG, Cama J, Soler JM et al (2015) Processes affecting the efficiency of limestone in passive treatments for AMD: column experiments. J Environ Chem Eng 3:304–316
Oh YS, Park HS, Kim DK et al (2020) Evaluation of Cu removal from mine water in passive treatment methods: field pilot experiments. Econ Environ Geol 53:235–244
Ordonez A, Loredo J, Pendas F (2012) A successive alkalinity producing system (SAPS) as operational unit in a hybrid passive treatment system for Acid Mine Drainage. Mine Water Environ:575–580
Pedersen F, Hansen PJ (2003) Effects of high pH on the growth and survival of six marine heterotrophic protists. Mar Ecol Prog Ser 260:33–41
Rambabu K, Banat F, Pham QM et al (2020) Biological remediation of acid mine drainage: review of past trends and current outlook. ESE 2:100024
Riefler RG, Krohn J, Stuart B, Socotch C (2008) Role of sulfur-reducing bacteria in a wetland system treating acid mine drainage. Sci Total Environ 394:222–229
Simate GS, Ndlovu S (2014) Acid mine drainage: challenges and opportunities. J Environ Chem Eng 2:1785–1803
Singh S, Chakraborty S (2020) Performance of organic substrate amended constructed wetland treating acid mine drainage (AMD) of North-Eastern India. J Hazard Mater 397:122719
Skousen JG, Ziemkiewicz PF, McDonald LM (2019) Acid mine drainage formation, control and treatment: approaches and strategies. Extr Ind Soc 6:241–249
Thisani SK, Kallon DVV, Byrne P (2021) A fixed bed pervious concrete anaerobic bioreactor for biological sulphate remediation of acid mine drainage using simple organic matter. Sustainability 13:6529
Trumm D, Watts M (2010) Results of small-scale passive system trials to treat acid mine drainage, West Coast Region, South Island, New Zealand. N Z J Geol Geophys 53:227–237
Vasquez Y, Escobar MC, Neculita CM et al (2016) Biochemical passive reactors for treatment of acid mine drainage: effect of hydraulic retention time on changes in efficiency, composition of reactive mixture, and microbial activity. Chemosphere 153:244–253
Verburg R, Bezuidenhout N, Chatwin T, Ferguson K (2009) The global acid rock drainage guide (GARD guide). Mine Water Environ 28:305
Watzlaf GR, Schroeder KT, Kairies CL (2000) Long-term performance of anoxic limestone drains. Mine Water Environ 19:98–110
Wolkersdorfer C, Bowell R (2005) Contemporary reviews of mine water studies in Europe, Part 2. Mine Water Environ 24:2–37
Wu G, Kang H, Zhang X et al (2010) A critical review on the bio-removal of hazardous heavy metals from contaminated soils: issues, progress, eco-environmental concerns and opportunities. J Hazard Mater 174:1–8
Younger PL, Banwart SA, Hedin RS (2002) Mine water: hydrology, pollution, remediation, vol 5. Kluwer Academic, Boston
Acknowledgements
This work was financially supported by the Korea Mine Rehabilitation and Mineral Resources Corporation (KOMIR), funded by the Ministry of Trade, Industry, and Energy.
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Oh, Y.S., Park, H.S., Ji, W.H. et al. Removal of Cu and Zn from mine water using bench-scale bioreactors with spent mushroom compost: a case study in an abandoned mine region, South Korea. Environ Earth Sci 82, 172 (2023). https://doi.org/10.1007/s12665-023-10839-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-023-10839-w