Abstract
Background
The clinical and pathological features of sporadic microsatellite instability-high (MSI) colorectal cancer (CRC) are still unclear. The present study aimed to clarify the clinicopathological features of sporadic MSI CRC in comparison with those of Lynch syndrome (LS) exploratorily.
Methods
The present study was a single-center, retrospective cohort study. Sporadic MSI CRC was defined as MSI CRC with aberrant promoter hypermethylation of the MLH1 gene, while hereditary MSI CRC was defined colorectal cancer in patients with LS.
Results
In total, 2653 patients were enrolled; of these, 120 (4.5%) had MSI CRC, 98 had sporadic MSI CRC, and 22 had LS. Patients with sporadic MSI CRC were significantly older (p < 0.001) than those with LS and had a right-sided colonic tumor (p < 0.001) which was pathologically poorly differentiated or mucinous (p = 0.025). The overall survival rate was significantly lower in patients with stage I, II or III MSI CRC than in those with LS (p = 0.024). However, the recurrence-free survival rate did not differ significantly (p = 0.85).
Conclusions
We concluded that patients with sporadic MSI are significantly older, tumors more likely to locate in the right-sided colon, pathologically poorly differentiated or mucinous, and worse overall survival than in those with LS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal carcinoma (CRC) is the third most common type of malignant tumor in the developed world and one of the leading causes of cancer-related deaths [1]. At the molecular level, CRC is a heterogeneous disease with several molecular subtypes harboring distinct, molecular, genetic, pathological, and clinical characteristics [2]. In recent years, microsatellite instability has become focal in the treatment of CRC. Microsatellite instability refers to the hypermutable state of cells caused by impaired DNA mismatch repair (MMR) and consists of insertion and deletion mutations in stretches of short tandem DNA repeats (i.e., microsatellites) as well as nucleotide substitutions throughout the genome [3]. Microsatellite instability-high (MSI) tumor, which is found in around 15% of CRC cases [4], consists of a hereditary form of MMR deficiency (dMMR) known as Lynch syndrome (LS) or sporadic MSI CRC. LS is caused by a germline mutation in a mismatch repair gene, resulting in the development of malignant tumors, such as colorectal and endometrial cancer. In contrast, the leading cause of sporadic MSI CRC is acquired aberrant methylation of the promoter region of the MLH1 gene.
Recently, a recommendation for universal tumor screening to test comprehensively for microsatellite instability in all patients with CRC has been advanced [5]. Testing for microsatellite instability during screening for LS is an important method of obtaining therapeutic and diagnostic information, because approximately 90% of LS tumors have MSI, and the screening results may be instrumental in determining the optimal therapeutic strategy [6]. Previous studies revealed that MSI CRC generally has a better prognosis, lower probability of metastasis, right-sided tumor location (i.e., the cecum or ascending and transverse colon), metachronous multiple CRC, and less favorable response to 5-FU-based chemotherapy [6,7,8].
However, the clinicopathological features of sporadic MSI CRC and LS, which are both manifestations of MSI, have not been thoroughly compared. The present study thus aimed to clarify the clinicopathological features of sporadic MSI CRC and LS exploratorily, both of which are subgroups of MSI CRC.
Patients and methods
Study design
The present study was a single-center, retrospective cohort study.
Setting
The present study was performed at Tokyo Metropolitan Cancer and Infectious Diseases Center Komagome Hospital between January 2008 and May 2020. The enrolled patients are currently being followed up.
Participants
Patients were included in the study if they met the following criteria: consecutive adult patients (≥ 18 years) with histologically confirmed colorectal adenocarcinoma who underwent surgery for CRC during the study period. All the patient enrolled underwent genetic and epigenetic analyses during universal tumor screening after submitting their informed consent. We performed microsatellite instability test as an examination for all patients with CRC, which is called universal tumor screening. Patients whose DNA was not analyzed or those with a history of inflammatory bowel disease or familial adenomatous polyposis were excluded. If the patients had previously undergone resection for two or more colorectal tumors, the most advanced tumor was selected for analysis.
Outcomes
Sex, age, primary tumor location, UICC stage, pathological T, pathological N, and pathological differentiation of the tumor were analyzed as outcomes in the descriptive statistical analysis. The survival outcome in patients with stage I–III CRC sporadic MSI or LS was the 5-year overall survival (OS) rate. Survival time was defined as the duration between the days of the first surgery for CRC to the latest confirmed date of survival. Patients with no events of interest were censored at the date of the final observation. Relapse-free survival (RFS), defined as the duration between the day of the first surgery for CRC to the diagnosis of a relapse of CRC or an LS-related tumor, was also analyzed.
Genetic and epigenetic analyses
MSI analysis, BRAF V600E mutation analysis and methylation analysis of MLH1 gene were performed using the methods described in previous reports [9]. In detail, CRCs and corresponding normal tissues, obtained after informed consent, were stored at − 80 °C immediately after resection. Genomic DNA samples from them were extracted using the QIAamp DNA mini kit (QIAGEN, Valencia, CA). Microsatellite status was determined using two microsatellite markers (BAT25 and BAT26). Polymerase chain reaction (PCR) was performed to amplify cancer and corresponding normal DNAs. The reaction mixture (25 μL) contained 50 ng of genomic DNA, 0.4 μM of each primer, 0.2 μM concentrations of each four deoxynucleotide triphosphate, 1 × PCR buffer, and Taq polymerase. PCR was performed as follows: 5 min at 95 °C once; 1 min at 94 °C, 1 min at 50 °C, and 1 min at 72 °C for 35 cycles; and 10 min at 72 °C once. Amplified PCR products were diluted with formamide, and run on an Applied Biosystems 3100xl automated capillary electrophoresis DNA sequencer. Allelic sizes for each of the markers were estimated using GeneMapper Software ver.4.0 (Applied Biosystems, Foster City, CA). Microsatellite instability status was defined as MSI (≥ two marker unstable of the informative markers), and MSS (none or only one unstable markers) in accordance with the National Cancer Institute guidelines for microsatellite instability testing [10].
In the mutation analysis, all samples were analyzed BRAF V600E through direct sequencing. The PCR reaction mixture (10 μL) contained 200 ng of genomic DNA, 0.4 μM of each primer, 5μL of the AmpliTaq Gold Fast PCR Master Mix (Applied Biosystems). Cycling steps were: initial Taq activation (97 °C) for 5 min, 35 cycles as follows: denature for 95 °C 1 s, annealing for 1 min at 60 °C, extend for 72 °C 1 min and final extension 72 °C 10 min. The PCR products were purified using the ExoSAP-IT (General Electric Company, Fairfield, CT). Amplified fragments went through a PCR sequencing amplification. The PCR product was amplified using the BigDye Terminator v1.1 Cycle Sequencing Kit, (Applied Biosystems). The resulting PCR product was purified with the BigDye XTerminator Purification kit (Applied Biosystems). The cleaned product was loaded into a 3130xl Genetic Analyzer (Applied Biosystems). Sequence histograms were analyzed searching for heterozygous and homozygous substitutions using Sequencing Analysis Software ver.3.7 (Applied Biosystems).
The bisulfite conversion and recovery of bisulfite-converted DNA steps were perfumed using the Zymo EZ DNA methylation kit (Zymo Research, CA, USA) according to the manufacturer’s instructions. Polymerase chain reaction (PCR) primers and probes for the CpG island locus of MLH1 gene were used as described previously [11]. DNA methylation analysis was performed for all patients who were MSI and by MethyLight as described previously. Briefly, the 20 μL MethyLight reaction mixture contained 10 μL 2 × EpiTect MethyLight Master Mix (Qiagen, Valencia, CA, USA), 0.4 μmol/L of each primer, 0.2 μmol/L probe, and 50 ng template. A QuantStudio 3 (Thermo Fisher Scientific, CA, USA) was used to conduct the PCR reactions using the following thermal conditions: 5 min at 95 °C once, followed by 45 cycles of 15 s at 95 °C and 1 min at 60 °C. The differences in the amounts of input genomic DNA were normalized by the COL2A1 gene. Duplicate tubes were used for each sample. The percentage methylated of reference (PMR) was calculated by dividing the MLH1: COL2A1 ratio of a sample by the MLH1: COL2A1 ratio of CpGenome Human Methylated DNA Standard (Millipore, Billerica, MA, USA) and multiplying by 100. The methylation status of each sample was determined to be positive when PMR > 4, a cut-off value based on validated data [11].
Definition of sporadic MSI CRC and hereditary MSI CRC
Sporadic MSI CRC was defined as a tumor with aberrant promoter hypermethylation of the MLH1 gene, and hereditary MSI CRC was defined as a colorectal tumor in patients with LS. In the present study, the patients with MSI were analyzed as a subgroup of patients with LS or sporadic MSI CRC. Patients whose microsatellite testing result was MSI and MLH1 promotor methylation was negative were performed a genetic test for MLH1, MSH2, MSH6, PMS2, or EPCAM after genetic counseling. As a result, patients with pathogenic variants in MLH1, MSH2, MSH6, PMS2, or EPCAM were diagnosed as LS. The patients’ informed consent was obtained for all these procedures.
Statistical analysis
The patient characteristics were reported as descriptive statistics, with continuous variables expressed as the median and range, and categorical variables expressed as a number and percentage. Continuous variables were compared using the Mann–Whitney U test, and categorical variables were compared using Fisher's exact test. The Kaplan–Meier curve and log-rank test were used for survival analysis. All statistical tests were two-sided, and P ≤ 0.05 was considered to indicate statistical significance. All statistical analyses were performed using STATA 15.1 (Texas, USA).
Loss to follow-up
In the present study, patients lost to follow-up were censored at the date of final confirmation of survival.
Ethics approval
The present study complied with the Declaration of Helsinki and all the applicable local laws and regulations. Approval for the protocol was obtained from the Tokyo Metropolitan Cancer and Infectious Diseases Center Komagome Hospital Ethical Committee (ID: 612, 1202, 1433, 1925, 2001).
Results
Patient recruitment
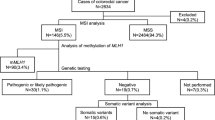
Figure 1 shows the patient flow. Patients who underwent surgical resection for CRC between January 2008 and December 2020 numbered 2656. Three patients had incomplete DNA information. Of the remaining 2653 patients, 2533 (95.5%) were excluded due to failure to meet the inclusion criteria, and 120 (4.5%) with MSI were included. Of the latter, 22 (18.3%) had LS, and 98 (81.7%) had sporadic MSI CRC.
Patient characteristics
The median days of follow-up were 1126 days (range: 7–3892). Table 1 shows the clinical and pathological characteristics of the patients. Patients with sporadic MSI CRC were significantly older (p < 0.001) than those with LS and had a right-sided CRC tumor (p < 0.001) that was pathologically poorly differentiated or mucinous (p = 0.025).
Genetic characteristics
Table 2 shows the genes in LS patients with a mutation.
Survival outcomes
Figures 2 and 3 show the survival outcomes in the study. Patients with Stage I–III sporadic MSI CRC and in those with LS was 91 and 22, respectively. Five-year OS in patients with Stage I–III sporadic MSI CRC and in those with LS was 87.8% [95% confidential interval (CI) 74.5–94.4] and 94.7% (95% CI 68.1–99.2), respectively. The OS was significantly worse in patients with sporadic MSI CRC than in patients with LS (log-rank test, p = 0.024). On the other hand, RFS did not differ significantly between the groups (log-rank test, p = 0.85).
Discussion
Our study produced the following significant findings: first, MSI CRC occurred less frequently than reported previously in studies enrolling Western (North American and European) patients. Second, universal tumor screening demonstrated that the LS prevalence was only 0.8%. Thus, sporadic MSI CRC accounted for a small proportion of the total CRC cases. Third, the differences in the clinicopathological features and prognosis were clarified by analyzing patients with sporadic MSI or LS in the same cohort. The present study is the first report to clarify the clinicopathological features of sporadic MSI CRC among CRC patients using universal tumor screening with microsatellite instability testing in a Japanese hospital-based cohort.
In the cohort in the present study, the MSI CRC frequency was 4.5%, which was closer to the frequency of 5.9% reported elsewhere in Asia in contrast to the frequency of 8.5–21.3% reported in the West [12,13,14]. Thus, our findings were in keeping with these data showing the frequency of MSI CRC in Asia to be lower than in the West. On the other hand, 3–5% of patients with CRC had LS, which has an estimated incidence of one in 279 in the general population [15, 16]. However, recent reports on universal tumor screening to detect patients with MSI demonstrated that the prevalence of patients with LS was 0.7–1.6% among those with CRC [17,18,19,20], demonstrating less difference between Asia and the West in terms of the prevalence of these patients. Therefore, in view of the frequency of MSI CRC and LS, the frequency of patients with sporadic MSI CRC might be lower in Asia than in the West.
Genetic testing was only performed in patients with MSI CRC without hypermethylation of the MLH1 gene and, therefore, may not have detected patients with LS who later experienced MSS CRC development. However, given that immunohistochemistry (IHC) is not the optimal screening tool for LS, a certain degree of inaccuracy may be unavoidable. The sensitivity of screening for patients with LS in MSI testing is similar to that of IHC [21, 22].
Previous studies demonstrated that MSI CRC was predominantly located at right-sided colon and was frequent in histology of poorly differentiated or mucinous type [23,24,25,26]. However, to date, few studies have reported the characteristics of MSI CRC in a large Asian cohort. The only study in Japan to assess CRC characteristics by MSI status in a large cohort (940 patients) stated that MSI-H cancers were observed more frequently in females, in the proximal colon, and in poorly differentiated or mucinous CRCs in comparison with MSS; however, the MSI-H CRC cases mentioned in the study included LS-associated CRCs as well as sporadic MSI CRCs, and no statistical comparison of between MSS CRCs and MSI-H CRCs was provided [19]. The present study prospectively collected more than 2,600 CRC patients and statistically analyzed the differences in the clinicopathological characteristics between MSS CRCs and MSI CRCs in the same cohort, resulting in demonstrating that the characteristics of MSI CRCs were comparable in Japanese between Western patients. Namely, sporadic MSI CRC was predominantly located on the right side of the colon and more often developed in elderly female patients.
To the best of our knowledge, only a few prospective studies have compared the comparison of clinicopathological characteristics between sporadic MSI CRCs and LS CRCs in the same cohort. Liu et al. reported the clinicopathological findings of sporadic dMMR CRCs and LS-associated CRCs; however, they analyzed neither BRAF V600E nor hypermethylation of the MLH1 gene in more than 60% of dMMR CRC cases and did not indicate the total number of cases for which dMMR testing was done, thus causing a selection bias1 [27]. The present study prospectively performed universal tumor screening for more than 2,600 patients with CRC prospectively since 2008, and compared MSI CRC and MSS CRC within the same cohort. No other study of comparable size has been done in East Asia, including Japan, and our findings showed that the MSI CRC characteristics in Asian patients were comparable to those in Western patients. Nonetheless, given the paucity of studies on this topic in Asia, including Japan, we believe that the present study is a meaningful contribution to this area of research. In this study, sporadic MSI CRCs predominantly developed in the right side of the colorectum, while hereditary MSI CRCs equally developed in both sides.
In the present study, sporadic MSI CRCs predominantly developed on the right side of the colorectum, while hereditary MSI CRCs developed on either side equally. From the embryological point-of-view, the right side of the colorectum develops from the midgut, while the left side develops from the hindgut. Comprehensive molecular analysis of gastrointestinal cancer throughout the gastrointestinal tract demonstrated that the genomic and epigenomic alterations differed at each location [28]. Thus, this difference in embryological origins may lead to differences in the frequency of the types of tumor.
The adenoma–carcinoma sequence, a well-known carcinogenesis pathway in colorectal cancer, proceeds stepwise through the accumulation of mutations due to the inactivation of tumor suppressor genes and the activation of oncogenes, with tumor development via this pathway occurring mainly in the left colorectum. On the other hand, in the serrated neoplastic pathway, which differs from the adenoma–carcinoma pathway, hyperplastic polyp development from normal mucosa due to BRAF gene mutation progresses to a sessile serrated adenoma/polyp due to genome-wide aberrant hypermethylation known as CIMP. Subsequently, the additional hypermethylation of the MLH1 gene leads to MSI CRC development, with tumor development via this pathway occurring mainly in the right colorectum. Recent evidence has shown that chronic infection by Fusobacterium nucleatum, which has been observed in MSI CRC, generates reactive oxygen species, resulting in aberrant hypermethylation of the genes [29,30,31]. Although it is unclear why CRC associated with F. nucleatum infection frequently occurs in the right colon [32], because of the higher frequency of MSI-H occurrence in elderly female patients than in male, female patients suggested that estrogen may have a protective effect against MSI-H CRC in women [33, 34]. Because LS is an autosomal dominant disorder, there is no difference in its frequency by gender. In addition, because patients with LS have monoallelic pathogenic variants in the MMR gene, CRC often develops at a young age.
In terms of prognosis, the OS was significantly worse in patients with sporadic MSI CRC than in those with LS in our study, in line with the results of previous studies [6, 35]. The difference in the prognosis may be explained by any of the following: (1) more elderly patients have MSI CRC than hereditary MSI CRC; (2) the majority of MSI CRC cases harbor a BRAF gene mutation; and (3) most MSI CRC cases show a poorly differentiated and/or mucinous histology [23]. Moreover, a comparison of MSI CRC and LS has shown that the two conditions differ in terms of genome-wide hypermethylation, although both have MSI due to disruption of the MMR system. In MSI CRC, aberrant hypermethylation occurs in various other genes besides MLH1, resulting in loss of function of various proteins. Therefore, the expression of genes without repeated sequences in the coding region are also suppressed in MSI CRC. Furthermore, the effect of chemotherapy reportedly varies according to the degree of microsatellite instability. Some studies have clinically demonstrated that genome-wide methylation in sporadic MSI CRC influenced the efficacy of chemotherapy and thus, the prognosis [27, 36,37,38,39]. Clinical trials have demonstrated that MSI tumors respond to immune checkpoint inhibitors [40,41,42], but the definitive difference in the drug response between MSI CRC and in LS has not been established. Since the frequency of programmed death-ligand 1 (PD-L1) expression differs between the CRC types [23], the effect of immune checkpoint inhibitors may differ in each type as well.
The current study has some limitations. First, the sample size was too small to allow any definitive conclusions to be drawn. However, the inclusion criteria were strict; therefore, patients without an unambiguous diagnosis of MSI CRC were excluded, thus maintaining high internal validity. In addition, there is a possibility that there were patients with Lynch-like syndrome among the excluded patients that was due to MSI and not MLH1 gene methylation [43, 44]. Second, genetic testing was performed only in patients with CRC with MSI, resulting in selection bias. Third, the present study analyzed only cases in which the tumor was present at surgery and did not include additional operations after endoscopic resection or the previously resected tumor. Nevertheless, our study was able to demonstrate the frequency of LS in patients with a tumor in surgery.
In conclusion, patients with sporadic MSI are significantly older, tumors more likely to locate in the right-sided colon, pathologically poorly differentiated or mucinous, and worse overall survival than in those with LS.
References
Siegel RL, Miller KD, Jemal A (2018) Cancer statistics, 2018. CA Cancer J Clin 68:7–30. https://doi.org/10.3322/caac.21442
Dienstmann R, Salazar R, Tabernero J (2014) The Evolution of Our Molecular Understanding of Colorectal Cancer: What We Are Doing Now, What the Future Holds, and How Tumor Profiling Is Just the Beginning. Am Soc Clin Oncol Educ Book 34:91–99. https://doi.org/10.14694/edbook_am.2014.34.91
Devaud N, Gallinger S (2013) Chemotherapy of MMR-deficient colorectal cancer. FamCancer 12:301–306. https://doi.org/10.1007/s10689-013-9633-z
Geiersbach KB, Samowitz WS (2011) Microsatellite instability and colorectal cancer. Arch Pathol Lab Med 135:1269–1277. https://doi.org/10.5858/arpa.2011-0035-RA
Canard G, Lefevre JH, Colas C et al (2012) Screening for lynch syndrome in colorectal cancer: are we doing enough? Ann Surg Oncol 19:809–816. https://doi.org/10.1245/s10434-011-2014-7
Ribic CM, Sargent DJ, Moore MJ et al (2003) Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med 349:247–257. https://doi.org/10.1056/nejmoa022289
Ward R (2001) Microsatellite instability and the clinicopathological features of sporadic colorectal cancer. Gut 48:821–829. https://doi.org/10.1136/gut.48.6.821
Shitoh K, Konishi F, Miyakura Y et al (2002) Microsatellite instability as a marker in predicting metachronous multiple colorectal carcinomas after surgery: a cohort-like study. Dis Colon Rectum 45:329–333. https://doi.org/10.1007/s10350-004-6177-1
Natsume S, Yamaguchi T, Takao M et al (2018) Clinicopathological and molecular differences between right-sided and left-sided colorectal cancer in Japanese patients. Jpn J Clin Oncol 48:609–618. https://doi.org/10.1093/jjco/hyy069
Perucho M, Boland CR, Thibodeau SN et al (1998) Correspondence re: C. R. Boland et al., A national cancer institute workshop on microsatellite instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in color. Cancer Res 59:5248–5257
Ogino S, Kawasaki T, Brahmandam M et al (2006) Precision and performance characteristics of bisulfite conversion and real-time PCR ( MethyLight ) for quantitative DNA methylation analysis. J Mol Diagnostics 8:209–217. https://doi.org/10.2353/jmoldx.2006.050135
Asaka SI, Arai Y, Nishimura Y et al (2009) Microsatellite instability-low colorectal cancer acquires a KRAS mutation during the progression from Dukes’ A to Dukes’ B. Carcinogenesis 30:494–499. https://doi.org/10.1093/carcin/bgp017
Nosho K, Kure S, Irahara N et al (2009) A prospective cohort study shows unique epigenetic, genetic, and prognostic features of synchronous colorectal cancers. Gastroenterology 137:1–23. https://doi.org/10.1053/j.gastro.2009.08.002
Vilar E, Gruber SB (2010) Microsatellite instability in colorectal cancer—the stable evidence. Nat Rev Clin Oncol 7:153–162
Matloff J, Lucas A, Polydorides AD, Itzkowitz SH (2013) Molecular tumor testing for lynch syndrome in patients with colorectal cancer. JNCCN J Natl Compr Cancer Netw 11:1380–1385. https://doi.org/10.6004/jnccn.2013.0161
Das C, Lucia MSHK, TJ, (2017) Prevalence and penetrance of major genes and polygenes for colorectal cancer. Cancer Epidemiol Biomarkers Prev 26:404–412
Li D, Hoodfar E, Jiang SF et al (2019) Comparison of universal versus age-restricted screening of colorectal tumors for lynch syndrome using mismatch repair immunohistochemistry: a cohort study. Ann Intern Med 171:19–26
Adar T, Rodgers LH, Shannon KM et al (2018) Universal screening of both endometrial and colon cancers increases the detection of Lynch syndrome. Cancer 124:3145–3153. https://doi.org/10.1002/cncr.31534
Chika N, Eguchi H, Kumamoto K et al (2017) Prevalence of Lynch syndrome and Lynch-like syndrome among patients with colorectal cancer in a Japanese hospital-based population. Jpn J Clin Oncol 47:108–117. https://doi.org/10.1093/jjco/hyw178
Jiang W, Cai MY, Li SY et al (2019) Universal screening for Lynch syndrome in a large consecutive cohort of Chinese colorectal cancer patients: High prevalence and unique molecular features. Int J Cancer 144:2161–2168. https://doi.org/10.1002/ijc.32044
Moreira L, Balaguer F, Lindor N et al (2012) Identification of Lynch syndrome among patients with colorectal cancer. JAMA-J Am Med Assoc 308:1555–1565. https://doi.org/10.1001/jama.2012.13088
Shia J (2008) Immunohistochemistry versus microsatellite instability testing for screening colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome: Part I. The utility of immunohistochemistry. J Mol Diagnostics 10:293–300. https://doi.org/10.2353/jmoldx.2008.080031
Yamada R, Yamaguchi T, Iijima T et al (2018) Differences in histological features and PD-L1 expression between sporadic microsatellite instability and Lynch-syndrome-associated disease in Japanese patients with colorectal cancer. Int J Clin Oncol 23:504–513
Slattery ML, Curtin PDK, Wolff PDRK et al (2009) A comparison of colon and rectal somatic DNA 0terations. Dis colon rectum. 52:1304–1311. https://doi.org/10.1007/DCR.0b013e3181a0e5df
Barault L, Funes M, Vega D et al (2008) Hypermethylator Phenotype in Sporadic Colon Cancer : Study on a Population-Based Series of 582 Cases. Cancer res 68:8541–8547. https://doi.org/10.1158/0008-5472.CAN-08-1171
Nosho K, Irahara N, Shima K et al (2008) Comprehensive biostatistical analysis of CpG island methylator phenotype in colorectal cancer using a large population-based sample. PLoS ONE 3:e3698. https://doi.org/10.1371/journal.pone.0003698
Liu GC, Liu RY, Yan JP et al (2018) The heterogeneity between lynch-associated and sporadic mmr deficiency in colorectal cancers. J Natl Cancer Inst 110:975–984. https://doi.org/10.1093/jnci/djy004
Liu Y, Sethi NS, Hinoue T et al (2018) Comparative molecular analysis of gastrointestinal adenocarcinomas. Cancer Cell 33:721–735. https://doi.org/10.1016/j.ccell.2018.03.010
Niwa T, Tsukamoto T, Toyoda T et al (2010) Inflammatory processes triggered by Helicobacter pylori infection cause aberrant DNA methylation in gastric epithelial cells. Cancer Res 70:1430–1440. https://doi.org/10.1158/0008-5472.CAN-09-2755
Niwa T, Ushijima T (2010) Induction of epigenetic alterations by chronic inflammation and its significance on carcinogenesis. Elsevier Inc., Lyon
Nosho K, Sukawa Y, Adachi Y et al (2016) Association of Fusobacterium nucleatum with immunity and molecular alterations in colorectal cancer. World J Gastroenterol 22:557–566. https://doi.org/10.3748/wjg.v22.i2.557
Mima K, Nishihara R, Rong Qian Z et al (2016) Fusobacterium nucleatum in colorectal carcinoma tissue and patient prognosis were responsible for collection of tumour tissue, and acquisition of epidemiologic, clinical and tumour tissue data, including histopathological and immunohistochemical character. Gut 65:1973–1980
Tsai YJ, Huang SC, Lin HH et al (2018) Differences in gene mutations according to gender among patients with colorectal cancer. World J Surg Oncol 16:1–5. https://doi.org/10.1186/s12957-018-1431-5
Slattery ML, Potter JD, Curtin K et al (2001) Estrogens reduce and withdrawal of estrogens increase risk of microsatellite instability-positive colon cancer. Cancer Res 61:126–130
Maccaroni E, Bracci R, Giampieri R, et al (2015) Prognostic impact of mismatch repair genes germline defects in colorectal cancer patients: Are all mutations equal? Oncotarget 6:38737–38748. https://doi.org/10.18632/oncotarget.5395
Sinicrope FA, Foster NR, Thibodeau SN et al (2011) DNA mismatch repair status and colon cancer recurrence and survival in clinical trials of 5-fluorouracil-based adjuvant therapy. J Natl Cancer Inst 103:863–875. https://doi.org/10.1093/jnci/djr153
Jover R, Nguyen TP, Pérez-Carbonell LZ et al (2011) 5-fluorouracil adjuvant chemotherapy does not increase survival in patients with CpG island methylator phenotype colorectal cancer. Gastroenterology 140:1174–1181
Dkk T, Burgermeister E, Ph D et al (2012) TFAP2E–DKK4 and chemoresistance in colorectal cancer. N Engl J Med 366:44–53
Perez-Carbonell L, Balaguer F, Toiyama Y et al (2014) IGFBP3 methylation is a novel diagnostic and predictive biomarker in colorectal cancer. PLoS ONE 9:1–11. https://doi.org/10.1371/journal.pone.0104285
André T, Shiu K-K, Kim TW et al (2020) Pembrolizumab in microsatellite-instability–high advanced colorectal cancer. N Engl J Med 383:2207–2218. https://doi.org/10.1056/nejmoa2017699
Marabelle A, Le DT, Ascierto PA et al (2020) Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/ mismatch repair–deficient cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol 38:1–10. https://doi.org/10.1200/JCO.19.02105
Overman MJ, Lonardi S, Wong KYM et al (2018) Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J Clin Oncol 36:773–779. https://doi.org/10.1200/JCO.2017.76.9901
Kang SY, Park CK, Chang DK et al (2015) Lynch-like syndrome: characterization and comparison with EPCAM deletion carriers. Int J Cancer 136:1568–1578. https://doi.org/10.1002/ijc.29133
Mensenkamp AR, Vogelaar IP, Van Zelst-Stams WAG et al (2014) Somatic mutations in MLH1 and MSH2 are a frequent cause of mismatch-repair deficiency in lynch syndrome-like tumors. Gastroenterology 146:643-646.e8. https://doi.org/10.1053/j.gastro.2013.12.002
Acknowledgements
We wish to express our gratitude to the Office of Metropolitan Hospital Management, Tokyo Metropolitan Government for their support. We are grateful to all the patients and their families for their participation in our study and would like to thank Mr. James R. Valera for his assistance in editing this manuscript.
Funding
The Office of Metropolitan Hospital Management, Tokyo Metropolitan Government provided funding for this study.
Author information
Authors and Affiliations
Contributions
YN, TI, TI, EK, MT, AT, SH, and TH: substantial contributions to the conception and design of the study and acquisition, analysis or interpretation of data. YN and TY: drafting or critical revision of the manuscript for important intellectual content. TY: final approval of the version to be published.
Corresponding author
Ethics declarations
Conflicts of interest
All the authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Nakayama, Y., Iijima, T., Inokuchi, T. et al. Clinicopathological features of sporadic MSI colorectal cancer and Lynch syndrome: a single-center retrospective cohort study. Int J Clin Oncol 26, 1881–1889 (2021). https://doi.org/10.1007/s10147-021-01968-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-021-01968-y