Abstract
Purpose
The aim of this study was to investigate the prevalence of Lynch syndrome among Japanese patients with surgically resected colorectal cancer at a single institution.
Methods
Of 616 colorectal cancer patients who underwent surgical operation in our institution from January 2005 to August 2010, immunohistochemistry analyses for mismatch repair proteins (MLH1, MSH2, MSH6, and PMS2) and microsatellite instability (MSI) testing for surgically resected, formalin-fixed paraffin-embedded colorectal cancer specimens from 138 colorectal cancer patients under 60 years of age were undertaken. Hypermethylation of the MLH1 promoter and BRAF mutation were analyzed where necessary.
Results
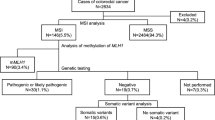
Seven patients were identified as candidates for genetic testing by mismatch repair protein loss (n = 7) or MSI-H (n = 6). Methylation of MLH1 was detected in one case. Three patients were diagnosed with Lynch syndrome, comprising 2.2 % of the total colorectal cancer patients younger than 60 years of age.
Conclusion
The prevalence of Lynch syndrome among hospital-based diagnosed cancer patients may therefore be lower than expected in Japan compared with Western populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lynch syndrome (LS) is the most common hereditary colorectal cancer (CRC) syndrome with an autosomal dominant inheritance pattern. Screening for LS is usually performed using the Amsterdam criteria II (AC II) [1] and the revised Bethesda guidelines (rBG) [2], leading to secondary screening consisting of microsatellite instability (MSI) testing or immunohistochemistry (IHC) to detect the inherited loss of function in DNA mismatch repair genes (MMRs), including MLH1, MSH2, MSH6, and PMS2. Patients with LS have an increased risk of developing colorectal, endometrial, ovarian, gastric, and other cancers. Therefore, LS screening is a promising strategy to reduce the mortality rate. A recent report [3] described that the prevalence of LS was 2.8 % among CRC patients in the United States. According to this data, one can estimate that the population-based prevalence of LS is approximately 1 in 370 [4]. In Japan, the prevalence of LS remains unknown due to the lack of a national database. With the recent increase in CRC in Japan [5], patients with LS are likely to have been included among so-called “sporadic” cancer patients. Clinically, the hallmarks of LS-related CRC are an early onset, multiple lesions, right hemicolon predominance, and histologically poorly differentiated adenocarcinoma compared with sporadic CRC. When a few of these clinical findings are recognized after surgical treatment, the possibility of LS should be considered in clinical practice.
Recently, several reports [3, 6–8] have recommended the implementation of universal population screening for LS among all patients with newly diagnosed CRC. It is important to obtain a medical and family history from patients with suspected LS so that initial screening for AC II and rBG is performed. However, patients sometimes do not know the status of relatives within even three degrees of relationship, since the trend toward nuclear families has been increasing recently. Considering these situations, it might be acceptable to perform MSI testing or IHC as a supplementary confirmation of a diagnosis of LS in all CRC patients. In fact, half of the LS cases that were discovered using universal screening did not fulfill the AC II and rBG criteria. Although IHC for the detection of MMR protein expression is not covered by health insurance in Japan, IHC is a more useful method in terms of cost effectiveness and simplicity, compared with MSI testing. In addition, IHC is a diagnostically effective method, since IHC can narrow down the pathogenic gene candidates in cases where germ line alterations of MMRs have been identified and LS has been confirmed.
In this study, we investigated the prevalence of LS among Japanese patients under 60 years of age with surgically resected CRC at a single institution using MSI testing and IHC as a primary screening method and considered the optimal method for detecting LS in municipal hospitals.
Patients and methods
Patients
This study was performed in accordance with the ethical guidelines for clinical and genetic research with the approval of our institutional ethics committee. Genetic testing was performed after obtaining the patient’s informed consent.
In this study, 138 unrelated colorectal cancer patients (82 males and 56 females) younger than 60 years of age, who underwent surgical resection in Saitama Medical Center between January 2005 and August 2010, were analyzed. Two female colorectal cancer patients younger than 60 years of age were excluded due to the diagnosis of familial adenomatous polyposis. A total of 616 colorectal cancer patients underwent surgical operation in our institution during the same period. The median age of the patients was 69 years (range 25–92 years). Of 138 patients examined, the localization of carcinoma was as follows: appendix: 4 cases, cecum: 6, ascending colon: 16, transverse colon: 11, descending colon: 2, sigmoid colon: 41, and rectum: 58. The histological diagnosis was well-differentiated adenocarcinoma: 53 cases, moderately: 73, poorly: 9, and others: 3. The carcinomas at the time of primary tumor resection were staged according to the UICC classification. These cases included stage I, 33 cases; stage II, 30 cases; stage III, 42 cases; and stage IV, 35 cases.
DNA extraction
Formalin-fixed paraffin-embedded (FFPE) specimens were obtained from the 138 colorectal cancer patients. Representative FFPE tumor and normal mucosa specimens were selected by a pathologist after examination of the hematoxylin and eosin-stained slides. Genomic DNA was then isolated using the QIAamp DNA FFPE Tissue Kit (Qiagen Inc, Valencia, CA, USA) following the manufacturer’s recommended protocols.
MSI testing
MSI testing was performed using extracted genomic DNA from cancer tissue and normal mucosa tissue embedded as FFPE specimens as described above. Five National Cancer Institute consensus microsatellite markers, including two mononucleotide (BAT-25 and BAT-26) and three dinucleotide (D5S346, D2S123, and D17S250), were analyzed for the detection of MSI as described previously [9]. In brief, the PCR conditions were 55 °C for 10 s and 95 °C for 10 min, followed by 42 cycles at 95 °C for 15 s and 60 °C for 1 min. The information of specific primer sequences and PCR conditions for BAT25, BAT26, D5S346, D2S123, and D17S250 is described in a previous report [9]. Cancerous tissue was designated as having high-level MSI (MSI-H) if novel PCR bands were identified in at least two of the five markers. Low-level MSI (MSI-L) was diagnosed when a single marker demonstrated novel PCR product bands. Cancerous tissue was considered to be microsatellite stable (MSS) if there was no evidence of MSI in any marker.
Immunohistochemistry for MMRs
Paraffin-embedded histological sections. (4 μm thick) were deparaffinized and washed with water, and then antigens were retrieved by autoclaving sections on slides in 0.01 M citrate buffer (pH 6.0) for 10 min. After endogenous peroxidase activity was blocked with 0.3 % hydrogen peroxide in methanol for 15 min, the sections were incubated with each primary antibody overnight at 4 °C. The primary antibodies for detecting MMRs were anti-hMLH1 antibody (G168-15, BD Pharmingen, San Diego, CA, USA, 1:50), anti-hMSH2 antibody (FE11, Calbiochem, La Jolla, CA, USA, 1:50), anti-hMSH6 antibody (44/MSH6, BD Pharmingen, 1:100), and anti-hPMS2 antibody (A16-4, BD Pharmingen, 1:50). After use of the DAKO Envision kit (Agilent Technologies Dako, Glostrup, Denmark), staining was visualized with diaminobenzidine (DAB), followed by counterstaining with hematoxylin. The expression of these proteins was evaluated as positive when the nuclei of the cancer cells were stained. Assessment of the staining was evaluated by two independent pathologists without knowledge of the clinical status of the patients.
Detection of MLH1 methylation and BRAF status
The combined bisulfite restriction analysis (COBRA) method was used to evaluate methylation of the MLH1 promoter. In brief, tumor DNA was bisulfite converted using commercially available kits (MethylEasy XceedRapid DNA Bisulphite Modification Kit, Human Genetic Signatures Pty Ltd, Australia) following the manufacturer’s recommended protocols. Target CpG-rich sequences in the MLH1 promoter region were amplified using TaKaRa EpiTaq™ HS (TAKARA BIO, Japan). A PCR reaction was performed using the following primers: forward primer: 5′-GTAAGGGGAGAGGAGGAGT-3′ and reverse primer: 5′-AAATACCTTCAACCAATCACCTC-3′. Restriction digest of the PCR products (384 bp) was then undertaken using TaqI enzymes that recognize sequences (T|CGA) potentially altered by methylation.
Genetic analysis
BRAF V600E mutation was analyzed by the direct sequencing method by LSI Medience Corporation (Tokyo, Japan). The full sequence analysis of MLH1, MSH2, and MSH6 was performed using the direct sequencing method by FALCO biosystems (Kyoto, Japan). When pathogenic mutations were not detected in the patients’ samples, multiplex ligation-dependent probe amplification (MLPA) was performed using the Salsa® MLPA® kit (MRC-Holland, Amsterdam, The Netherlands) by the Research Center for Genomic Medicine at our institution.
Results
MSI testing and IHC detection
Of 138 patients, 6 patients (4.3 %) showed MSI-H (Table 1). MSI-L was observed in 2 patients (1.4 %).
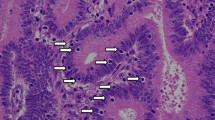
In the IHC evaluation, the loss of any MMR expression was observed in 7 patients (5.1 %) (Table 2). The loss of both MLH1 and PMS2 expression was observed in 4 patients (Nos. 19, 24, 77, and 113). Both MSH2 and MSH6 were not expressed in 2 patients (Nos. 5 and 62). Only one patient had undetectable MSH6 expression (No. 137). Representative staining is shown in Fig. 1.
Typical staining of MMRs in colorectal cancerous tissue. The loss of both MLH1 and PMS2 expression was observed in cancerous tissue from patient No. 19. The loss of both MSH2 and MSH6 was observed in cancerous tissue from patient No. 62. Only MSH6 expression was deleted in cancerous tissue from patient No. 137
All patients with MSI-H had a complete overlap with patients with any loss of MMR expression.
MLH1 promoter methylation and BRAF mutation
Of 4 patients with the loss of MLH1 expression, MLH1 methylation was detected in one patient (No. 24) (Fig. 2). No BRAF mutation was found in the remaining three patients.
Genetic analysis for confirming LS
A genetic analysis was performed for 6 patients with both the loss of MMR expression and MSI-H, except one patient (No. 24) where the loss of MLH1 expression was implicated by the methylation analysis (Fig. 3). Of 6 patients, pathogenic mutations were identified in three patients (Nos. 19, 62 and 137) (Table 3). The patient with the loss of MLH1 expression (No. 77) had a missense mutation, which has not been shown to affect MLH1 function. There was no mutation in the MLH1 gene of another patient (No. 113). The patient with the loss of MSH2 expression (No. 62) had two mutations in the MSH2 gene. However, these mutations have also not been shown to be pathogenic mutations. As a candidate of a pathogenic mutation, the duplication of exon 1 was observed using the MLPA method (No. 62). Unfortunately, PCR amplification was not seen in the patient (No. 5) due to DNA degradation.
Discussion
The number of CRC patients has increased in parallel with the increasing number of elderly individuals in Japan, and CRC is now the leading cause of death from cancer among Japanese women and the third leading cause among Japanese men. In clinical practice, hereditary CRC is usually considered when a younger patient develops CRC. However, the exact number of LS patients with CRC in Japan is unknown. Recently, several reports [3, 6–8] have recommended the implementation of universal population screening for LS among all patients with newly diagnosed CRC. Therefore, we retrospectively conducted LS screening using MSI testing and IHC in CRC patients younger than 60 years of age. At our institution, approximately 20 % of all CRC patients were younger than 60 years of age. Among 138 CRC patients, 3 patients (2.2 %) were identified as having LS in the present study. The ages of these patients at the time of the primary diagnosis of CRC were 24, 37, and 55 years of age. The 24-year-old female patient fulfilled only the criterion of “diagnosed under the age of 50 years,” as she did not have a family history of CRC or other LS-related cancers. The 37-year-old male patient had a family history of endometrial cancer in his mother and relatives. Only the 55-year-old male patient did not meet any of the clinical criteria. These three LS patients, who were selected from among CRC patients younger than 60 years of age, accounted for only 0.5 % of all the CRC patients treated during the same period. Even if screening was extended to patients older than 60 years of age, the prevalence of LS might be lower than expected in Japan, compared with a reported prevalence of LS of 0.5–13 % among CRC patients in Western countries [3, 10, 11]. In Japan, Furukawa et al. [12] reported that mutations in MLH1 or MSH2 were identified in 8 LS patients (1.7 %) among 452 CRC patients. Among these 8 patients, 7 patients (87.5 %) had an age of onset of younger than 50 years of age. Chang et al. [13] demonstrated that the prevalence of LS was 13 patients (2.3 %) among 561 CRC patients in Taiwan. Among these 13 patients, 5 patients (38.5 %) were diagnosed at younger than 50 years of age. Hampel et al. [3] performed universal screening and identified 18 patients (3.6 %) with LS among 500 CRC patients in a United States cohort. Among these 18 patients, 8 patients (44.4 %) were diagnosed at younger than 50 years of age. Consequently, 44 patients (2.8 %) were identified as having LS among 1,566 CRC patients when previously reported data were added to the current data. Musulén et al. [14] also performed universal screening for LS among 1624 CRC patients in Spain. A genetic analysis detected pathogenic mutations in the MMR genes in 18 patients (1.1 %). Among these 18 patients, 7 patients (38.9 %) were diagnosed at younger than 50 years of age. These results suggested that the prevalence of LS differs depending on the country, or even the region within the country. Further investigation of the prevalence of LS according to the region will be important. Therefore, our current results are clinically significant as an example of a regional cohort in Japan.
As mentioned above, several reports [3, 13, 14] have shown that patients 50 years of age and older were included among patients with confirmed LS, similar to our results. In clinical practice, patients are screened for LS using the AC II and rBG criteria. We previously evaluated the usefulness of these clinical criteria for LS screening in 890 CRC patients between 2005 and 2012 [15]. Approximately 25 % of the patients were selected as candidates for LS using the rBG criteria, while less than 1 % of the patients remained after applying the AC II criteria. The reason why only a few LS candidates met the AC II criteria is that most of the patients did not know the accurate age at diagnosis of their relatives with LS-related cancers. We concluded that the rBG criteria were suitable for LS screening. However, the application of only the rBG criteria is not sufficient for the selection of LS candidates. Several reports [16–18] have suggested that the problem with the rBG criteria is that candidates are limited to an age of younger than 50 years. Recent guidelines for LS screening, including the US Multi-Society Task Force on colorectal cancer [19], the ESMO Clinical Practice Guidelines [20], and the National Comprehensive Cancer Network [21], may overcome these problems and select suitable candidates using MSI testing or IHC prior to genetic testing. These guidelines recommend that universal screening be performed for LS in patients younger than 70 years of age who have CRC and in CRC patients older than 70 years of age who fulfill the rBG criteria. Sie et al. [22] reported that testing CRC patients younger than 70 years of age resulted in a cost-effective outcome, compared with only testing patients younger than 50 years of age. These concepts regarding effective LS screening are certainly favorable, including medical economy. However, the prevalence of LS must be high for these benefits to be realized. In this study, we found that the prevalence of LS might be lower than expected among Japanese patients younger than 60 years of age, although our results were analyzed in a limited regional population. Our CRC series included 206 CRC patients between 60 and 70 years of age. If screening is extended to patients younger than 70 years of age, as the guidelines suggest, the cost of the analysis might be out of proportion to the benefit. Regarding CRC patients older than 60 years of age, only patients with a family history would be investigated for LS screening. Thus, we believe that universal screening should be applied to Japanese CRC patients younger than 60 years of age, as others have suggested [13, 16, 17, 23].
MSI testing is considered to be essential for the selection of LS candidates. Previous reports have demonstrated that mononucleotide markers, including BAT25 and BAT26, were sufficient to determine the MSI status [24, 25]. In this study, both BAT25 and BAT26 were positive in all the patients with MSI-H. Therefore, regarding MSI testing, the examination of BAT25 and BAT26 might be sufficient to determine the MSI status as a minimum method for primary screening of LS. Moreover, IHC is also a useful method for selecting LS candidates in terms of cost and time. The expression of target MMRs can be estimated based on the MMR staining pattern as shown in Table 2. Although we examined all MMR protein expressions in this study, Shia et al. [26] suggested that the examination of both MSH6 and PMS2 expression could be as predictive as that of all MMRs using IHC. Since MSH6 and PMS2 are obligate binding partners of MSH2 and MLH1, respectively, they are not expressed at the protein level in the absence of their partner proteins. Therefore, the loss of MSH6 expression can detect both defects in MSH6 and MSH2, whereas the loss of PMS2 may indicate defects of both PMS2 and MLH1. This approach might be more effective in terms of time and cost. In the present study, the LS candidates were selected based on both MSI testing and IHC methods. Several reports have concluded that the IHC results are concordant with the MSI status [27–29]. Regau et al. [28] summarized the relationship between the IHC results for MMR expression and the MSI status in 3,494 CRC cases. The sensitivity, specificity, positive predictive value, and negative predictive value of the IHC results for the MSI status were 92.4, 99.6, 98.5, and 97.8 %, respectively. Lindor et al. [29] also reported that the predictive value of normal IHC for an MSS/MSI-L phenotype was 96.7 %, and the predictive value of abnormal IHC was 100 % for an MSI-H phenotype in 1,144 CRC patients. The disadvantage of MSI testing was that patients with an MSH6 deficiency were occasionally not detected [30], while the IHC method was able to resolve this issue. IHC is a more useful method in terms of its cost effectiveness and simplicity compared with MSI testing. In addition, IHC is a diagnostically effective method, since IHC can narrow down pathogenic gene candidates when the germ line alteration of MMRs has been used to confirm a diagnosis of LS. However, one potential disadvantage of IHC is that cases with positive MMR expression are observed when patients have rare MLH1 or MSH6 mutations despite MSI [30, 31]. In these cases, MSI testing is still required. MSI testing for BAT25 and BAT26 and IHC for MSH6 and PMS2 may be sufficient for primary LS screening without clinical criteria. Moreover, an MLH1 promoter methylation analysis and a BRAF (V600E) mutation analysis can distinguish a sporadic CRC from CRC associated with LS when MLH1 is thought to exhibit a pathogenic mutation [32]. We observed MLH1 promoter methylation in a 51-year-old female patient. When we checked to confirm that she had a germ line mutation in the MLH1 gene, no mutations were detected.
We found an MSH6 mutation in a younger patient in this study. Seventeen CRC patients with MSH6 mutations were recently reported in a Japanese population [33]. The mean age at diagnosis was 53.6 years (range 36–86 years), leading to the suggestion that the late onset of CRC was compatible with reports from Western counties [31]. The 24-year-old female patient in whom we identified as having a MSH6 mutation exhibited a relatively early onset of CRC compared with other reported patients with MSH6 mutations. In experimental murine studies, a deficiency of both MSH6 and MSH3 increased the incidence of intestinal cancer compared with that in single nullizygous mice [34]. This experimental data suggested that the above-mentioned patient might have had an MSH3 mutation. We analyzed the MSH3 gene in this patient; however, a pathogenic mutation in MSH3 was not detected. Terui et al. [33] reported that the mean age at the onset of endometrial cancer was 49.2 years in 8 female patients, while it was 56.5 years in patients from Western countries. Thus, further follow-up of the above-mentioned patient for LS-related cancer, especially endometrial cancer, is needed.
In the present study, we could not confirm the presence of pathogenic mutations in two patients with MSI-H and/or the loss of MMR expression as LS. There are two subsets of disease that should be distinguished from LS. CRC with MSI-H and/or a loss of MMR expression, but without a detectable germ line mutation or hypermethylation in the MMR genes can be classified as Lynch-like syndrome (LLS) [35, 36]. Patients who met the Amsterdam criteria I but lacked MMR mutations were diagnosed as having familial colorectal cancer type X [37]. According to the definition, these patients can be categorized as having LLS. The mutation of p.V384D (GTT>GAT) in MLH1, which was recognized in one of our patients (No. 77), was classified as class 1 in the database of the International Society for Gastrointestinal Hereditary Tumours Incorporated (InSiGHT) (http://insight-group.org/variants/database/) [38]. Ohsawa et al. reported that this mutation was detected in 39 (5.8 %) of 670 CRC patients, suggesting tumor susceptibility [39]. Rodríguez-Soler et al. [36] reported that the risk of cancer in families with LLS was lower than that of families with LS but higher than that of families with sporadic CRC. Therefore, special screening and surveillance strategies for these patients and their relatives are needed.
We speculated that the prevalence of LS would be less than or comparable to 1 % among all CRC patients in a Japanese hospital-based population, since the prevalence of LS was 0.5 % among CRC patients younger than 60 years of age at our institution. This finding suggests that the guidelines used in the US and Europe do not necessarily apply to CRC patients in Japan, since the prevalence of LS in Japan might be lower than that in the US and Europe. Although our analysis was limited in that only a regional population was analyzed, the present results might be useful for determining the management of younger patients with CRC. We recommend that CRC patients younger than 60 years of age undergo universal screening for LS using MSI testing for BAT25 and BAT26 and IHC for MSH6 and PMS2.
References
Vasen HF. Clinical diagnosis and management of hereditary colorectal cancer syndromes. J Clin Oncol. 2000;18:81S–92S.
Umar A, Boland CR, Terdiman JP, Syngal S, de la Chapelle A, Rüschoff J, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004;96:261–8.
Hampel H, Frankel WL, Martin E, Arnold M, Khanduja K, Kuebler P, et al. Feasibility of screening for Lynch syndrome among patients with colorectal cancer. J Clin Oncol. 2008;26:5783–8.
Hampel H, de la Chapelle A. The search for unaffected individuals with Lynch syndrome: do the ends justify the means? Cancer Prev Res (Phila). 2011;4:1–5.
Long N, Moore MA, Chen W, Gao CM, Lai MS, Mizoue T, et al. Cancer epidemiology and control in north-East Asia—past, present and future. Asian Pac J Cancer Prev. 2010;11:107–48.
Heald B, Plesec T, Liu X, Pai R, Patil D, Moline J, et al. Implementation of universal microsatellite instability and immunohistochemistry screening for diagnosing lynch syndrome in a large academic medical center. J Clin Oncol. 2013;31:1336–40.
Pérez-Carbonell L, Ruiz-Ponte C, Guarinos C, Alenda C, Payá A, Brea A, et al. Comparison between universal molecular screening for Lynch syndrome and revised Bethesda guidelines in a large population-based cohort of patients with colorectal cancer. Gut. 2012;61:865–72.
Morrison J, Bronner M, Leach BH, Downs-Kelly E, Goldblum JR, Liu X. Lynch syndrome screening in newly diagnosed colorectal cancer in general pathology practice: from the revised Bethesda guidelines to a universal approach. Scand J Gastroenterol. 2011;46:1340–8.
Loukola A, Eklin K, Laiho P, Salovaara R, Kristo P, Järvinen H, et al. Microsatellite marker analysis in screening for hereditary nonpolyposis colorectal cancer (HNPCC). Cancer Res. 2001;61:4545–9.
Aaltonen LA, Sankila R, Mecklin JP, Järvinen H, Pukkala E, Peltomäki P, et al. A novel approach to estimate the proportion of hereditary nonpolyposis colorectal cancer of total colorectal cancer burden. Cancer Detect Prev. 1994;18:57–63.
Houlston RS, Collins A, Slack J, Morton NE. Dominant genes for colorectal cancer are not rare. Ann Hum Genet. 1992;56:99–103.
Furukawa T, Konishi F, Shitoh K, Kojima M, Nagai H, Tsukamoto T. Evaluation of screening strategy for detecting hereditary nonpolyposis colorectal carcinoma. Cancer. 2002;94:911–20.
Chang SC, Lin PC, Yang SH, Wang HS, Liang WY, Lin JK. Taiwan hospital-based detection of Lynch syndrome distinguishes 2 types of microsatellite instabilities in colorectal cancers. Surgery. 2010;147:720–8.
Musulén E, Sanz C, Muñoz-Mármol AM, Ariza A. Mismatch repair protein immunohistochemistry: a useful population screening strategy for Lynch syndrome. Hum Pathol. 2014;45:1388–96.
Tajima Y, Kumamoto K, Ito T, Matsuzawa T, Ishiguro T, Kumagai Y, et al. Pit Fall of First Screening of Lynch Syndrome from Medical Records (in Japanese with English abstract). Nihon Gekakeirengo Gakkaishi (Journal of Japanese College of Surgeons). 2013;38:944–9.
Urso E, Pucciarelli S, Agostini M, Maretto I, Mescoli C, Bertorelle R, et al. Proximal colon cancer in patients aged 51–60 years of age should be tested for microsatellites instability. A comment on the Revised Bethesda Guidelines. Int J Colorectal Dis. 2008;23:801–6.
Chou CL, Lin JK, Wang HS, Yang SH, Li AF, Chang SC. Microsatellite instability screening should be done for right-sided colon cancer patients less than 60 years of age. Int J Colorectal Dis. 2010;25:47–52.
Morrison J, Bronner M, Leach BH, Downs-Kelly E, Goldblum JR, Liu X. Lynch syndrome screening in newly diagnosed colorectal cancer in general pathology practice: from the revised Bethesda guidelines to a universal approach. Scand J Gastroenterol. 2011;46:1340–8.
Giardiello FM, Allen JI, Axilbund JE, Boland CR, Burke CA, Burt RW, et al. Guidelines on genetic evaluation and management of Lynch syndrome: a consensus statement by the US Multi-Society Task Force on colorectal cancer. Gastroenterology. 2014;147:502–26.
Balmaña J, Balaguer F, Cervantes A, Arnold D, ESMO Guidelines Working Group. Familial risk-colorectal cancer: ESMO clinical practice guidelines. Ann Oncol. 2013;24:vi73–80.
National Comprehensive Cancer Network. Lynch syndrome. NCCN Clinical Practice Guidelines in Oncology. Genetic/Familial High-Risk Assessment: colorectal Version 2. 2014. National Comprehensive Cancer Network, 2014.
Sie AS, Mensenkamp AR, Adang EM, Ligtenberg MJ, Hoogerbrugge N. Fourfold increased detection of Lynch syndrome by raising age limit for tumour genetic testing from 50 to 70 years is cost-effective. Ann Oncol. 2014;25:2001–7.
Schofield L, Grieu F, Goldblatt J, Amanuel B, Iacopetta B. A state-wide population-based program for detection of lynch syndrome based upon immunohistochemical and molecular testing of colorectal tumours. Fam Cancer. 2012;11:1–6.
Xicola RM, Llor X, Pons E, Castells A, Alenda C, Piñol V, et al. Performance of different microsatellite marker panels for detection of mismatch repair-deficient colorectal tumors. J Natl Cancer Inst. 2007;99:244–52.
Hatch SB, Lightfoot HM Jr, Garwacki CP, Moore DT, Calvo BF, Woosley JT, et al. Microsatellite instability testing in colorectal carcinoma: choice of markers affects sensitivity of detection of mismatch repair-deficient tumors. Clin Cancer Res. 2005;11:2180–7.
Shia J, Tang LH, Vakiani E, Guillem JG, Stadler ZK, Soslow RA, et al. Immunohistochemistry as first-line screening for detecting colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome: a 2-antibody panel may be as predictive as a 4-antibody panel. Am J Surg Pathol. 2009;33:1639–45.
Bartley AN, Luthra R, Saraiya DS, Urbauer DL, Broaddus RR. Identification of cancer patients with Lynch syndrome: clinically significant discordances and problems in tissue-based mismatch repair testing. Cancer Prev Res (Phila). 2012;5:320–7.
Rigau V, Sebbagh N, Olschwang S, Paraf F, Mourra N, Parc Y, et al. Microsatellite instability in colorectal carcinoma. The comparison of immunohistochemistry and molecular biology suggests a role for hMSH6 immunostaining. Arch Pathol Lab Med. 2003;127:694–700.
Lindor NM, Burgart LJ, Leontovich O, Goldberg RM, Cunningham JM, Sargent DJ, et al. Immunohistochemistry versus microsatellite instability testing in phenotyping colorectal tumors. J Clin Oncol. 2002;20:1043–8.
Pino MS, Chung DC. Microsatellite instability in the management of colorectal cancer. Expert Rev Gastroenterol Hepatol. 2011;5:385–99.
Klarskov L, Holck S, Bernstein I, Okkels H, Rambech E, Baldetorp B, et al. Challenges in the identification of MSH6-associated colorectal cancer: rectal location, less typical histology, and a subset with retained mismatch repair function. Am J Surg Pathol. 2011;35:1391–9.
Kastrinos F, Syngal S. Screening patients with colorectal cancer for Lynch syndrome: what are we waiting for? J Clin Oncol. 2012;30:1024–7.
Terui H, Tachikawa T, Kakuta M, Nishimura Y, Yatsuoka T, Yamaguchi K, et al. Molecular and clinical characteristics of MSH6 germline variants detected in colorectal cancer patients. Oncol Rep. 2013;30:2909–16.
de Wind N, Dekker M, Claij N, Jansen L, van Klink Y, Radman M, et al. HNPCC-like cancer predisposition in mice through simultaneous loss of Msh3 and Msh6 mismatch-repair protein functions. Nat Genet. 1999;23:359–62.
Kang SY, Park CK, Chang DK, Kim JW, Son HJ, Cho YB, et al. Lynch-like syndrome: characterization and comparison with EPCAM deletion carriers. Int J Cancer. 2015;136:1568–78.
Rodríguez-Soler M, Pérez-Carbonell L, Guarinos C, Zapater P, Castillejo A, Barberá VM, et al. Risk of cancer in cases of suspected lynch syndrome without germline mutation. Gastroenterology. 2013;144:926–32.
Yamaguchi T, Furukawa Y, Nakamura Y, Matsubara N, Ishikawa H, Arai M, et al. Comparison of clinical features between suspected familial colorectal cancer type X and Lynch syndrome in Japanese patients with colorectal cancer: a cross-sectional study conducted by the Japanese Society for Cancer of the Colon and Rectum. Jpn J Clin Oncol. 2015;45:153–9.
Thompson BA, Spurdle AB, Plazzer JP, Greenblatt MS, Akagi K, Al-Mulla F, et al. Application of a 5-tiered scheme for standardized classification of 2,360 unique mismatch repair gene variants in the InSiGHT locus-specific database. Nat Genet. 2014;46:107–15.
Ohsawa T, Sahara T, Muramatsu S, Nishimura Y, Yathuoka T, Tanaka Y, et al. Colorectal cancer susceptibility associated with the hMLH1 V384D variant. Mol Med Rep. 2009;2:887–91.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Kumamoto, K., Ishida, H., Suzuki, O. et al. Lower prevalence of Lynch syndrome in colorectal cancer patients in a Japanese hospital-based population. Surg Today 46, 713–720 (2016). https://doi.org/10.1007/s00595-015-1232-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-015-1232-1