Abstract
Background
Specific circulating autoantibodies are produced by host immune systems to respond to antigens that arise during tumorigenesis. To achieve auxiliary diagnosis, the present study was designed to test whether circulating autoantibodies against tumor-associated antigens (TAAs) were altered in early breast cancer.
Methods
A total of 102 breast cancer patients and 146 age-matched healthy volunteers were recruited to participate in this study. Autoantibody expression was tested using in-house developed enzyme-linked immunosorbent assay (ELISA) with linear peptide envelope antigens derived from TAAs.
Results
Student’s t tests showed that expression of autoantibodies against the panel (p16, c-myc, TP53, and ANXA-1) was significantly higher in the breast cancer group, stage I and II breast cancer group, and stage III and IV breast cancer group than in the healthy control group (p < 0.001, p < 0.001, p < 0.001). The sensitivities of detection of the panel (90% specificity) in these groups were 33.3%, 31.7%, and 33.3%, respectively, significantly higher than that of any single autoantibody.
Conclusion
The panel of autoantibodies is more sensitive than single TAA autoantibody detection and may be used as biomarkers for early diagnosis of breast cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer, the most common cancer in females, has been the leading cause of cancer-related deaths in women worldwide, and the incidence has increased in recent years [1, 2]. Imaging is the primary means of screening for breast cancer, but this method routinely fails to detect tiny lesions. Recently, tumor biomarkers have been shown to have a function in predicting the risks of breast cancer recurrence and metastasis and response to treatment. For example, in addition to traditional pathological classification and clinical staging, gene microarray analysis or immunohistochemistry [to detect the estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2), and cell proliferation marker Ki-67] can be used to divide breast cancer into four subtypes: luminal A, luminal B (ER+/PR+, HER2+), Erb-B2 overexpression (ER−/PR−/HER2+), and basal-like (ER−/PR−/HER2−). Based on these different molecular subtypes, physicians can more accurately determine the prognosis and best treatment regimen for individual patients [3]. In addition to the foregoing four breast cancer biomarkers, carbohydrate antigen 15-3 and 27.29 (CA15-3 and CA27.29) have also been approved for evaluation of the prognosis and treatment of breast cancer [4]. CA15-3 and CA27.29 are different epitopes on the transmembrane glycoprotein expressed by the breast cancer-related gene MUC1. Most studies have shown a positive relationship between CA15-3 levels and breast cancer recurrence and metastasis, and changes in CA15-3 levels are indicative of therapeutic effect. Thus, this marker is a good predictor of the risk of postoperative recurrence and effectiveness of treatment. However, the sensitivity and specificity of this marker in breast cancer are relatively low, which greatly limits its application in early diagnosis of breast cancer [5–7]. Carcinoembryonic antigen (CEA) is a type of glycoprotein expressed in the digestive tube of the embryonic stage and is also widely used as a marker in breast cancer diagnosis and treatment, but its low specificity and sensitivity similarly do not allow for early detection of breast cancer [8]. Therefore, the current clinical use of tumor biomarkers, such as ER, PR, Her-2, CA15-3, CA27.29, and CEA, fails to detect early-stage breast cancer. Early diagnosis and treatment of breast cancer can reduce medical costs, control tumor development, and improve patient prognosis. Hence, it is necessary to find a panel of sensitive and specific biomarkers for early diagnosis of breast cancer.
Abnormal expression of certain proteins occurs in tumor cells during the process of carcinogenesis. These antigenic substances, known as tumor-associated antigens (TAAs), trigger immune responses in patients. Specific circulating autoantibodies are produced by the host immune system to respond to these antigens. A number of studies suggest that circulating autoantibodies against tumor-associated antigens (TAAs) may be detectable several years before radiographic detection or screening is able to identify the tumors [9, 10]. Recent studies have highlighted the importance of circulating autoantibodies against tumor antigens as biomarkers in tumor identification, risk assessment, and prognosis evaluation [11, 12]. In the present study, we probed for a panel of autoantibodies against TAAs (p16, c-myc, TP53, and ANXA1) to evaluate its diagnostic value in early-stage breast cancer.
Materials and methods
Subjects
Female subjects were selected for both the case and the control groups. A total of 102 female patients, including 57 patients with stage I and II breast cancer and 45 patients with stage III and IV breast cancer, as confirmed by radiographic examination and histology, participated in the study. All 102 patients had complete clinical information and were recruited from the second hospital of Jilin University. Blood samples were taken before any anticancer treatment. One hundred forty-six age-matched healthy female subjects (Table 1) were also recruited from local communities as controls. The clinical diagnosis of breast cancer is well established and effective. Clinical interviews and imaging examinations were used to exclude patients with other tumors and control subjects with a history of tumors. Patients with a history of severe autoimmune disease were also excluded. All research subjects were of Chinese Han origin and provided written informed consent before participating in the study. This work was approved by the Ethics Committee of Jilin University.
Autoantibody testing
A linear peptide antigen was designed according to the computational prediction of human leukocyte antigen class II (HLA-II)-restricted epitopes, which can be recognized by HLA-II molecules among >90% of the Chinese population. The autoantibody specific for TAA was measured using a relative enzyme-linked immunosorbent assay (ELISA) approach, as described in our recent publication [13–18]. A specific binding index (SBI) was used to express the levels of circulating autoantibody.
Data analysis
The mean SBI ± SD was used to present data. Microsoft Excel 2010 was used to construct a database with individual SBI values and to graphically analyze the distributions of individual autoantibody levels. IBM SPSS Statistics 19.0 was used to perform Student’s t test to compare testing results, such as the SBIs, for each anti-TAA antibody with those for the panel of multiple TAA autoantibodies among the breast cancer group, stage I and II breast cancer group, stage III and IV breast cancer group, and healthy controls. Receiver operating characteristic curve (ROC) analysis was used to calculate the area under the ROC curve (AUC); ELISA detected autoantibodies with >90% specificity.
Results
After testing the expression of p16, c-myc, TP53, and ANXA-1 autoantibodies in the breast cancer group, stage I and II breast cancer group, stage III and IV breast cancer group, and healthy control group (Table 2), p16 autoantibody expression was found to be significantly higher in the breast cancer group than in the control group (p = 0.046), whereas no differences were found between the stage I and II breast cancer group and stage III and IV breast cancer group and the healthy control group (p = 0.100, p = 0.166). Anti c-myc antibody expression showed no difference in each of the three groups compared with the control group (p = 0.160, p = 0.109, p = 0.586). The expression of autoantibodies against TP53 was significantly higher in the three groups than in the healthy control group (p < 0.001, p < 0.001, p < 0.001). The results of the panel of TAAs consisted of the mean of the expression of each autoantibody against p16, c- myc, TP53, and ANXA-1. Autoantibody expression of the panel was significantly higher in the breast cancer group, stage I and II breast cancer group, and stage III and IV breast cancer group than in the healthy control group (p < 0.001, p < 0.001, p < 0.001) (Table 2).
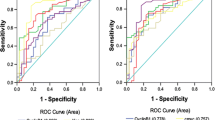
Next, ROC analysis was performed on p16, c-myc, TP53, and ANXA-1 and on the panel of all four markers to assess the diagnostic value of each marker and the panel of markers. The sensitivities of detection are listed in Table 3. The sensitivity of the panel was significantly higher than the sensitivity of any single TAA autoantibody in the breast cancer group (33.3%), stage I and II breast cancer group (31.7%), and stage III and IV breast cancer group (33.3%) (Fig. 1; Table 3).
Receiver operating characteristic (ROC) analysis of different autoantibodies in patients with breast cancer and different stages of breast cancer. a ROC analysis of different autoantibodies in patients with breast cancer (AUC of P16, 0.575; c-myc, 0.574; P53, 0.705; ANXA1, 0.733; mean, 0.725). b ROC analysis of different autoantibodies in patients with stage I and II breast cancer (AUC of P16, 0.568; c-myc, 0.584; P53, 0.687; ANXA1, 0.717; mean, 0.714). c ROC analysis of different autoantibodies in patients with stage III and IV breast cancer (AUC of P16, 0.583; c-myc, 0.561; P53, 0.726; ANXA1, 0.753; mean, 0.737)
HER2, ER, PR, and Ki-67 are also critical biological determinants in breast cancer molecular subtyping. Thus, we included them in the correlation analysis. Because some of the collected samples were from patients who were diagnosed with breast cancer for the first time, many patients did not complete molecular subtyping diagnosis. The analysis results showed that four types of autoantibodies did not significantly associate with HER2, ER, PR, and Ki-67. Thus, these results were not shown.
Discussion
As early as 1955, Baldwin et al. confirmed the tumor monitoring and killing effects of the immune system [19]. Immune responses induced by tumor extracts can destroy tumor tissues and maintain the growth of nonrecurrent tumors in animal models. In the same year, Graham et al. detected 48 cases of serum antibody titers of gynecological tumor patients (including cervical cancer and ovarian cancer), found significantly increased antibody titers in 12 patients, and first proposed the “antibody” as a tool for the diagnosis of cancer [20]. Qiu et al. applied protein chip technology to detect patient serum samples 1 year before diagnosis of lung cancer and found significantly increased autoantibodies to annexin 1, 14-3-3 theta, and LAMR1 in patients without any clinical symptoms [21]. The use of autoantibodies against TAAs has drawn increasing attention. Because of immune surveillance, the autoantibodies induced by tumors allow patients to recognize TAAs before any clinical signs emerge and can be detected even at very low levels [22]. Autoantibodies can be detected in peripheral blood serum, furthermore, the samples are stable and easy to collect, the detection instrument is very common, and the method is relatively well established, all of which make this strategy a potentially effective means of early cancer detection and diagnosis.
To achieve auxiliary diagnosis, TAAs detected by autoantibodies must be different in different tumor types. In our previous studies, we found that single autoantibody tests are not very sensitive [14–18]. However, detecting autoantibodies against multiple TAAs in a panel significantly improved specificity and sensitivity. In our previous study on TAA autoantibodies in cervical cancer, we tested 111 cervical cancer patients and 160 healthy subjects using a panel of five TAAs (survivin, cyclin B-1, ANXA-1, c-myc, and TP53) and obtained a specificity of 90% and a sensitivity of 37.8% [13]. In the present study, we tested a panel of autoantibodies, including p16, c-myc, TP53, and ANXA-1 in patient serum.
p16 is a cyclin-dependent kinase (CDK) inhibitor that can regulate cell-cycle progression from G1 to S phase. Mutation or deletion of the p16 gene can lead to a wide range of cancers. In our previous studies, we found that expression of the p16 gene significantly changed in lung cancer and breast cancer [15, 18]. The present results showed that expression of the anti-p16 autoantibody was significantly higher in the breast cancer group than in the control group, but there were no significant differences among the stage I and II breast cancer group, stage III and IV breast cancer group, and control group. The detection sensitivity of p16 autoantibodies in the breast cancer group was 27.5% (at 90% specificity), consistent with our previous results and higher than the results that we obtained in our non-small cell lung cancer study [15]. The c-myc gene encodes nuclear transcription factors. The proteins encoded by this gene are important in the regulation of cell-cycle progression and cell transformation. Overexpression and mutations of the c-myc gene may lead to abnormalities in the regulation of multiple genes and eventually cause the formation of carcinomas [23]. Our results showed that serum c-myc autoantibody levels were not significantly elevated in the breast cancer, stage I and II breast cancer, and stage III and IV breast cancer groups, similar to the results of a non-small cell lung cancer study [24]. TP53 is one of the most frequently mutated genes in cancer; the protein is crucial in regulating apoptosis and maintaining genomic stability. Mutations and epigenetic changes in TP53 are associated with a significantly increased risk for a variety of human malignancies, including breast cancer, cervical cancer, and lung cancer [25–27]. The anti-TP53 antibody has been the most frequently studied autoantibody as a diagnostic tool, followed by autoantibodies against MUC1, HER2, and cyclin B1 [28]. Our results showed that serum TP53 autoantibody levels were significantly higher in the breast cancer group, stage I and II breast cancer group, and stage III and IV breast cancer group than in the control group, similar to the results that we obtained in the cervical cancer study. Annexin 1 (ANXA-1), a calcium- and phospholipid-binding protein, has general functions in cell differentiation, apoptosis inhibition, and proliferation of cancerous cells [29]. Abnormal expression of ANXA-1 has been found in many types of tumors, including breast cancer [30]. The results showed that the serum levels of anti-ANXA-1 autoantibody in the breast cancer group, stage I and II breast cancer group, and stage III and IV breast cancer group were significantly higher than those in the control group, which were also similar to the results that we obtained from the cervical cancer study [13]. Based on the results, combined analysis showed that expression of the panel of anti-TAA autoantibodies was significantly higher in patients with malignant tumors, stage I and II breast cancer, and stage III and IV breast cancer than in healthy controls. In all groups, sensitivity was significantly higher for the panel than for any single autoantibody, reaching 33.3%, 31.6%, and 33.3%, respectively. The results suggested that investigation using the autoantibody panel is more suitable and consistent than single autoantibody analysis.
More recently, multiple studies have confirmed that the use of autoantibodies toward autologous TAAs has been gathering momentum, as these markers have been detected in the asymptomatic stage of cancer and may therefore serve as diagnostic biomarkers. In breast cancer, several studies of individual TAAs showed that many autoantibodies have high sensitivity, including p53, MUC-1, HSP-27, HSP-60, HSP90, HER2/neu/c-erg B2, GIPC-1, c-myc, BRCA1, BRCA2, endostatin, lipophilin B, cyclin B1, and cyclin D1. Similarly to these reports, we found that the sensitivity and specificity of the “autoantibody detection” is the maximum limiting factor. Typically, only 10–30% of cancer patients elicit a specific humoral response against a single TAA [31]. Therefore, several studies have evaluated the usefulness of detecting various autoantibodies as a panel to increase the accuracy of a potential diagnostic test. Desmetz et al. reported a multimarker signature, combining HSP60, MUC1, FKBP52, PPIA, and PRDX2, that reached sensitivity, specificity, and accuracy of 72.2%, 72.6%, and 72.4%, respectively, in breast cancer patients compared to healthy individuals [32]. However, in our study, MUC1, SOX2, FOXP3, survivin, and so on did not show good sensitivity. To advance the discovery of novel combinations of autoantibody biomarkers and improve sensitivity, we plan to use four approaches: (1) to design other important epitope peptides for key breast cancer-associated genes, thereby improving the detection efficiency of the autoantibodies; (2) to use the serological analysis of tumor antigens by recombinant cDNA expression cloning (SEREX) for further effective screening of overexpressed autoantibodies in breast cancer to increase the specificity and sensitivity of breast cancer detection; (3) to screen and optimize TAAs from this study, further combining multiple TAAs for the detection of autoantibodies in breast cancer; and (4) to expand sample size and perfect cases of molecular subtyping, for example, based on HER2, ER, PR, and Ki-67, to define the expression of autoantibodies in different clinical molecular subtyping samples and improve the specificity of autoantibody detection. We expect to be able to effectively raise panel sensitivity further through the detection of multiple TAAs.
References
Jemal A, Bray F, Center MM et al (2011) Global cancer statistics. CA Cancer J Clin 61(2):69–90
Torre LA, Bray F, Siegel RL et al (2015) Global cancer statistics, 2012. CA Cancer J Clin 65(2):87–108
Goldhirsch A, Wood WC, Coates AS et al (2011) Strategies for subtypes—dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol 22(8):1736–1748
Fuzery AK, Levin J, Chan MM et al (2013) Translation of proteomic biomarkers into FDA approved cancer diagnostics: issues and challenges. Clin Proteomics 10(1):13
Keshaviah A, Dellapasqua S, Rotmensz N et al (2007) CA15-3 and alkaline phosphatase as predictors for breast cancer recurrence: a combined analysis of seven International Breast Cancer Study Group trials. Ann Oncol 18(4):701–708
Kim HS, Park YH, Park MJ et al (2009) Clinical significance of a serum CA15-3 surge and the usefulness of CA15-3 kinetics in monitoring chemotherapy response in patients with metastatic breast cancer. Breast Cancer Res Treat 118(1):89–97
Ali HQ, Mahdi NK, Al-Jowher MH (2013) The value of CA15-3 in diagnosis, prognosis and treatment response in women with breast cancer. J Pak Med Assoc 63(9):1138–1141
Shao Y, Sun X, He Y et al (2015) Elevated levels of serum tumor markers CEA and CA15-3 are prognostic parameters for different molecular subtypes of breast cancer. PLoS One 10(7):e0133830
Chapman C, Murray A, Chakrabarti J et al (2007) Autoantibodies in breast cancer: their use as an aid to early diagnosis. Ann Oncol 18(5):868–873
Tan HT, Low J, Lim SG et al (2009) Serum autoantibodies as biomarkers for early cancer detection. FEBS J 276(23):6880–6904
Zuo X, Chen L, Liu L et al (2016) Identification of a panel of complex autoantigens (LGALS3, PHB2, MUC1, and GK2) in combination with CA15-3 for the diagnosis of early-stage breast cancer. Tumor Biol 37(1):1309–1317
Chapman C, Murray A, Chakrabarti J et al (2007) Autoantibodies in breast cancer: their use as an aid to early diagnosis. Ann Oncol 18(5):868–873
Huangfu M, Xu S, Li S et al (2016) A panel of autoantibodies as potential early diagnostic serum biomarkers in patients with cervical cancer. Tumor Biol 37(7):8709–8714
Xu S, Huangfu M, Jia X et al (2015) FOXP3 autoantibody as a potential early prognostic serum biomarker in patients with cervical cancer. Int J Clin Oncol 20(5):982–988
Zhang C, Ye L, Guan S et al (2014) Autoantibodies against p16 protein-derived peptides may be a potential biomarker for non-small cell lung cancer. Tumour Biol 35(3):2047–2051
Ye L, Guan S, Zhang C et al (2013) Circulating autoantibody to FOXP3 may be a potential biomarker for esophageal squamous cell carcinoma. Tumour Biol 34(3):1873–1877
Guan S, Liu B, Zhang C et al (2013) Circulating autoantibody to CD25 may be a potential biomarker for early diagnosis of esophageal squamous cell carcinoma. Clin Transl Oncol 15(10):825–829
Chen C, Huang Y, Zhang C et al (2015) Circulating antibodies to p16 protein? derived peptides in breast cancer. Mol Clin Oncol 3(3):591–594
Baldwin RW (1955) Immunity to transplanted tumour: the effect of tumour extracts on the growth of homologous tumours in rats. Br J Cancer 9(4):646–651
Graham JB, Graham RM (1955) Antibodies elicited by cancer in patients. Cancer (Phila) 8(2):409–416
Qiu J, Choi G, Li L et al (2008) Occurrence of autoantibodies to annexin I, 14-3-3 theta and LAMR1 in prediagnostic lung cancer sera. J Clin Oncol 26(31):5060–5066
Cunha LL, Morari EC, Nonogaki S et al (2012) Foxp3 expression is associated with aggressiveness in differentiated thyroid carcinomas. Clinics (Sao Paulo) 67(5):483–488
Farrell AS, Sears RC (2014) MYC degradation. Cold Spring Harb Perspect Med 4:a014365
Ye L, Wang W, Chen C et al (2015) Study of circulating IgG antibodies to BIRC5 and MYC in non-small cell lung cancer. FEBS Open Bio 5:809–812
Tornesello ML, Buonaguro L, Buonaguro FM (2013) Mutations of the TP53 gene in adenocarcinoma and squamous cell carcinoma of the cervix: a systematic review. Gynecol Oncol 128(3):442–448
Muller PA, Vousden KH (2014) Mutant p53 in cancer: new functions and therapeutic opportunities. Cancer Cell 25(3):305–317
Miller LD, Smeds J, George J et al (2005) An expression signature for p53 status in human breast cancer predicts mutation status, transcriptional effects, and patient survival. Proc Natl Acad Sci USA 102(38):13550–13555
Xia J, Shi J, Wang P et al (2016) Tumor-associated autoantibodies as diagnostic biomarkers for breast cancer: a systematic review and meta-analysis. Scand J Immunol 83(6):393–408
Zhang Z, Huang L, Zhao W et al (2010) Annexin 1 induced by anti-inflammatory drugs binds to NF-kappaB and inhibits its activation: anticancer effects in vitro and in vivo. Cancer Res 70(6):2379–2388
Shen D, Nooraie F, Elshimali Y et al (2006) Decreased expression of annexin A1 is correlated with breast cancer development and progression as determined by a tissue microarray analysis. Hum Pathol 37(12):1583–1591
Casiano CA, Mediavilla-Varela M, Tan EM (2006) Tumor-associated antigen arrays for the serological diagnosis of cancer. Mol Cell Proteomics 5(10):1745–1759
Desmetz C, Bascoul-Mollevi C, Rochaix P et al (2009) Identification of a new panel of serum autoantibodies associated with the presence of in situ carcinoma of the breast in younger women. Clin Cancer Res 15(14):4733–4741
Acknowledgements
This work was supported by the Norman Bethune Program of Jilin University [2015313].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that they have no conflict of interest.
About this article
Cite this article
Liu, Y., Liao, Y., Xiang, L. et al. A panel of autoantibodies as potential early diagnostic serum biomarkers in patients with breast cancer. Int J Clin Oncol 22, 291–296 (2017). https://doi.org/10.1007/s10147-016-1047-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-016-1047-0