Abstract
The study was designed to test whether circulating autoantibodies against associated antigens (TAAs) were altered in early cervical cancer and benign cervical tumors. A total of 111 cervical cancer patients, 137 cervical benign tumor patients, and 160 healthy volunteers matched in age were recruited in this study. The expression of autoantibodies was tested using in-house developed enzyme-linked immunosorbent assay (ELISA) with linear peptide envelope antigens derived from TAAs. One-way ANOVA test showed that there was no difference in the CD25 autoantibody expression among the cervical cancer group, benign tumor group, and healthy control group (P = 0.063; P = 0.191). The expression of autoantibodies against survivin and TP53 in the cervical cancer group was significantly higher than that in the benign tumor group (P < 0.001; P < 0.001). The levels of autoantibodies against cyclinB-1 and ANXA-1 were higher in the cervical cancer group than in the healthy control group (P = 0.010; P = 0.001), while autoantibodies in the cervical cancer group showed no difference in expression compared with that in the benign tumor group. The panel of five TAAs showed a sensitivity of 37.8 % and a specificity of 90 %, which was much higher than the sensitivity of the single-TAA testing group. The data from this study further support our previous hypothesis that the detection of autoantibodies for the diagnosis of a specific cancer type can be enhanced using a panel of several selected TAAs as target antigens.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cervical cancer is one of the most common female cancers worldwide, and the incidence is second only to breast cancer. In recent years, the incidence of cervical cancer has shown a regional increasing trend and an earlier onset age [1]. The pathological types and clinical stages are closely related to the prognosis of patients with cervical cancer. The early diagnosis of patients with cervical cancer is in favor to receive proper, timely, and effective treatment, control disease progression, and improve the health and quality of life. Hematologic testing is the simplest invasive examination. A sensitive and targeted tumor marker for the early diagnosis of cervical cancer can help clinicians improve the early diagnostic rate.
When a tumor develops, abnormally proliferated tumor cells will be considered by the immune system as exotic abnormal antigens to stimulate immune responses. It has been demonstrated that cancer sera contain antibodies that react with a unique group of autologous cellular antigens, and the above antigens are generally known as tumor-associated antigens (TAAs) [2]. Antigenic changes in cancer cells can be recognized by the patient’s immune system, which responds by producing autoantibodies. There are autoantibodies that react with the unique autologous tissue cells in a patient’s serum. Tumor cells may be killed by autoantibodies against TAAs, achieving the aim of destroying tumors [3]. Based on the antigen–antibody reaction, TAA autoantibodies could be detected at a higher sensitivity. Therefore, compared with TAAs, the detection of TAA autoantibodies is more sensitive than that of tumor antigen detection in the early tumor diagnosis. These autoantibodies can be used to identify aberrant cellular mechanisms in tumorigenesis and also serve as immunodiagnostic markers for early cervical cancer detection.
Currently, a series of TAAs was identified through proteomic technology and serological analysis of a cDNA expression library (SEREX). For example, survivin is a member of the inhibitor of apoptosis (IAP) family, which can inhibit apoptosis, promote cell transformation, and induce tumor cells to be drug resistant [4]. CyclinB-1 is one of the main regulatory factors of the G2/M cell cycle check point. CyclinB-1 can show unscheduled expression in tumor cells, which will promote the progression of the cell cycle, induce tumor cells to proliferate excessively, and lead to cell carcinomatous changes [5, 6]. Annexin I belongs to the annexin protein superfamily that comprises a multigene family of Ca2+ and phospholipid binding proteins. It plays a significant role in the development of tumorigenesis by regulating cell signal transduction [7–9]. c-Myc is a nuclear transcription factor that is directly involved in the regulation of DNA replication, cell proliferation, cell differentiation, and apoptosis. The excessive accumulation of c-myc protein in a malignant tumor is closely associated with tumor progression [10, 11]. The wild-type p53 gene is an important tumor suppressor gene. While mutant p53 becomes a tumor-promoting factor, which activates tumor cell growth and promotes tumor angiogenesis [12]. CD25+ T cells can inhibit the immune system and promote tumor immune escape. Meanwhile, CD25+ T cells are closely associated with HPV infection [13].
To achieve a feasible auxiliary diagnosis, the tumor antigens of autoantibodies are differentially associated with tumor types. Additionally, in previous studies [14–19], we found that the sensitivity of single autoantibody detection was low, lacking the support of a single autoantibody as a tumor marker. Thus, researchers have combined multiple TAAs to detect autoantibodies in studies [20, 21]; this strategy has significantly improved specificity and sensitivity. At present, most studies contain insufficient samples and shortages of benign tumor samples. In our study, we have greatly increased the number of samples. We also compared autoantibody expression between the benign group and cervical cancer group.
Materials and methods
Subjects
A total of 248 female patients first diagnosed with cervical cancer, comprising 137 patients with cervical benign tumors and 111 patients with malignant tumors, were confirmed by radiographic examination and histological confirmation with staging information. All 258 patients had complete clinical data information and were recruited from the second hospital of Jilin University. Blood samples were taken prior to any anticancer treatment. One hundred sixty healthy subjects, matched in age (Table 1), were also recruited as controls from the local communities. The clinical diagnosis means of advanced cervical cancer (including stages III and IV) have been very mature and effective. The application of clinical interviews and imaging examination has been taken to exclude the patients with any other tumors and control subjects with a history of any tumors. Among the patient and control groups, those with a history of severe autoimmune diseases were excluded. All of the research subjects were of Chinese Han origin and provided written informed consent to participate in the study. This work was approved by the Ethics Committee of Jilin University.
Autoantibody testing
A linear peptide antigen was designed according to the computational prediction of human leukocyte antigen class II (HLA-II)-restricted epitopes, which can be recognized by the HLA-II molecules among >90 % of the Chinese population. The autoantibody specific for TAA was measured using a relative enzyme-linked immunosorbent assay (ELISA) approach as described in our recent publication (14–19). A specific binding index (SBI) was used to express the levels of circulating autoantibody. SBI = TAA antigen (OD) − NC (OD)/control antigen (OD) − NC (OD).
Data analysis
The mean ± SD in SBI was used to present data. Microsoft Excel 2010 was used to construct a database with individual SBI values and to graphically analyze the distributions of individual autoantibody levels. IBM SPSS Statistics 19.0 was applied to perform one-way ANOVA test to compare the testing results for each anti-TAA antibody and the panel of multiple TAAs in SBI among the cancer group, the benign group, and healthy control, and to analyze using receiver operating characteristic curve (ROC) analysis for the calculation of the area under the ROC curve (AUC) with the ELISA sensitivity against a specificity of >90 %.
Results
After testing the expression of survivin, cyclinB-1, ANXA-1, c-myc, TP53, and CD25 autoantibodies in the cervical cancer group, benign tumor group, and healthy control group (Table 2), no difference was found in CD25 autoantibody expression among the cervical cancer group, benign tumor group, and healthy control group (P = 0.063; P = 0.191). The expression of autoantibodies against survivin and TP53 in the cervical cancer group was significantly higher than that in the benign tumor group (P < 0.001; P < 0.001). The level of autoantibodies against cyclinB-1 and ANXA-1 was higher in the cervical cancer group than in the healthy control group (P = 0.010; P = 0.001), while autoantibodies in the cervical cancer group showed no difference compared with those in the benign tumor group. The panel of multiple TAAs comprised survivin, cyclinB-1, ANXA-1, c-myc, and TP53, but not CD25. The autoantibody expression of the panel was statistically higher in the cervical cancer group than in the benign group and healthy control group (P < 0.001; P < 0.001; Table 2).
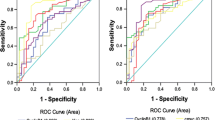
Next, ROC analysis was tested in survivin, cyclinB-1, ANXA-1, c-myc, and TP53 and the panel of five to assess the diagnostic value between the case and healthy control groups. The sensitivities against 90.0 % specificity of TAAs in the benign tumor group and cervical cancer group are listed in Table 3. The sensitivity of the panel autoantibody expression in the cervical cancer group (37.8 %) was significantly higher than that in the benign tumor group (28.5 %) and was also higher than any other single TAA autoantibody (Fig. 1 and Table 3). Subgroup analysis of the panel demonstrated that the sensitivity of the stage I cervical cancer group (44.2 %) was higher than that of the stage II cervical cancer group (32.4 %). However, in the benign tumor group, the panel of five did not show obvious advantages, and the sensitivity was below that of c-myc (34.1 %; Table 3 and Fig. 1).
Discussion
Currently, the early diagnosis of cervical cancer has drawn increasing attentions. Tiny lesions are often misdiagnosed in imaging examination, and traditional tumor markers cannot meet the clinical demand for early diagnosis. Thus, more sensitive autoantibodies against TAAs based on antigen–antibody theory are of great interest. Most methods to detect autoantibodies utilize ELISA with recombinant proteins. In our study, autoantibodies against TAAs were detected by peptide antigen fragments that were designed based on antigen–antibody binding epitopes. The special method has been confirmed to be effective in previous reports [14–19]. Although TAAs have many advantages as tumor markers, they are not sufficiently sensitive for clinical application. Consequently, researchers consider a group of multiple TAAs and use them for the joint detection and diagnosis of different types of tumors. In the study on TAA autoantibodies in esophageal cancer, researchers tested 388 esophageal cancer patients and 125 healthy subjects using a panel of six TAAs (p53, NY-ESO-1, MMP-7, Hsp70, Prx VI, and Bmi-1) and obtained a specificity of 95 % and a sensitivity of 45 % [20]. In the autoantibody study of breast cancer, they found the sensitivity of detecting a single autoantibody against the TAAs of Imp1, p16, Koc, survivin, cyclinB-1, and c-myc to be between 12.8 and approximately 18.4 %, while the sensitivity and specificity of the panel of six TAAs achieved 67.3 and 92.2 %, respectively. However, the study comprised 49 patients with breast cancer and 35 patients with benign breast tumor [22]. The early lung cancer diagnostic kit EARLY-CDT has been successfully marketed in North America and Europe. The kit was made with a panel of six TAAs, including p53, NY-ESO-1, CAGE, GBU4-5, SOX2, HuD, and MAGE A4. It has a specificity of 93 % and a sensitivity of 49 % in 165 patients, values that are almost similar to those of imaging diagnosis [21]. Therefore, we tested six autoantibodies against TAAs in our study. To improve the detection rate of cervical cancer, a panel of multiple TAAs was tested simultaneously.
We tested six TAA autoantibodies—survivin, cyclinB-1, ANXA-1, c-myc, TP53, and CD25—all of which are involved in tumorigenesis. Previous studies have shown that survivin is expressed increasingly in chronic cervical inflammation, cervical intraepithelial neoplasia (CIN), and cervical cancer [23]. It is a prognostic marker for patients with early cervical cancer, in which it is overexpressed [24]. In our study, the expression of survivin autoantibody was significantly higher in the cervical cancer group than in the benign tumor group and healthy control group, findings that are consistent with the above studies. However, the expression of survivin autoantibody was lower in the stage I cervical cancer group than in the stage II cervical cancer group. CyclinB-1 can promote cell cycle progression and mitosis, resulting in carcinogenesis [5, 6]. Hoffmann TK et al. found that cyclinB-1 expression was significantly increased in head and neck cancer [25]. In our study, we found that the expression of the autoantibody against cyclinB-1 in the cervical cancer group was statistically higher than that in the healthy control group but showed no difference compared with that in the benign tumor group. Mussunoor S reported that ANXA expression was significantly higher in patients with invasive tumors [26]. Our results showed that the autoantibody against ANXA expression was significantly higher in the cervical cancer group than in the benign tumor group and healthy control group, and autoantibody expression was higher in the stage I cervical cancer group than in the stage II cervical cancer group, a finding that is also similar to that of our previous study on lung cancer [18]. HPV infection in cervical epithelial tissue has been found to be associated with abnormal expression of c-myc, indicating that c-myc expression was abnormal in HPV-positive cervical cancer. Moreover, it has been found that the expression of c-myc showed an increasing trend from normal cervical tissue, CIN, and cervical cancer, which is accompanied by abnormal expression of Ki-67, p16INK4a, and cyclinB-1 [11]. That the expression of c-myc autoantibody increased with staging further validated the above reports. The autoantibody expression of survivin, cyclinB-1, ANXA-1, c-myc, and TP53 was significantly higher in patients with cervical cancer than in healthy controls. Additionally, the autoantibody expression of survivin, c-myc, and TP53 was also statistically higher in the cervical cancer group than in the benign tumor group, while autoantibodies to cyclinB-1 and ANXA-1 showed no difference between the above groups. Based on the results, the panel of multiple TAAs comprised survivin, cyclinB-1, ANXA-1, c-myc, and TP53. The autoantibody panel was tested in patients with malignant tumors, patients with benign tumors and healthy controls. The results showed that the expression level of the panel of five was significantly higher in patients with cervical cancer than in patients with a benign tumor and healthy controls (P < 0.001; P < 0.001).
It has been reported that CD4+CD25+FOXP3+ T cells could promote tumor progression in nasopharyngeal carcinoma and thyroid cancer [27, 28], and the proportion of CD4+CD25+ T cells was successively increased in patients with CIN and cervical cancer [13]. Based on the above studies, we tested CD3+CD25+ T cells and CD4+CD25+ T cells by flow cytometry in 55 patients with cervical cancer, 48 patients with benign cervical cancer, and 27 healthy controls and found no significant differences among them (Table 4). Meanwhile, test of CD25 autoantibodies also showed no differences among patients with cervical cancer, patients with a benign tumor, and healthy controls. Although inconsistent with previous reports, these results fully confirmed that changes in autoantibody expression of TAAs in serum may reflect changes in TAA expression in tumor tissues. Thus, the CD25 autoantibody was excluded in the panel of multiple TAAs.
The results showed that the expression of the panel TAAs was significantly higher in patients with malignant tumors than in patients with benign tumors and healthy controls. The sensitivity was significantly higher than that of each single autoantibody, reaching 37.8 % and showing the same trend of multiple TAAs in other tumors [20–22]. The mechanism underlying the increased expression of TAAs is unclear and has been suggested to involve (1) the host response to TAA, (2) abnormal immune gene regulation in the process of tumorigenesis, and (3) antigenic stimulation resulting from destruction of tumor cells [29]. It is worth noting that, although the results of single TAAs are different in stage I and II cervical cancer, the level and sensitivity of the panel were significantly higher in stage I cervical cancer than in stage II cervical cancer. The sensitivity was 44.2 % for stage I cancer and 34.2 % for stage II cancer against a 90 % specificity, showing the advantages of TAAs in early cervical cancer detection. Our results support the previous hypothesis that the detection of TAA for the diagnosis of a specific type of cancer can be enhanced using a panel of several carefully selected TAAs as target antigens, and a panel of multiple TAAs would be a useful approach in the detection and diagnosis of cervical cancer.
References
Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108.
Tan EM, Zhang J. Autoantibodies to tumor-associated antigens: reporters from the immune system. Immunol Rev. 2008;222:328–40.
Zaenker P, Ziman MR. Serologic autoantibodies as diagnostic cancer biomarkers—a review. Cancer Epidemiol Biomarkers Prev. 2013;22:2161–81.
Wu SF, Zhang JW, Qian WY, Yang YB, Liu Y, Dong Y, et al. Altered expression of survivin, Fas and FasL contributed to cervical cancer development and metastasis. Eur Rev Med Pharmacol Sci. 2012;16:2044–50.
Barrett KL, Demiranda D, Katula KS. CycinB1 promoter activity and functional in G1 phase of human breast cancer cells. J Cell Biol Int. 2002;26:19–28.
Prystowsky M, Feeney K, Kawachi N, Montagna C, Willmott M, Wasson C, et al. Inhibition of Plk1 and Cyclin B1 expression results in panobinostat-induced G2 delay and mitotic defects. Sci Rep. 2013;3:2640. doi:10.1038/srep02640.
de Graauw M, van Miltenburg MH, Schmidt MK, Pont C, Lalai R, Kartopawiro J, et al. Annexin A1 regulates TGF-beta signaling and promotes metastasis formation of basal-like breast cancer cells. Proc Natl Acad Sci U S A. 2010;107(14):6340–5.
Yu G, Wang J, Chen Y, Wang X, Pan J, Li Q, et al. Tissue microarray analysis reveals strong clinical evidence for a close association between loss of annexin A1 expression and nodal metastasis in gastric cancer. Clin Exp Metastasis. 2008;25:695–702.
Cao Y, Li Y, Edelweiss M, Arun B, Rosen D, Resetkova E, et al. Loss of annexin A1 expression in breast cancer progression. Appl Immunohistochem Mol Morphol. 2008;16:530–4.
Protrka Z, Arsenijevic S, Dimitrijevic A, Mitrovic S, Stankovic V, Milosavljevic M, et al. Co-overexpression of bcl-2 and c-myc in uterine cervix carcinomas and premalignant lesions. Eur J Histochem. 2011;55(1):44–9.
Samir R, Asplund A, Tot T, Pekar G, Hellberg D. High-risk HPV infection and CIN grade correlates to the expression of c-myc, CD4+, FHIT, E-cadherin, Ki-67, and p16INK4a. J Low Genit Tract Dis. 2011;15:280–6.
Tornesello ML, Buonaguro L, Buonaguro FM. Mutations of the TP53 gene in adenocarcinoma and squamous cell carcinoma of the cervix: a systematic review. Gynecol Oncol. 2013;128:442–8.
Chen ZF, Xu Q, Ding JB, Zhang Y, Du R, Ding Y. CD4+CD25+Foxp3+ Treg and TGF-beta play important roles in pathogenesis of Uygur cervical carcinoma. Eur J Gynaecol Oncol. 2012;33:502–7.
Liu L, Liu N, Liu B, Yang Y, Zhang Q, Zhang W, et al. Are circulating autoantibodies to ABCC3 transporter a potential biomarker for lung cancer? J Cancer Res Clin Oncol. 2012;138:1737–42.
Cheng Y, Xu J, Guo J, Jin Y, Wang X, Zhang Q, et al. Circulating autoantibody to ABCC3 may be a potential biomarker for esophageal squamous cell carcinoma. Clin Transl Oncol. 2013;15:398–402.
Zhang C, Ye L, Guan S, Jin S, Wang W, Sun S, et al. Autoantibodies against p16 protein-derived peptides may be a potential biomarker for non-small cell lung cancer. Tumour Biol. 2014;35:2047–51.
Jin Y, Guan S, Liu L, Sun S, Lee KH, Wei J. Anti-p16 autoantibodies may be a useful biomarker for early diagnosis of esophageal cancer. Asia Pac J Clin Oncol. 2014. doi:10.1111/ajco.12198.
Wang W, Guan S, Sun S, Jin Y, Lee KH, Chen Y, et al. Detection of circulating antibodies to linear peptide antigens derived from ANXA1 and DDX53 in lung cancer. Tumor Biol. 2014;35:4901–5.
Ye L, Guan S, Zhang C, Lee KH, Sun S, Wei J, et al. Circulating autoantibody to FOXP3 may be a potential biomarker for esophageal squamous cell carcinoma. Tumour Biol. 2013;34:1873–7.
Xu YW, Peng YH, Chen B, Wu ZY, Wu JY, Shen JH, et al. Autoantibodies as potential biomarkers for the early detection of esophageal squamous cell carcinoma. Am J Gastroenterol. 2014;109:36–45.
Macdonald IK, Murray A, Healey GF, Parsy-Kowalska CB, Allen J, McElveen J, et al. Application of a high throughput method of biomarker discovery to improvement of the EarlyCDT(®)-Lung Test. PLoS One. 2012;7(12):e51002. doi:10.1371/journal.pone.0051002.
Liu W, De La Torre IG, Gutiérrez-Rivera MC, Wang B, Liu Y, Dai L, et al. Detection of autoantibodies to multiple tumor-associated antigens (TAAs) in the immunodiagnosis of breast cancer. Tumour Biol. 2015;36(2):1307–12.
Cao XQ, Lu HS, Zhang L, Chen LL, Gan MF. MEKK3 and survivin expression in cervical cancer: association with clinicopathological factors and prognosis. Asian Pac J Cancer Prev. 2014;15:5271–6.
Sukpan K, Settakorn J, Khunamornpong S, Cheewakriangkrai C, Srisomboon J, Siriaunkgul S. Expression of survivin, CD117, and C-erbB-2 in neuroendocrine carcinoma of the uterine cervix. Int J Gynecol Cancer. 2011;21:911–7.
Hoffmann TK, Trellakis S, Okulicz K, Schuler P, Greve J, Arnolds J, et al. Cyclin B1 expression and p53 status in squamous cell carcinomas of the head and neck. Anticancer Res. 2011;31(10):3151–7.
Mussunoor S, Murray GI. The role of annexins in tumour development and progression. J Pathol. 2008;216:131–40.
Shi J, Zhou J. Role of CD4+ CD25+ regulatory T cells in peripheral blood from patients with papillary thyroid carcinoma. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2012;26(21):965–9. 972.(Article in Chinese).
Gu Y, Wang C, Han D, Zhang L. Increased frequencies of CD4+ CD25+ FOXP3+ regulatory T cells in human nasal inverted papilloma. Head Neck. 2011;33:1005–12.
Caron M, Choquet-Kastylevsky G, Joubert-Caron R. Cancer immunomics using autoantibody signatures for biomarker discovery. Mol Cell Proteomics. 2007;6:1115–22.
Acknowledgments
This study was supported by the Jilin Pharmaceutical Industry Development Special Fund Project (No. 130701YY01066802), and by Glory Biomedical Co. Ltd., Taipei, Taiwan. We would like to acknowledge Dr. Cui Manhua and colleagues for their help and support with serum sample processing.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
None
Rights and permissions
About this article
Cite this article
Huangfu, M., Xu, S., Li, S. et al. A panel of autoantibodies as potential early diagnostic serum biomarkers in patients with cervical cancer. Tumor Biol. 37, 8709–8714 (2016). https://doi.org/10.1007/s13277-015-4472-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-4472-1