Abstract
Overexpression of tumor-associated antigens (TAAs) has been reported in many types of cancer and may trigger secretion of their autoantibodies. The present work was thus designed to test whether circulating antibody to p16 protein-derived antigens was altered in lung cancer. Two hundred seventy-one patients with non-small cell lung cancer (NSCLC) and 226 control subjects matched in age, gender, and smoking history were recruited in this study. The levels of circulating anti-p16 IgA and IgG antibodies were tested using an enzyme-linked immunosorbent assay (ELISA) developed in-house with linear peptide antigens derived from p16 protein. Student’s t test showed that patients with NSCLC had a significant higher level of anti-p16 IgG antibody than control subjects (t = 2.74, P = 0.0063) but did not have a significant increase in IgA antibody levels (t = 1.92, P = 0.056). The sensitivity against >90 % specificity was 19.7 % for the IgG assay with an inter-assay deviation of 11.6 %, and 10.3 % for the IgA assay with an inter-assay deviation of 14.7 %. Based on a cut-off value determined by the 99th percentile of control IgG levels, the anti-p16 IgG positivity was 6.7 % in patients with NSCLC compared to 0.88 % in control subjects (χ 2 = 10.58, P = 0.001, OR = 7.97, 95 % CI 1.84–34.85). Circulating anti-p16 IgG levels were increased with stages of NSCLC, and patients with stage IV NSCLC had the highest IgG level among all four stages (t = 2.42, P = 0.016, compared with the control group). Pearson correlation analysis showed a significant correlation between circulating levels of IgA and IgG in the patient group (r = −0.2, df = 236, P = 0.0021) but not in the control group (r = −0.1, df = 205, P = 0.146). Circulating IgG antibody to p16 protein may be a potential biomarker with prognostic values for lung cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The p16 protein is a cyclin-dependent kinase (CDK) inhibitor that has been found to be involved in the down-regulation of the cell cycle by inactivating the CDK [1–3]. This CDK inhibitor mainly plays a role in suppressing CDK4 and CDK6 activities during the G1 phase of the cell cycle and arresting cells in this phase [2, 4]. A number of studies indicated that the expression of p16 protein was significantly increased in patients with non-small cell lung cancer (NSCLC) [5, 6], but meta-analysis showed that abnormal methylation of the p16 gene was an indicator of poor prognosis of NSCLC [7]. While the relationship between p16 expression and lung cancer remains to be established, humoral immune response to p16 protein may have a potential in serving as a biomarker for diagnosis and prognosis of the malignant disease.
A number of studies demonstrated that circulating autoantibodies to particular tumor-associated antigens (TAAs) were positive in patients with malignant tumors [8–12]. TAAs involved in the specific immune response may vary between tumor types and between individuals with a tumor, but spontaneous tumor-related antibodies are still useful biomarkers for diagnosis and prognosis of malignancy [10–16]. For example, EarlyCDT-Lung was the first autoantibody-based diagnostic tool in lung cancer [14, 15]; it was made with a panel of seven recombinant TAAs, and the panel antibody positivity has achieved up 50 % in patients with lung cancer [15]. A previous study developed an enzyme-linked immunoassay (ELISA) in-house using recombinant p16 proteins as antigens to detect anti-p16 IgG in plasma [17] but failed to show a significant increase in prevalence of the IgG antibody in patients with lung cancer as compared with control subjects although the prevalence of anti-p16 antibodies have been found to be increased in breast cancer, nasopharyngeal cancer, and heptocellular carcinoma [16, 17]. Based on our recent work, application of linear peptides as antigens may be more sensitive for ELISA of circulating autoantibodies against some TAAs [18, 19]. The linear peptide antigens can be completely exposed to antibodies and specifically bound to these antibodies. Accordingly, the present work was undertaken to develop an in-house ELISA with human leukocyte antigen class II (HLA-II) restricted peptide antigens to detect circulating antibodies to p16 protein.
Materials and methods
Subjects
A total of 271 patients who were newly diagnosed as having NSCLC were recruited for this study by the Department of Pulmonary Oncology, Third Affiliated Hospital of Harbin Medical University, Harbin, China. Of these 271 patients aged 57.4 ± 9.2 years, 176 were male and 95 were female. Their diagnosis was made based on radiographic examination and histological confirmation with staging information; inclusion of patients was restricted to those with adenocarcinoma (n = 158) and squamous carcinoma (n = 113) only. Blood samples were taken prior to any anticancer treatment. Two hundred twenty-six healthy subjects, well matched in age (57.1 ± 10.4 years), gender, and smoking history, were also recruited as controls from local communities, of whom 134 were male and 92 were female. Clinical interview and radiographic examination were applied to rule out the control subjects who had history of lung cancer or any other malignant tumors. All the subjects were of Chinese Han origin, and all gave written consent to participation in this study. This work was approved by the Ethics Committee of Harbin Medical University and conformed to the requirements of the Declaration of Helsinki.
Autoantibody testing
A linear peptide antigen was designed according to the computational prediction of HLA-II epitopes [20, 21]. Its sequence used was as follows: H-DVARYLRAAAGGTRRPIQVMMMGSARVAEL-OH, which is corresponding to human p16 protein sequence (accession NP_000068) and carries 11 overlapping HLA-II restricted epitopes that can be recognized by >90 % of the Chinese population; a synthetic peptide, H-VFQKLKDLKDYGGVSLPEWVCIAFHTSG-OH, that is corresponding to goat alpha-lactalbumin protein (accession 1FKV_A) was used as control antigen. Both IgA and IgG autoantibodies specific for p16 protein were measured by a relative ELISA approach as described in our recent publications [18, 19]. Briefly, synthetic peptides were dissolved in 67 % acetic acid to obtain a concentration of 5 mg/ml as stock solution. The antigens were diluted with phosphate-buffered saline (PBS, Product No P4417, Sigma-Aldrich), containing 0.1 % sodium azide just before use, and a working solution was optimized using a series of concentrations ranging from 0 to 25 μg/ml. The optimal working solution was 10 μg/ml for p16-derived antigens and 20 μg/ml for the control antigen. Coaster 96-Well Microtiter EIA Plates (ImmunoChemistry Technologies, Bloomington, MN, USA) were half-coated in 0.1 ml per well of the p16 antigen (1 μg per well) and half-coated in 0.1 ml per well of the control antigen (2 μg per well). The antigen-coated 96-well microplates were covered and incubated overnight at 4 °C. After the antigen-coated plate was washed three times with PBS containing 0.05 % Tween-20 (PBS-T), 100 μl plasma sample diluted 1:200 in Assay Buffer (DS98200, Life Technologies) was added to the sample wells, and 100 μl Assay Buffer was added to the negative control (NC) wells. Following 3-h incubation at room temperature, the plate was washed three times and 100 μl peroxidase-conjugated goat antibody either to human IgG (A8667, Sigma-Aldrich) diluted 1:40,000 in Assay Buffer or to human IgA (A0295, Sigma-Aldrich) diluted 1:30,000 in Assay Buffer was added to each well, respectively. After incubation at room temperature for 2 h, color development was initiated by adding 100 μl Stabilized Chromogen (SB01, Life Technologies) and terminated 25 min later by adding 50 μl Stop Solution (SS03100, Life Technologies). The measurement of optical density (OD) was completed on a microplate reader (BioTeck, USA) within 10 min at 450 nm with a reference wavelength of 620 nm.
Each sample was tested in duplicate. To reduce the interference from a non-specific signal produced by passive absorption of various antibodies in plasma to the surface of 96-well microplate, a specific binding index (SBI) was used to express the levels of circulating antibodies to p16 protein. SBI was calculated as follows:
Data analysis
The mean ± SD in SBI was used to present data. IBM SPSS Statistics 21.0 was used to perform Student’s t test for the difference in SBI between the patient group and the control group, to perform Pearson correlation analysis of the correlation between circulating levels of anti-p16 IgA ang IgG, to perform Pearson’s chi-squared (χ 2) test for the difference in antibody positivity between the patient group and the control group, with calculation of odds ratio (OR) and 95 % confidence interval (CI), and to perform receiver operating characteristic (ROC) analysis in order to work out the area under the ROC curve (AUC) with ELISA sensitivity against a specificity of >90 %. The anti-p16 antibody positivity was determined by a cut-off value above the 99th percentile of antibody levels in control subjects.
To minimize the intra-assay deviation, the ratio of the difference between duplicated OD values to their sum was used to assess the precision for assay of each sample; if the ratio was >10 %, the test of this sample was treated as being invalid and would not be used for data analysis. The inter-assay deviation was estimated using a pooled plasma sample, namely quality control (QC) sample, which was randomly collected from >200 unrelated healthy subjects and tested on every 96-well plate.
Results
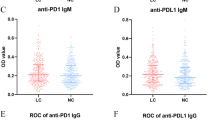
Patients with NSCLC had a significant higher level of IgG antibody to p16 protein than control subjects (t = 2.74, P = 0.0063) but did not have a significant increase in IgA antibody levels (t = 1.92, P = 0.056). As shown in Table 1, adenocarcinoma appeared to mainly contribute to the increased levels of anti-p16 IgG (t = 2.65, P = 0.0084). As shown in Fig. 1, Pearson correlation analysis showed a significant correlation between circulating levels of IgA and IgG in the patient group (r = −0.2, df = 236, P = 0.0021) but not in the control group (r = −0.1, df = 205, P = 0.146).
ROC analysis gave an AUC of 0.57 (SE = 0.027, 95%CI 0.51–0.62) in the IgG assay and an AUC of 0.46 (SE = 0.026, 95 % CI 0.41–0.51) in the IgA assay; the in-house ELISA antibody test had a sensitivity of 19.7 % with 90.4 % specificity for the IgG assay and a sensitivity of 10.3 % with 90.3 % specificity for the IgA assay. Analysis of QC samples gave an inter-assay deviation of 11.6 % for the IgG assay and 14.7 % for the IgA assay (Table 2).
Circulating anti-p16 IgG levels were increased with stages of NSCLC (Table 3), in which patients with stage IV NSCLC had the highest IgG level among all four stages (t = 2.42, P = 0.016, compared to the control group) and those at stage III also had an increase in IgG levels as compared with control subjects (t = 2.24, P = 0.026).
Based on the cut-off values (SBI = 1.53) for circulating anti-p16 IgG positivity, about 6.7 % of patients with NSCLC were positive for the IgG antibody as compared to 0.88 % of control subjects (χ 2 = 10.58, P = 0.001, OR = 7.97, 95%CI 1.84–34.85).
Discussion
The p16 protein plays a crucial role in negatively regulating cell proliferation [1]. Overexpression of p16 protein has been reported in many types of cancer although the normal tissues show a low or undetectable level [17, 22]. Possibly, this is a feedback reaction to the development of malignancy in the body. In this study, however, we identified a significant increase in anti-p16 IgG levels in patients with NSCLC as compared to control subjects (Table 1), especially those with stages III and IV of NSCLC (Table 3). This finding raises a possibility that the anti-p16 IgG antibody may have a prognostic value and could be applied as a potential biomarker for lung cancer. A question is what mechanism may underlie the increased anti-p16 IgG levels in lung cancer? It has been widely recognized that the development of cancer is a multi-step process that may undergo abnormal regulation of cell growth, differentiation, and apoptosis. The immune system is also involved in monitoring potential cancer cells through its function of immune surveillance. It is possible that overexpression of such a TAA may stimulate the immune system to secret antibodies against itself [23]. In addition, somatic p16 mutation in primary tumors may be another possible reason for the autoimmune response to p16 protein due to increased immunogenicity [24].
While circulating levels of the anti-p16 IgG antibody were significantly increased in NSCLC, its low positivity in the patient group suggests that such an antibody alone is unlikely to serve as a biomarker for diagnosis and prognosis of the malignant disease. It is therefore important to identify a panel of TAA-derived linear peptide antigens for the development of diagnostic testing with a high sensitivity. Just like the EarlyCDT-Lung test, the panel antibody positivity is more powerful for diagnosis than individual antibody positivity [14, 15]. Moreover, the cost for synthetic peptides is much lower than that for recombinant TAAs. So, the peptide-based ELISA antibody test may be more suitable for population screening.
References
Hall M, Bates S, Peters G. Evidence for different modes of action of cyclin-dependent kinase inhibitors: p15 and p16 bind to kinases, p21 and p27 bind to cyclins. Oncogene. 1995;11:1581–8.
Baldi A, De Luca A, Esposito V, Campioni M, Spugnini EP, Citro G. Tumor suppressors and cell-cycle proteins in lung cancer. Pathology Res Int. 2011. doi:10.4061/2011/605042.
Johnson JL, Pillai S, Chellappan SP. Genetic and biochemical alterations in non-small cell lung cancer. Biochem Res Int. 2012. doi:10.1155/2012/940405.
Matsushime H, Ewen ME, Strom DK, Kato JY, Hanks SK, Roussel MF, et al. Identification and properties of an atypical catalytic subunit (p34PSK-J3/cdk4) for mammalian D type G1 cyclins. Cell. 1992;71:323–45.
Tong J, Sun X, Cheng H, Zhao D, Ma J, Zhen Q, et al. Expression of p16 in non-small cell lung cancer and its prognostic significance: a meta-analysis of published literatures. Lung Cancer. 2011;74:155–63.
Zhao W, Huang CC, Otterson GA, Leon ME, Tang Y, Shilo K, et al. Altered p16(INK4) and RB1 expressions are associated with poor prognosis in patients with nonsmall cell lung cancer. J Oncol. 2012. doi:10.1155/2012/957437.
Lou-Qian Z, Rong Y, Ming L, Xin Y, Feng J, Lin X. The prognostic value of epigenetic silencing of p16 gene in NSCLC patients: a systematic review and meta-analysis. PLoS One. 2013. doi:10.1371/journal.pone.00549708.
Zhong L, Coe SP, Stromberg AJ, Khattar NH, Jett JR, Hirschowitz EA. Profiling tumor-associated antibodies for early detection of non-small cell lung cancer. J Thorac Oncol. 2005;1:513–9.
Reuschenbach M, von Knebel DM, Wentzensen N. A systematic review of humoral immune responses against tumor antigens. Cancer Immunol Immunother. 2009;58:1535–44.
Kobold S, Lütkens T, Cao Y, Bokemeyer C, Atanackovic D. Autoantibodies against tumor-related antigens: incidence and biologic significance. Hum Immunol. 2010;71:643–51.
Järås K, Anderson K. Autoantibodies in cancer: prognostic biomarkers and immune activation. Expert Rev Proteomics. 2011;8:577–89.
Liu W, Peng B, Lu YXW, Qian W, Zhang J-Y. Autoantibodies to tumor-associated antigens as biomarkers in cancer immunodiagnosis. Autoimmun Rev. 2011;10:331–5.
Chapman C, Murray A, Chakrabarti J, Thorpe A, Woolston C, Sahin U, et al. Autoantibodies in breast cancer: their use as an aid to early diagnosis. Ann Oncol. 2007;18:868–73.
Lam S, Boyle P, Healey GF, Maddison P, Peek L, Murray A, et al. EarlyCDT-Lung: an immunobiomarker test as an aid to early detection of lung cancer. Cancer Prev Res (Phila). 2011;4:1126–34.
Chapman CJ, Healey GF, Murray A, Boyle P, Robertson C, Peek LJ, et al. EarlyCDT®-Lung test: improved clinical utility through additional autoantibody assays. Tumour Biol. 2012;33:1319–26.
Ye H, Sun C, Ren P, Dai L, Peng B, Wang K, et al. Mini-array of multiple tumor-associated antigens (TAAs) in the immunodiagnosis of breast cancer. Oncol Lett. 2013;5:663–8.
Looi K, Megliorino R, Shi FD, Peng XX, Chen Y, Zhang JY. Humoral immune response to p16, a cyclin-dependent kinase inhibitor in human malignancies. Oncol Rep. 2006;16:1105–10.
Liu L, Liu N, Liu B, Yang Y, Zhang Q, Zhang W, et al. Are circulating autoantibodies to ABCC3 transporter a potential biomarker for lung cancer? J Cancer Res Clin Oncol. 2012;138:1737–42.
Ye L, Guan S, Zhang C, Lee K-H, Sun L, Wei J, et al. Circulating autoantibody to FOXP3 may be a potential biomarker for esophageal squamous cell carcinoma. Tumour Biol. 2013;34:1873–7.
Söllner J, Heinzel A, Summer G, Fechete R, Stipkovits L, Szathmary S, et al. Concept and application of a computational vaccinology workflow. Immunome Res. 2010;6 suppl 2:S7.
Wang P, Sidney J, Kim Y, Sette A, Lund O, Nielsen M, et al. Peptide binding predictions for HLA DR, DP and DQ molecules. BMC Bioinformatics. 2010. doi:10.1186/1471-2105-11-568.
Lee CT, Capodieci P, Osman I, Fazzari M, Ferrara J, Scher HI, et al. Overexpression of the cyclin-dependent kinase inhibitor p16 is associated with tumor recurrence in human prostate cancer. Clin Cancer Res. 1999;5:977–83.
Chen YT, Scanlan MJ, Sahin U, Türeci O, Gure AO, Tsang S, et al. A testicular antigen aberrantly expressed in human cancers detected by autologous antibody screening. Proc Natl Acad Sci USA. 1997;94:1914–8.
Kanda M, Matthaei H, Wu J, Hong SM, Yu J, Borges M, et al. Presence of somatic mutations in most early-stage pancreatic intraepithelial neoplasia. Gastroenterology. 2012;142:730–3.
Acknowledgments
We thank patients and healthy volunteers for their support and participation. This work was supported by the Natural Science Foundation of Heilongjiang Province, Harbin, China (grant number D201238), by YingJi Biotechnology & Exploitation Ltd, Shenzhen, China, and by Glory Biomedical Co. Ltd, Taipei, Taiwan.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Cong Zhang and Leiguang Ye contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhang, C., Ye, L., Guan, S. et al. Autoantibodies against p16 protein-derived peptides may be a potential biomarker for non-small cell lung cancer. Tumor Biol. 35, 2047–2051 (2014). https://doi.org/10.1007/s13277-013-1271-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-013-1271-4