Abstract
High-grade meningiomas in ventricles are rare, where most published series only include a few patients. A retrospective analysis was performed on the clinical features, radiological findings, and treatment outcomes of 26 patients with high-grade meningiomas in lateral ventricles who were surgically treated in our hospital between July 2008 and July 2016. A female predilection (female/male = 1.4:1) was observed with a mean age of 42.4 years. Headache and/or vomiting (65.3%) were the most common initial symptom, and with symptom duration time ranging between 7 days and 5 years (mean 8.5 months). The lateral ventricle trigone area was the most common site (80.7%). Twenty-two patients (84.6%) obtained gross total resection. The 2007 WHO classification was used to classify 22 (84.6%) meningiomas as grade II and the remaining four tumors were graded III. These tumors accounted for a recurrence rate of 38.5% (10 of 26 patients) and a mortality rate of 11.5% (3 deaths) during the follow-up periods. The recurrence rate after the gross total resection was 27.3% (6 of 22 patients). Radiotherapy was administered as an adjuvant treatment in 12 patients (46.2%) after surgery. There were 4 recurrences out of the 12 patients who received radiotherapy and 6 of the 14 patients relapsed without radiotherapy (p = 0.58). The subtotal resection was considered a risk factor for recurrence. The postoperative radiotherapy seemed to have little significance for the high-grade meningiomas in the lateral ventricles. Long-term follow-up is required, regardless of the resection grade, and reoperation is feasible for patients with recurrence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Meningiomas account for 13–40% of intracranial tumors with an incidence of 1.5 to 5.5 per 100,000, making them the second most common intracranial tumors in adults [1,2,3]. A majority of meningiomas are benign, with non-benign meningiomas comprising about 5% of the total [4, 5]. Intraventricular meningiomas (IVMs) originate from the choroid plexus and the velum interpositum, which grow strictly within the ventricles without dural attachment. They show clinical characteristics that distinguish them from meningiomas attached to the dura mater, including low prevalence, as they occupy 0.5–3% of all intracranial meningiomas and 9.8–14% of all intraventricular tumors [6, 7]. They also present a neurosurgical challenge because of their localization within the ventricular system, where a complete resection without complications like visual impairment is challenging, due to the proximity to the optic radiations [8,9,10]. Moreover, 88% of IVMs are located in the lateral ventricles, with the remaining 12% located in the third and fourth ventricles. WHO II and III meningiomas in the ventricular system account for 7.4% and 2.8% of all IVMs [11].
There are only a small number of high-grade lateral ventricular meningiomas (LVMs) reported. A majority of them are sporadic cases within a series of IVMs, and the remaining are case reports. The clinical characteristics, radiological findings, and treatment outcomes of the high-grade LVMs remain unknown. In this study, we retrospectively analyzed the medical records of 26 patients with high-grade LVMs as well as their long-term follow-up data in our hospital to investigate the clinical characteristics, radiological findings, and their long-term treatment outcomes.
Material and methods
Data collection
All 120 patients with histologically diagnosed LVMs underwent surgery in our hospital between July 2008 and July 2016. The preoperative radiological images and the histological results showed that 26 patients had WHO grade II or III LVMs. The clinical data were extracted from the medical records, including age, gender, initial symptom, symptom duration time, tumor location and size, Simpson resection grade, and histological characteristics. The data of preoperative magnetic resonance imaging (MRI) and the computed tomography (CT) scan were examined via PACS.
Age was recorded according to at the time of the first operation. The size of the lesions was recorded by measuring the maximum diameter of the tumors on the preoperative CT or MRI. The symptom duration time was calculated as the time period from onset to admission. The Simpson resection grade classification was used to evaluate the extent of resection according to the surgical reports and the postoperative MRI. The gross total resection (GTR) included the Simpson resection grades I and II, and the subtotal resection (STR) included the Simpson resection grades III and IV.
In all cases, the tumor specimens were formalin-fixed, paraffin-embedded, and cut into sections that were 4-μm thick. Hematoxylin-eosin and immunohistochemical staining were then conducted using the following markers in the differential diagnosis: epithelial membrane antigen (EMA), progesterone receptor (PR), S-100, glial fibrillary acidic protein (GFAP), and MIB-1(Ki-67), among others. The cases were analyzed by an experienced independent neuropathologist and diagnosed using the 2007 WHO grading system. The high-grade meningiomas included the WHO grade II and III meningiomas.
The Karnofsky performance score (KPS) was assessed prior to surgery and at discharge, according to the medical records. Follow-up data was collected through regular outpatient review and telephone interviews. The recurrence-free survival (RFS) was calculated from the surgical date to the date of first recurrence or progression based on either postoperative radiological image assessments or clinical symptoms developments, whichever occurred first.
Statistical analysis
Continuous variables were presented as the mean ± standard deviation or the median and ranges. The Student’s t test or the Mann–Whitney U test was used to test continuously evaluated parameters, and the Fisher’s exact test was used to analyze categorical data. The Kaplan–Meier curves and the log-rank test were used to assess the factors that influenced the tumor recurrence. A GraphPad Prism 6 (GraphPad Software, La Jolla, CA) was used for statistical analysis, and p values < 0.05 were considered statistically significant.
Results
Clinical features
Out of the 120 patients with LVMs, 26 (21.7%) were diagnosed with high-grade LVMs. Their clinical data is summarized in Table 1. This cohort included 15 females and 11 males (female/male = 1.4) with an age range from 15 to 71 years (mean 42.4 ± 15.1 years). The symptom duration time ranged from 7 to 5 years (mean 8.5 months). The size of the tumors ranged from 2.6–10.6 cm (mean 5.3 ± 1.6 cm). Headache and/or vomiting were the most common initial symptoms in 17 (65.3%) patients, followed by visual impairment in 2 (7.7%) patients, memory decay in 2 (7.7%) patients, limb weakness or numbness in 2 (7.7%) patients, and accidental discovery in 2 (7.7%) patients. One patient (3.9%) suffered from dizziness. The left and right lateral ventricles accounted for 38.5% (n = 10) and 61.5% (n = 16) of all LVMs in terms of tumor location. There were 20 in the trigone region, 4 in the occipital horn, 1 in the temporal horn, and 1 in the foramen of Monro within the lateral ventricle tumors. The patients were all diagnosed with a single lesion. One patient presented lesions that partially grew into the third ventricle from the unilateral ventricle. No patient was diagnosed with neurofibromatosis type 2. Twenty-two of the patients (84.6%) had a preoperative KPS ≥ 70. Comparisons between the clinical features of the high-grade LVMs and the WHO grade I LVMs were analyzed, as shown in Table 2.
Radiological findings
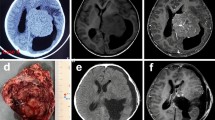
Seventeen patients had preoperative MRI examinations, and 11 patients had preoperative CT scan in our PACS database. All patients had postoperative CT scans and MRI examinations. The radiological findings are summarized in Table 3. Either iso- (n = 13) or hypo-intense (n = 4) on the T1-weighted images and either iso- (n = 8) or hyper-intense (n = 8) on the T2-weighted images were presented in most of the LVMs. MRI contrast enhanced presented in all of the tumors, including 7 cases that were homogenous and 10 cases that were heterogeneous. There were 10 masses with an irregular lobulation, and the remaining 7 masses had a regular shape (Fig. 1). The foci of calcification were found in four cases (4 of 11, 36.4%). The tumor size ranged from 2.6 to 10.6 cm, with 12 tumors (42.6%) < 5.0 cm, and 14 tumors (53.8%) ≥ 5.0 cm in diameter. The local dilatation of the temporal and the occipital horns of the ipsilateral lateral ventricles was presented on the MRI in 14 patients (14 of 17, 82.4%). Varying degrees of peritumoral edema was seen on the MRI in 13 patients (13 of 17, 76.5%). The solid and cystic appearance on the MRI was observed in 4 of our patients. We attempted to use magnetic resonance spectroscopy (MRS) to diagnose the high-grade LVMs. The MRS results showed a high choline peak, a significantly reduced N-acetyl-aspartate peak, and a suspicious lipid peak. There was a characteristic upside-down alanine peak in the meningioma (Fig. 1).
MRI and MRS characteristics of the high-grade lateral ventricular meningiomas from 9 patients. The axial T1-enhanced images show homogeneous or heterogeneous enhancement with the spherical, regular, and irregular lobulated shapes. The locations were distributed in the trigone area, the occipital horn and the temporal horn. The bottom row (case 12) shows that the axial, coronal, and sagittal T1-enhanced images have heterogeneous enhancement and adjacent ependyma and brain tissue involvement. The MRS shows a high choline peak and upside-down alanine peaks
Pathological features
The resected tumors were subjected to immunohistochemistry examinations (see Table 4). According to the 2007 WHO classification, 22 tumors were diagnosed grade II (21 atypical meningiomas and 1 chordoid meningioma) and the remaining 4 tumors were diagnosed as grade III (anaplastic meningioma). The immunohistochemical examinations revealed that the tumor cells were positive or locally positive for EMA (26 of 26 cases), PR (10 of 26), and vimentin (4 of 26), as well as partially positive for cytokeratin (CK, 7 of 26) and S-100 (6 of 26). They were negative for GFAP (21 of 26) and CD34 (14 of 26). The MIB-1 (Ki-67) proliferation index ranged from 3 to 25% (mean 11.5 ± 5.7%) and was significantly higher than the WHO grade I counterparts (p < 0.01).
Treatment and outcomes
Out of the 26 LVMs, 15 were resected via a parietooccipital transcortical approach, 9 via a temporoparietal approach, and 1 via a middle temporal gyrus approach. There was 1 meningioma located in the foramen of Monro that partially grew into the third ventricle, which was resected via the transcallosal approach. GTR was achieved in 22 (84.6%) cases and STR was performed in 4 cases. The cause of STR was two tumors exhibiting invasive growths, breaking through the ependymal membrane and the boundary with the surrounding brain tissue becoming obscured. Tumor residue was to avoid serious complications caused by damage to white matter around the ventricles. The remaining 2 tumors invaded the septum pellucidum to cause serious adhesions that resulted in the tumor not being completely resected. An intraventricular drain device was left in all patients for 3–6 days after the lesions were removed. Postoperative hydrocephalus occurred in 5 patients (19.2%) and 4 patients (15.3%) required surgical intervention. Out of the 4 patients requiring surgical intervention for postoperative hydrocephalus, 1 patient received ventriculoperitoneal shunt. The remaining 3 patients underwent reoperation to dredge the cerebrospinal fluid circulation pathway assisted by endoscopy. Hydrocephalus resolved after the surgery and did not occur during the follow-up. Twenty-one of the patients (80.8%) had KPS ≥ 70 at discharge.
For patients that had STR and an obscure border between the tumor and the surrounding brain tissue, radiotherapy was recommended as an adjuvant treatment after initial surgery. Twelve patients received radiotherapy after initial surgery. The time of adjuvant radiotherapy began about 2–8 weeks after surgery (mean, 5 weeks). Nine patients received postoperative fractionated stereotactic radiotherapy after their initial GTR using an average dose of 52.8 Gy (range, 48.4–60.4 Gy). Three patients received postoperative single-fraction gamma knife radiotherapy after their initial STR using an average marginal dose of 15 Gy. The follow-up period ranged from 2 to 97 months with a mean duration of 44 months. During the follow-up period, tumors recurred in 10 of the cases (Table 1 and Fig. 2a). The recurrence rate with GTR was 27.3% (6 patients), and the recurrence rate with STR was 100% (4 patients) (p < 0.01, Fig. 2b). However, there were 4 recurrences (3 STR and 1 GTR) in 12 patients who received radiotherapy and 6 (1 STR and 5 GTR) of the 14 patients relapsed without radiotherapy (p = 0.58, Fig. 2c). The age (p = 0.67), gender (p = 0.73), and WHO grade (p = 0.08, Fig. 2d) did not have a significant effect on recurrence.
Kaplan–Meier analysis in the 26 LVM patients. a Kaplan–Meier analysis shows the percentage of LVMs with recurrence versus time after resection in months. b A comparison between the RFS based on the extent of resection. c A comparison between the RFS based on radiotherapy or not. d A comparison between the RFS based on WHO grade. (RT: radiotherapy)
Three patients died during the follow-up period. One patient (case 13) required surgical evacuation of a postoperative hematoma and died of pneumonia 2 months after surgery. Case 3 underwent two relapses 12 months and 30 months after the first surgical procedure. This patient developed multiple intraspinal metastases during the second recurrence and died of pneumonia 1 month after the third surgical procedure. Case 10 experienced recurrence 7 months after the initial operation and underwent a second surgery with a tumor malignancy increasing from grade II to grade III. This patient died of multiple intracranial metastasis 28 months after the first surgical procedure (Fig. 3).
Discussion
Incidence and clinical characteristics
The appearance of meningiomas in the ventricles was explained by the inclusion of arachnoid cells in the choroid plexus and the velum interpositum [6]. They commonly occurred in the trigone area of the lateral ventricles and rarely occurred in the third or fourth ventricles [7, 12, 13]. The IVMs are infrequent, so the number of published series on the subject remains low and most series refer only to a few patients. Up until March 2018, Pereira identified 602 LVMs in the literature on Medline [11]. However, only 51 patients (8.5%) presented with high-grade LVMs (34 grade II and 17 grade III). In our study, 26 high-grade LVMs, accounting for 21.7% of all LVMs (120 cases), were described to provide additional insight into these tumors.
Meningiomas that arose within the ventricular system were reported to have an incidence of 0.5–3% of all intracranial meningiomas [6, 7]. Female predilection is previously reported, ranging from 41 to 82%, with an average female-to-male ratio of 2:1 [5, 9, 13]. The LVMs are more common on the left lateral ventricle than on the right lateral ventricle [8, 13]. In our study, female predilection was observed in the WHO I LVMs (female/male = 3.3). This was not prominent in the high-grade LVMs (female/male = 1.4). The age of all patients at diagnosis ranged from 15 to 71 years (mean 42.4 ± 15.1 years). This was similar to the previous literature regarding IVMs [11, 14, 15]. For the high-grade LVMs in our study, it seems that the left lateral ventricle (n = 10) had fewer occurrences than the right lateral ventricle (n = 16).
The LVMs did not present with specific clinical presentations. The tumors often reached an extensive size prior to becoming symptomatic, unless located in a way where the cerebrospinal fluid circulation was blocked at an early stage [3, 6]. Gassel stated that the lateral ventricles are the most silent sites of the meningiomas and those IVMs are often among the largest intracranial tumors [16]. The symptom duration time of an intraventricular tumor lasts from a few days to 20 years with variable clinical manifestation. The clinical presentation correlated with the tumor localization within the lateral ventricles, the size of the tumor, and its growth direction. The most frequently reported symptoms described in the literature are headaches and/or vomiting, as well as memory decay, sensorimotor deficits, visual impairment, and seizures [7, 12, 17]. Most of these clinical symptoms are not only related to increased intracranial pressure, but also to compression of the surrounding brain structures. In our study, the LVMs reached larger sizes (mean 5.3 ± 1.6 cm) than the WHO grade I (mean 4.3 ± 1.5 cm, p < 0.01). According to the WHO edition (2007), high-grade meningioma exhibits high proliferative activity or general invasive growth. Grade I meningiomas are benign and its growth pattern is generally expansive with low proliferative activity and growth rates. The lateral ventricles can provide plenty of space for growth, so the size of high-grade LVMs is larger than the grade I meningioma due to its high growth rate. The symptom duration time varied greatly from 7 days to 5 years (mean 8.5 months). The most common clinical symptom was related to increased intracranial pressure followed by memory decay, visual impairment, and numbness and weakness in the limbs.
Radiological and pathological characteristics
Depending on the advanced radiographic techniques, a high percentage of IVMs can be diagnosed from an imaging perspective [18,19,20]. Irregular lobulation and heterogenous enhancement on MRI are considered to be radiological characteristics for most of the atypical or malignant meningiomas [12, 21]. Our study found that smaller tumors tend to be spherical or regular lobulated and exhibit homologous enhancement, while larger tumors tend to exhibit irregular lobulation and heterogeneous enhancement. Irregular lobulated shapes are more likely to be accompanied by heterogeneous enhancement in the gadolinium-enhanced T1-weighted images (Fig. 1). This could be because the LVMs had relatively more free space to expand. When the tumor size was large, the growth space was limited, and the growth rate in all directions was inconsistent, eventually leading to heterogeneous enhancement and irregular lobulation. The solid and cystic appearance on the MRI was observed in 4 of our patients. This could be related to the rapid growth of the high-grade tumors and the intratumoral necrosis. Local dilatation in the lateral ventricle of the affected side was presented in 14 patients (82.4%). This was because the cerebrospinal fluid pathway of the temporal horn and the occipital horn were blocked by the tumor in the ipsilateral ventricular triangle area.
The definitive diagnosis required pathological support. These 26 pathological diagnoses included 21 atypical meningiomas, 1 chordoid meningioma, and 4 anaplastic meningiomas. Although the immunohistochemical features were not identical (Table 4), these tumors were no different histologically from tumors that were dural in origin. Of the 10 PR-positive patients, 5 of them experienced tumor recurrence. It remains unknown if there was a relationship between PR and meningioma recurrence [22, 23]. The MIB-1 (Ki-67) proliferation index (mean 11.5 ± 5.7%) was significantly higher than the WHO grade I counterparts (2.4 ± 2.1%, p < 0.01). In the WHO edition (2007), grade I meningiomas (benign) are recognized by their histologic subtype and lack of anaplastic features. Grade II meningiomas (atypical) are defined by one or more of the following criteria: increased mitotic activity with four mitoses to 19 per ten high-power fields (HPFs), brain infiltration, and at least three of the following characteristics: sheet-like growth, spontaneous necrosis, increased cellularity, prominent nucleoli, or small cells [24]. Grade III meningiomas (anaplastic) is a histological picture of frank malignancy, or 20 or more mitosis per ten HPFs. In grade II and III meningioma, there are significantly more mitotic tumor cells than in a grade I meningioma. Therefore, the proliferation index MIB-1(Ki-67) of the high-grade meningioma was statistically significant compared with WHO grade I meningioma.
Surgical treatment and prognosis
GTR is the standard treatment for meningiomas. The main influencing factors for GTR are the location, the size, and the vascularity of the tumors. A previous study reported that IVMs are completely resected in 94.7% (541 of 571) patients [11]. There are many different surgical approaches for these tumors, primarily including parietooccipital, posterior middle temporal gyrus, temporoparietal, transcallosal, and transfrontal [3, 8, 9, 13, 15]. The purpose of these surgical approaches is to minimize surgical damage and postoperative complications, by designing the shortest route, avoiding the functional area, early blocking of the blood supply artery, and piecemeal resection. In our series, there were 9 resections via a temporoparietal approach and 1 via a middle temporal gyrus approach. There was 1 meningioma located in the foramen of Monro that partially grew into the third ventricle, which was resected via a transcallosal approach. The remaining 15 tumors were resected via the parietooccipital transcortical approach. We observed a relatively low GTR rate (84.6%). The cause of STR included the large size of high-grade LVMs (mean 5.3 ± 1.6 cm, p < 0.01), the unclear border with the surrounding ependymal membrane and brain tissue, and the irregular growth patterns into deep structures (Fig. 1).
Postoperative bleeds of the surgical bed and hematomas are important factors in the occurrence of postoperative hydrocephalus [3, 6, 13, 15]. In our study, an intraventricular drain device was left in the LVM patients for 3–6 days and 4 patients (15.3%) required reoperation intervention for hydrocephalus drainage. As previous intraventricular neoplasm reported stated, a postoperative intraventricular drain was effective in avoiding postoperative hydrocephalus by draining the blood cerebrospinal fluid in the ventricular system [13]. The effectiveness of post-surgery adjuvant radiotherapy as the standard treatment for the high-grade meningiomas remains controversial [24,25,26]. For high-grade meningioma in the ventricular system, only several case reports and some sporadic cases within a series of IVMs are reported in the literature. Radiotherapy as an adjuvant therapy for high-grade LVM is reported in some patients, but the long-term effects remain unknown [6, 12, 27,28,29]. In the present study, radiotherapy was recommended as an adjuvant treatment for high-grade LVMs after initial surgery in patients with STR and an obscure border between the tumor and the surrounding brain tissue. We were unable to demonstrate a significant improvement in the tumor recurrence after post-surgical radiotherapy for the high-grade LVMs (Fig. 2c).
The latest literature review states that 494 cases presented information regarding the follow-up, and 26 (5.3%) tumors recurred with the recurrence period ranging from 3 to 84 months (mean 26 months) [11]. The majority of recurrent IVMs occurred at the initial tumor site, but some cases transfer to other parts of the brain and the spinal canal through the cerebrospinal fluid circulation [27, 30, 31]. There are reports that state the tumors are transferred to the extracranial areas, such as to the liver [32]. The outcome of patients with malignant IVM with metastases is poor. The survival time is typically within 1 year from confirmation of metastasis to death with or without radiotherapy [27,28,29]. In our study, the recurrence rate was as high as 38.5% (10 of 26 cases). This was mainly related to the WHO grade and the extent of resection of the tumors. The recurrence period ranged from 4 to 34 months, with an average of 15.4 months. The recurrence of the high-grade LVMs was concentrated within 2 years (Fig. 2a). The intraspinal metastases (case 3) and the multiple intracranial metastases (case 10) were observed in our series.
There was recurrence of 4 patients with STR, and 6 of 22 patients with GTR also experienced recurrence (27.3%). The resection degree remarkably affected the prognosis of the high-grade LVMs (Fig. 2b). The risk of recurrence existed even when the GTR was performed. We recommended reoperation for cases with relapse, because the tumor is amenable to this procedure and the effect of radiotherapy is unknown. Previous literature reports state that IVMs mortality is about 4.0% (25 of 625 cases) [11]. In our study, three patients (11.5%) died during the follow-up period. The deaths were divided into two types. The first type died from clinical deterioration after surgery (case 13). The other type died from the progression of the disease itself (cases 3 and 10).
High-grade LVMs are large by the time when they are detected. They present as increased intracranial pressure with no localizing features, so the diagnosis is based on both imaging findings and pathological features. The GTR is the standard treatment for LVMs, although patients with GTR are still likely to relapse. Postoperative radiotherapy seems to have little significance for the high-grade LVMs, and the regular head and the spinal MRI follow-ups are required regardless of the resection status. Reoperation is feasible for patients with recurrence.
Limitations
This study has several limitations. First, our research is a retrospective study, and the inherent bias is inevitable. Second, the number of WHO grade III is only 4 patients in our study, and there may be bias in statistical analysis. Third, more patients are needed to better understand the clinical and radiological features of this disease. Fourth, there could be a bias in the analysis of postoperative radiotherapy effects since some postoperative patients refused radiotherapy. Finally, longer clinical and imaging follow-up periods are necessary for high-grade LVMs to obtain a clearer prognosis.
References
Ostrom QT, Gittleman H, Liao P, Vecchione-Koval T, Wolinsky Y, Kruchko C, Barnholtz-Sloan JS (2017) CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2010-2014. Neuro-Oncology 19:v1–v88. https://doi.org/10.1093/neuonc/nox158
Chen WC, Magill ST, Englot DJ, Baal JD, Wagle S, Rick JW, McDermott MW (2017) Factors associated with pre- and postoperative seizures in 1033 patients undergoing supratentorial meningioma resection. Neurosurgery 81:297–306. https://doi.org/10.1093/neuros/nyx001
Odegaard KM, Helseth E, Meling TR (2013) Intraventricular meningiomas: a consecutive series of 22 patients and literature review. Neurosurg Rev 36:57–64; discussion 64. https://doi.org/10.1007/s10143-012-0410-5
Kshettry VR, Ostrom QT, Kruchko C, Al-Mefty O, Barnett GH, Barnholtz-Sloan JS (2015) Descriptive epidemiology of World Health Organization grades II and III intracranial meningiomas in the United States. Neuro-Oncology 17:1166–1173. https://doi.org/10.1093/neuonc/nov069
Wiemels J, Wrensch M, Claus EB (2010) Epidemiology and etiology of meningioma. J Neuro-Oncol 99:307–314. https://doi.org/10.1007/s11060-010-0386-3
Li Z, Li H, Jiao Y, Ma J, Wang S, Cao Y, Zhao J (2018) Clinical features and long-term outcomes of pediatric intraventricular meningiomas: data from a single neurosurgical center. Neurosurg Rev 41:525–530. https://doi.org/10.1007/s10143-017-0884-2
Bhatoe HS, Singh P, Dutta V (2006) Intraventricular meningiomas: a clinicopathological study and review. Neurosurg Focus 20:E9–E6. https://doi.org/10.3171/foc.2006.20.3.10
Nayar VV, DeMonte F, Yoshor D, Blacklock JB, Sawaya R (2010) Surgical approaches to meningiomas of the lateral ventricles. Clin Neurol Neurosurg 112:400–405. https://doi.org/10.1016/j.clineuro.2010.02.005
Ma J, Cheng L, Wang G, Lin S (2014) Surgical management of meningioma of the trigone area of the lateral ventricle. World Neurosurg 82:757–769. https://doi.org/10.1016/j.wneu.2014.05.026
Nanda A, Bir SC, Maiti T, Konar S (2016) Intraventricular meningioma: technical nuances in surgical management. World Neurosurg 88:526–537. https://doi.org/10.1016/j.wneu.2015.10.071
Pereira BJA, de Almeida AN, Paiva WS, de Aguiar PHP, Teixeira MJ, Marie SKN (2018) Natural history of intraventricular meningiomas: systematic review. Neurosurg Rev. https://doi.org/10.1007/s10143-018-1019-0
Kim EY, Kim ST, Kim HJ, Jeon P, Kim KH, Byun HS (2009) Intraventricular meningiomas: radiological findings and clinical features in 12 patients. Clin Imaging 33:175–180. https://doi.org/10.1016/j.clinimag.2008.09.005
Liu M, Wei Y, Liu Y, Zhu S, Li X (2006) Intraventricular meningiomas: a report of 25 cases. Neurosurg Rev 29:36–40. https://doi.org/10.1007/s10143-005-0418-1
Menon G, Nair S, Sudhir J, Rao R, Easwer HV, Krishnakumar K (2009) Meningiomas of the lateral ventricle - a report of 15 cases. Br J Neurosurg 23:297–303. https://doi.org/10.1080/02688690902721862
Grujicic D, Cavallo LM, Somma T, Illic R, Milicevic M, Raicevic S, Gazibara MS, Villa A, Savic D, Solari D, Cappabianca P (2017) Intraventricular meningiomas: a series of 42 patients at a single institution and literature review. World Neurosurg 97:178–188. https://doi.org/10.1016/j.wneu.2016.09.068
Gassel MM, Davies H (1961) Meningiomas in the lateral ventricles. Brain 84:605–627
Bertalanffy A, Roessler K, Koperek O, Gelpi E, Prayer D, Neuner M, Knosp E (2006) Intraventricular meningiomas: a report of 16 cases. Neurosurg Rev 29:30–35. https://doi.org/10.1007/s10143-005-0414-5
Jelinek J, Smirniotopoulos JG, Parisi JE, Kanzer M (1990) Lateral ventricular neoplasms of the brain: differential diagnosis based on clinical, CT, and MR findings. AJR Am J Roentgenol 155:365–372. https://doi.org/10.2214/ajr.155.2.2115270
Majós C, Cucurella G, Aguilera C, Coll S, Pons LC (1999) Intraventricular meningiomas: MR imaging and MR spectroscopic findings in two cases. AJNR Am J Neuroradiol 20:882–885
Vuckovic N, Kozic D, Vulekovic P, Vuckovic D, Ostojic J, Semnic R (2010) MR and MRS characteristics of intraventricular meningioma. J Neuroimaging 20:294–296. https://doi.org/10.1111/j.1552-6569.2008.00345.x
Lin BJ, Chou KC, Kao HW, Lin C, Tsai WC, Feng SW, Lee MS, Hueng DY (2014) Correlation between magnetic resonance imaging grading and pathological grading in meningioma. J Neurosurg 121:1201–1208. https://doi.org/10.3171/2014.7.JNS132359
Iplikcioglu AC, Hatiboglu MA, Ozek E, Ozcan D (2014) Is progesterone receptor status really a prognostic factor for intracranial meningiomas? Clin Neurol Neurosurg 124:119–122. https://doi.org/10.1016/j.clineuro.2014.06.015
Nanda A, Bir SC, Konar S, Maiti T, Kalakoti P, Jacobsohn JA, Guthikonda B (2016) Outcome of resection of WHO Grade II meningioma and correlation of pathological and radiological predictive factors for recurrence. J Clin Neurosci 31:112–121. https://doi.org/10.1016/j.jocn.2016.02.021
Champeaux C, Wilson E, Shieff C, Khan AA, Thorne L (2016) WHO grade II meningioma: a retrospective study for outcome and prognostic factor assessment. J Neuro-Oncol 129:337–345. https://doi.org/10.1007/s11060-016-2181-2
Aghi MK, Carter BS, Cosgrove GR, Ojemann RG, Aminhanjani S, Martuza RL, Jr CW, Nd BF (2009) Long-term recurrence rates of atypical meningiomas after gross total resection with or without postoperative adjuvant radiation. Neurosurgery 64:56–60. https://doi.org/10.1227/01.NEU.0000330399.55586.63
Stessin AM, Schwartz A, Judanin G, Pannullo SC, Boockvar JA, Schwartz TH, Stieg PE, Wernicke AG (2012) Does adjuvant external-beam radiotherapy improve outcomes for nonbenign meningiomas? A Surveillance, Epidemiology, and End Results (SEER)-based analysis. J Neurosurg 117:669–675. https://doi.org/10.3171/2012.7.JNS111439
Eom KS, Kim HS, Kim TY, Kim JM (2009) Intraventricular malignant meningioma with CSF-disseminated spinal metastasis: case report and literature review. J Korean Neurosurg Soc 45:256–259. https://doi.org/10.3340/jkns.2009.45.4.256
Peh WC, Fan YW (1995) Case report: intraventricular meningioma with cerebellopontine angle and drop metastases. Br J Radiol 68:428–430. https://doi.org/10.1259/0007-1285-68-808-428
Kamiya K, Inagawa T, Nagasako R (1989) Malignant intraventricular meningioma with spinal metastasis through the cerebrospinal fluid. Surg Neurol 32:213–218
Darwish B, Munro I, Boet R, Renaut P, Abdelaal AS, MacFarlane MR (2004) Intraventricular meningioma with drop metastases and subgaleal metastatic nodule. J Clin Neurosci 11:787–791. https://doi.org/10.1016/j.jocn.2004.02.008
Shintaku M, Hashimoto K, Okamoto S-i (2007) Intraventricular meningioma with anaplastic transformation and metastasis via the cerebrospinal fluid. Neuropathology 27:448–452. https://doi.org/10.1111/j.1440-1789.2007.00786.x
Garcia-Conde M, Roldan-Delgado H, Martel-Barth-Hansen D, Manzano-Sanz C (2009) Anaplastic transformation of an atypical intraventricular meningioma with metastases to the liver: case report. Neurocirugia (Astur) 20:541–549
Acknowledgments
The authors wish to thank all of the patients who trusted them and all the physicians and staff who helped with this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
For this type of study, formal consent is not required.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jiang, Y., Lv, L., Li, J. et al. Clinical features, radiological findings, and treatment outcomes of high-grade lateral ventricular meningiomas: a report of 26 cases. Neurosurg Rev 43, 565–573 (2020). https://doi.org/10.1007/s10143-019-01078-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-019-01078-4