Abstract
Purpose
Lateral ventricle meningiomas (LVM) in children are very rare. The current research is mostly limited to adults, and there are very few related studies on children. The purpose of this study was to analyze the clinicopathological and imaging features of lateral ventricle meningiomas in children.
Methods
A retrospective analysis of five children with pathologically confirmed lateral ventricle meningioma was performed, and we collected clinical data, including clinicopathological data, treatment prognosis data, and imaging features (including tumor location, signal intensity, enhancement degree, intratumoral cyst, calcification, peritumoral edema, and associated hydrocephalus).
Results
Among the 5 patients with LVM, 4 were male and 1 was female with an average age of 7.6 years (range 2 to 12 years). All CT scans showed slight hyperintensity or isodensity, and only 1 patient had calcification. Two patients demonstrated cyst changes. Four patients had varying degrees of peritumoral edema. The average tumor volume was 164.1 cm3 (1.4–314.9 cm3). All 5 patients with LVM were iso- or hypointense on T1WI. The T2WI signals had no obvious features. Four patients had a high signal on DWI (80%). The contrast-enhanced signals were mostly homogeneously strong (80%). MRI showed hydrocephalus in 3 patients. All patients underwent gross total resection, and they were followed up regularly after the operation. The average follow-up time was 47.4 months. No recurrence was found in any of the children. All patients were pathologically confirmed to have meningiomas, and WHO grades were all grade I.
Conclusion
Lateral ventricle meningiomas in children are very rare, and the imaging manifestations of the tumor have certain characteristics, but the clinical diagnosis is still difficult, and the diagnosis still requires pathological analysis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Meningiomas in children are very rare, accounting for approximately 0.4 ~ 4.6% of intracranial tumors in children [1]. Intraventricular meningiomas are most often seen in the lateral ventricles. Lateral ventricle meningioma (LVM) originates from arachnoid cells contained within the choroid plexus, which is rarer in children than in adults [2]. Meningiomas in the lateral ventricle are mostly benign, grow slowly, and generally have no clinical symptoms in the early stage due to the large space in the trigone area of the lateral ventricle. When the tumor grows to a certain extent, local compression of brain tissue or obstructive hydrocephalus occurs, and symptoms of increased intracranial pressure or focal neurological impairment occur. Lateral ventricle meningiomas are often associated with large tumor sizes, MRI diagnosis is very difficult, and the preoperative misdiagnosis rate is very high. At present, the related research on LVM is mostly limited to adults, and there are very few related studies in children, with only a few patient reports. To further summarize the clinical features of LVM in children, this study retrospectively analyzed the relevant clinical data, especially imaging and pathological features, of pediatric patients with lateral ventricular meningiomas in our institution.
Methods
Five patients with pathologically confirmed lateral ventricle meningioma who were admitted to the Department of Pediatric Neurosurgery, Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, between December 2013 and February 2022, were retrospectively analyzed. We collected clinical data of patients from hospital information systems, including medical history, imaging examinations, pathological examination data, and prognostic data. All patients underwent head CT examination and SIEMNES-MRI (3.0 T) examination before the operation, and axial spin-echo T1-weighted (T1W) images, axial fast spin-echo T2-weighted (T2W) images, diffusion-weighted images (DWI) in the axial plane, and axial or coronal T2W fluid attenuated inversion recovery sequence (T2FLAIR) images were acquired. Imaging features were collected, including tumor location, contour, signal intensity relative to gray matter, degree of enhancement, intratumoral cysts and necrosis, tumor calcification, tumor borders, peritumoral edema, and associated hydrocephalus. All images were reviewed and agreed upon by two neuroradiologists with more than 10 years of experience. According to postoperative magnetic resonance imaging (MRI) and surgical records, the resection range was defined as gross total resection (GTR) and subtotal resection (STR). GTR was defined as the removal of 95% of tumors by surgery, and STR was defined as resection of 75% of tumors. All patients underwent histopathological examination after surgery, and histological diagnosis was made based on the unique histological morphology of meningiomas. Specimens were subjected to immunohistochemical studies to confirm histogenesis and tumor grade. This study was approved by the Institutional Review Board of Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, and informed consent was obtained from the patients’ parents or guardians.
Results
The general clinical data of the patients are presented in Table 1. Among the 5 patients with LVM, there were 4 males and 1 female, with a male-to-female ratio of 4:1. The mean age was 7.6 years (range 2 to 12 years). The clinical symptoms were noted as follows: 1 patient with strabismus, 1 patient with unsteady walking, 2 patients with dizziness, and the other patient was first diagnosed because of a mass in the right temporal head.
All surgical procedures were carried out by a senior physician (Doctor Ma Jie), the transcortical approaches were applied in this study with the help of neuronavigation and intraoperative electrophysiological monitoring, and all surgical procedures were performed by classical standard methods. Two patients underwent digital subtraction angiography (DSA) to characterize the tumor blood supply before tumor resection (1 patient underwent embolization). All 5 patients underwent total resection (GTR rate 100%). Regular follow-up was conducted after the operation was performed. The average follow-up time was 47.4 months (range 3–100 months), and none of the children received radiotherapy or chemotherapy after the operation. Symptoms improved in the patients who presented with hydrocephalus, and all patients had no significant neurological impairments during follow-up. To date, none of the children had recurrence, death, or neurological damage complications (the results of pre- and postoperative examinations of patient 4 are shown in Fig. 1).
MR images of a 7-year-old boy with meningioma in the left lateral ventricle (case 4) a Preoperative, noncontrast CT showing a large hyperdense pathological mass with peritumoral edema and hydrocephalus in the left ventricular trigone (arrow). b Axial T1 FLAIR image shows a slightly low signal. c Gd-enhanced T1W images show a heterogeneous strong signal. d, e, f Postoperative CT and MRI examination show no obvious residual tumor after surgery
All patients were pathologically confirmed to have meningiomas with WHO grade I, including 1 patient with fibrous meningioma, 1 patient with transitional meningioma, 1 patient with meningothelial, and the other 2 patients had unknown findings. The Ki67 index of all patients was ≤ 5%, with a mean of 2.8% (range 1 to 5%). Both OLIG2 and GFAP tests were negative. EMA was positive or focally positive. Only 1 of 4 patients (one patient was not evaluated) had positive S100 expression. PR: 4 patients were positive (one patient was not evaluated). SYN: 3 patients were negative (two patients were not evaluated). VIM: Two of 3 patients were positive (two patients were not evaluated). NeuN: Four patients were negative (one patient was not evaluated). SSTR2: Three patients were positive (two patients were not evaluated). CD34: 3 of 5 patients were positive. It can be seen from the above results that the positive rates of EMA, PR, SSTR2, and CD34 are relatively high (the photomicrograph of case 5 is shown in Fig. 2, and the immunohistochemical results are shown in Table 2).
Analysis of imaging features
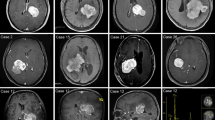
The imaging characteristics of all patients in this study are shown in Table 3. In general, all 5 patients with LVM had tumors located in the lateral ventricle (3 patients had tumors on the left side, and 2 patients had tumors on the right side), with clear borders (round or relatively round). Two patients showed cysts and necrotic changes, including 1 patient with large cysts and 1 patient with multiple small cyst changes. Four children (80%) had varying degrees of peritumoural edema. Three patients presented with hydrocephalus underwent fundoscopy examinations (one of them showed optic disc protrusion), and none of them underwent shunt surgery before the operation. All patients had no visual field deficits in pre- and post-operation. According to the tumor volume calculated by MRI, the average tumor volume was 164.1 cm3 (1.4–314.9 cm3). On T1WI, there were 2 patients with isointense signals, 2 patients with slightly hypointense signals, and 1 patient with hypointense signals. All 5 patients with LVM in this study had isointense or hypointense regions on T1WI. Hypointense T2WI signals were observed in 1 patient, isointense signals were found in 1 patient, slightly hypointense signals were noted in 1 patient, and slightly hyperintense signals were observed in 2 patients. The T2WI signal of 5 patients with LVM had no obvious characteristics. All patients were scanned by DWI sequence; 4 patients (80%) had a hyperintense signal, and 1 patient had a slightly hypointense signal. Enhanced scanning was performed after injection of contrast agent (Gd-DTPA, 0.2 mmol/kg), of which 1 patient was homogeneously strong, and 4 patients (80%) demonstrated high heterogeneity. From the above data, it can be seen that pediatric LVM in this study was mainly evident an isointense or hypointense signal on T1WI, and there was no obvious feature of the T2WI signal. CT scans showed that 3 patients showed slightly high-density shadows, 2 patients had isodense regions, and only 1 patient showed calcification (the CT and MRI images of 4 patients with lateral ventricle meningioma are shown in Fig. 3). Combined with CT and MRI findings that led to a comprehensive diagnosis, no meningioma was diagnosed in any tumors, 2 patients were initially diagnosed as choroid plexus tumors, and 3 patients were initially diagnosed as having ependymoma. The preoperative diagnosis of lateral ventricle meningioma in this study was misdiagnosed. This study additionally reviewed case reports of children with lateral ventricle meningiomas in the past 10 years. The relevant data are shown in Table 4.
The top rows of images are CT images, and the bottom rows of images are Gd-enhanced T1W images.
Discussion
Meningiomas account for approximately 30% of all primary brain tumors, are the largest subgroup of intracranial tumors, are most common in middle-aged individuals, and are extremely rare in childhood [18]. Unlike adult meningiomas, which mostly occur in women, meningiomas in children are more common in men [19]. The ratio of males to females in this group was 4:1, which is consistent with reports in the literature. The reason may be attributed to the role of estrogen in the tumorigenesis of meningiomas [20]. In children with meningioma, the effect of estrogen is not evident. Unlike adult meningiomas, which often manifest on the convexity of the brain or parasagittal, childhood and juvenile meningiomas often manifest in areas such as the intraventricular and anterior/middle fossae [21]. Intraventricular meningiomas are tumors derived from the choroid plexus tissue in the ventricle. They are rare in intracranial tumors and have a low incidence, accounting for approximately 0.5 to 5% of all intracranial meningiomas [22]. Among intraventricular meningiomas, lateral ventricle meningiomas are the most common, accounting for approximately 80% of the incidence of intraventricular meningiomas [23]. Lateral ventricle meningiomas are more common in children than in adults [8]. Lateral ventricle meningiomas grow from choroid plexus tissue, which, together with the dura mater, originates from the embryonic ectoderm. Lateral ventricle meningiomas are mostly benign and grow slowly. In addition, the lateral ventricle is located in the deep part of the brain that has a certain amount of space, so the lateral ventricle meningioma can grow to a large size. Most of its clinical manifestations are atypical, and most of them do not elicit specific symptoms and signs. In addition, children’s lack of a clear description of the disease and the lack of cooperation in physical examination make the diagnosis difficult. The most common symptom is increased intracranial pressure, manifesting as headache, vomiting, and papilledema, which is related to the slow growth and large size of the tumor. Another factor is that the tumor occurs in the ventricle, which can easily lead to the obstruction of cerebrospinal fluid circulation and cause elevated intracranial pressure. When the tumor compresses the internal capsule, the patient may have contralateral hemiplegia, sensory disturbance of the contralateral limb, and even contralateral hemianopia. Memory impairment, neuropsychological impairment, and personality changes are rare.
Because of the abundant choroid plexus tissue in the trigone area of the lateral ventricle, meningiomas of the lateral ventricle are slightly more likely to occur in the trigone area on the left side than on the right side [24]. The diagnosis of lateral ventricle meningioma mainly relies on imaging examinations, including head CT and MRI, and angiography is also valuable for diagnosis. Among them, contrast-enhanced brain MRI has the greatest diagnostic value, as it can accurately display the tumor texture, location whether it is associated with hydrocephalus, and other information. In this study, all 5 patients underwent head CT examination to determine the location of the tumor. To obtain precise information about the tumor, they all underwent MRI examination. On MRI T1WI, tumors all showed isointense or hypointense signals, with relatively clear borders (round or quasi-round). Most of them showed hyperintense signals on T2WI (80%), and 4 patients (80%) had peritumoural edema, which showed obvious hyperintense signals on T2WI around the tumor, and all the tumors were obviously enhanced on contrast-enhanced scans. Digital subtraction angiography can provide doctors with information about the blood supply inside the meningioma (it can show dilation, tortuosity, and position changes of the anterior choroidal artery). In this study, 2 patients underwent preoperative DSA examination. Pediatric meningiomas have different imaging and histological features from adult meningiomas; however, there have been no systematic imaging studies of patients with LVM in the past, with only a few case reports. Meningiomas in children are more prone to necrosis and cystic changes than those in adults [25]. This study found that 40% of meningiomas in the lateral ventricle had cystic changes. Tumors often cause enlargement of the temporal angle of the lateral ventricle and localized calcification of the choroid plexus. Proton magnetic resonance spectroscopy (MRS) is unable to discriminate low- and high-grade meningiomas. The literature reports that patients with meningiomas have a high Cho signal (long TE). There were very low signals of Naa and Cr in the spectra of meningioma patients [26]. Mild to moderate edema was common in the peripheral brain parenchyma, and 4/5 of the patients in this group had peritumoral edema. Analyses of previous studies have shown that sex, tumor size, and peritumoral edema are predictors of high-grade LVM in univariate analysis [27]. Meningioma in the trigone area of the lateral ventricle is closely related to the choroid plexus [28], which is one of the important features, similar to the dural tail sign, or can be called the “choroid plexus tail sign.” In this study, we did not find a significant relationship between MRI signal and WHO grade, probably because the sample size was too small. It has been suggested that the use of imaging features of meningiomas to determine the grade of LVM is inaccurate.
LVM is easily misdiagnosed, and the diagnosis is mainly based on MRI findings. Therefore, pathological examination remains the gold standard for the diagnosis of LVM. Children’s meningiomas have different molecular pathological features compared with adults. Meningiomas are more likely to be malignant in children than in adults [29], but in this study, all 5 meningiomas in the lateral ventricle were benign (WHO grade I). A review of published data in the past 10 years found that 14.3% of children with lateral ventricle meningiomas were pathologically grade II or higher. Commonly used immunohistochemical indicators for the diagnosis of meningeal tumors include S100, D2-40, Nestin, claudin-1, CK, EMA, CEA, Vimentin, and Ki-67 [30]. Meningeal-derived tumors are characterized by coexpression of EMA, CK, and S100. Other markers, such as D2-40, are mainly used for the diagnosis of aggressive tumor types, such as atypical meningioma and anaplastic meningioma [31]. The evaluation of the Ki-67 proliferative index is critical for the determination of tumor grade. CEA expression is characteristic of pseudopsammoma bodies in secretory meningiomas. This study found that the positive rates of EMA, PR, SSTR2, and CD34 were higher in children with lateral ventricle meningiomas. Progesterone receptor (PR) expression was found in approximately 40% of meningiomas and was found to be associated with the patient’s prognosis. Moreover, studies found that PR expression was significantly correlated with recurrence, so it was suggested that PR indicators should be included in the routine pathological diagnosis of meningiomas [32]. SSTR is common in neuroendocrine tumors, and SSTR2 has developed into an important marker of central nervous system tumors; studies have shown that it is also present in most meningiomas [33]. As a class of highly glycosylated type I transmembrane glycoproteins, CD34 plays a role in cell adhesion and cell signal transduction. CD34 can be expressed in meningioma tissue and can be used to evaluate the prognosis of patients [34]. The continuous proliferation, invasion, and metastasis of tumors are closely related to microangiogenesis and blood supply. The production and secretion of factors by tumor cells need to be regulated by a variety of vascular factors, such as CD34. At present, the treatment of tumors by inhibiting tumor microangiogenesis has become a hot topic, and CD34 may be a key point to prevent the microangiogenesis of meningiomas [35].
There have also been many recent reports on the genetic mutational signature of meningiomas in children. Pediatric meningiomas share a high frequency of NF2 alterations which are distinct from adult counterparts. One study analyzed tumor specimens from 38 children with childhood meningioma by next-generation sequencing and found that loss-of-function mutations in NF2 and chromosome 22 losses were common in children meningiomas. Pathogenic variants in other genes, including SMARCB1, FUBP1, BRAF, and TERT, were found in a minority of patients. H3K27 hypomethylation was not found in the cohort, and it is a useful biomarker in adult tumors [36]. Sporadic childhood and adolescent meningiomas are less common than meningiomas in adulthood. Genetic syndromes associated with meningiomas include neurofibromatosis type 2 with germline mutations in NF2, Gorlin syndrome with mutations in SUFU, and clear cell meningioma with germline mutations in SMARCE1 [37]. One study showed that pediatric meningiomas frequently harbored NF2 deletions (82%) along with more aggressive histological features, and the prognosis was worse than that of patients without NF2 mutations [38]. However, none of the LVM patients in this group were found to also have neurofibromatosis type 2. The driver genes of meningioma reported in recent years include AKT1, TRAF7, SMO, KLF4, and PIK3CA [39]. However, a study showed that intraventricular meningiomas frequently harbor NF2 mutations but lack common genetic alterations in TRAF7, AKT1, SMO, KLF4, PIK3CA, and TERT [40]. The driver genes of sporadic pediatric meningioma have not yet been revealed. Recently, researchers identified YAP1 fusions as a potential oncogenic driver in the development of pediatric meningioma patients, which strengthens the hypothesis that deregulation of the HIPPO pathway is a central mechanism in meningioma tumorigenesis. YAP1 fusions appear to be a surrogate for NF2 inactivation, and YAP1 fusion-positive meningiomas are closer to NF2-mutated patients than other pediatric meningiomas [41]. In the past 10 years, a total of 30 cases of pediatric patients with meningioma were admitted to our hospital; only two showed NF2 alterations (data not shown). In this study, none of the five patients with LVM did genetic testings, and all were not diagnosed with neurofibromatosis type 2.
Lateral ventricle meningiomas have atypical imaging manifestations and inconspicuous clinical symptoms, which can easily lead to misdiagnosis. Therefore, LVM needs to be differentiated from other types of tumors that occur in the lateral ventricle. For tumors in the trigone of the lateral ventricle, choroid plexus tumors are most common in younger children, and ependymoma and astrocytoma are most common in older children. To date, first-line treatments for meningiomas are observation and surgery, but adjuvant radiotherapy/radiosurgery is also necessary for atypical or anaplastic meningiomas [42]. Surgical treatment of lateral ventricle meningiomas is a challenge for neurosurgeons. Experience and thorough anatomical knowledge are determinants of resection. Successful surgical resection can allow patients to live a normal life, while any surgical approach selection or errors during the surgical procedure can lead to neurological dysfunction and even death. Before surgery, it is necessary to fully understand the anatomy of the lateral ventricle, the course of arteries and veins, and the relationship these structures have with the optic tract and the dominant hemisphere. Principles of successful surgical resection include early devascularization, less brain tissue pulling to allow for space for tumor resection, in-depth understanding of the function of surrounding anatomy, avoidance of important neural structures, and minimal distance from the tumor; however, there is no single surgical approach that satisfies all conditions.
Availability of data and material
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
References
Szychot E, Goodden J, Whitfield G, Curry S (2020) Children’s Cancer and Leukaemia Group (CCLG): review and guidelines for the management of meningioma in children, teenagers and young adults. Br J Neurosurg 34:142–153
McDermott MW (2003) Intraventricular meningiomas. Neurosurg Clin N Am 14:559–569
Rai HIS, Singh J, Singh M et al (2022) Surgical track and scalp implantation following intraventricular meningiomas excision: a report with review of literature. Neurol India 70:31–36
Kumar J, Lakshmanan R, Dyke JM, Lee S, Shipman P (2022) Case report: congenital intraventricular meningioma demonstrated with fetal MRI. Childs Nerv Syst 38:191–194
Shah A, Dandpat S, Vutha R, Goel A (2020) Recurrence of intraventricular meningioma along the surgical track. Neurol India 68:1188–1191
Mizrahi CJ, Benenson S, Moscovici S, Candanedo C, Benifla M, Spektor S (2019) Combination treatment with intravenous tigecycline and intraventricular and intravenous colistin in postoperative ventriculitis caused by multidrug-resistant Acinetobacter baumannii. Cureus 11:e3888
Hong S, Usami K, Hirokawa D, Ogiwara H (2019) Pediatric meningiomas: a report of 5 cases and review of literature. Childs Nerv Syst 35:2219–2225
Li Z, Li H, Jiao Y et al (2018) Clinical features and long-term outcomes of pediatric intraventricular meningiomas: data from a single neurosurgical center. Neurosurg Rev 41:525–530
Munjal S, Vats A, Kumar J, Srivastava A, Mehta VS (2016) Giant pediatric intraventricular meningioma: case report and review of literature. J Pediatr Neurosci 11:219–222
Dash C, Pasricha R, Gurjar H, Singh PK, Sharma BS (2016) Pediatric intraventricular meningioma: a series of six cases. J Pediatr Neurosci 11:193–196
Prodromou N, Alexiou GA, Stefanaki K, Moraiti A, Sfakianos G (2016) Giant atypical intraventricular meningioma in a child. Pediatr Neurosurg 51:306–308
Wang HH, Luo CB, Guo WY et al (2013) Preoperative embolization of hypervascular pediatric brain tumors: evaluation of technical safety and outcome. Childs Nerv Syst 29:2043–2049
Moiyadi AV, Shetty P (2012) Giant velum interpositum meningioma in a child. Indian J Med Paediatr Oncol 33:173–175
Okechi H, Albright AL (2012) Intraventricular meningioma: case report and literature review. Pediatr Neurosurg 48:30–34
Chohan MO, Rehman T, Medina-Flores R, Clericuzio C, Heideman R, Marchand E (2011) 16 month-old female with intraventricular mass. Brain Pathol 21:349–350
Cleary C, Curtin D (2010) Giant atypical intraventricular meningioma presenting with visual loss in a child. Ir J Med Sci 179:617–619
Nayar VV, DeMonte F, Yoshor D, Blacklock JB, Sawaya R (2010) Surgical approaches to meningiomas of the lateral ventricles. Clin Neurol Neurosurg 112:400–405
Pereira BJA, Oba-Shinjo SM, de Almeida AN, Marie SKN (2019) Molecular alterations in meningiomas: literature review. Clin Neurol Neurosurg 176:89–96
Liu Y, Li F, Zhu S, Liu M, Wu C (2008) Clinical features and treatment of meningiomas in children: report of 12 cases and literature review. Pediatr Neurosurg 44:112–117
Giraldi L, Fugleholm K, Munch TN (2018) The impact of hormonal factors in meningioma development. Ugeskr Laeger 180:V02180153
Gao X, Zhang R, Mao Y, Wang Y (2009) Childhood and juvenile meningiomas. Childs Nerv Syst 25:1571–1580
Ramraje S, Kulkarni S, Choudhury B (2012) Paediatric intraventricular meningiomas. A report of two cases. Australas Med J 5:126–129
Pereira BJA, de Almeida AN, Paiva WS, de Aguiar PHP, Teixeira MJ, Marie SKN (2020) Natural history of intraventricular meningiomas: systematic review. Neurosurg Rev 43:513–523
Curry WT Jr, Cosgrove GR, Buchbinder BR, Ojemann RG (2001) Resection of a dominant-hemisphere intraventricular meningioma facilitated by functional magnetic resonance imaging. Case report Neurosurg Focus 10:E1
Phillips D, Auguste KI, Gupta N (2020) Meningiomas in children. Handb Clin Neurol 169:253–259
Jaskolski DJ, Fortuniak J, Stefanczyk L et al (2013) Differential diagnosis of intracranial meningiomas based on magnetic resonance spectroscopy. Neurol Neurochir Pol 47:247–255
Ressel A, Fichte S, Brodhun M, Rosahl SK, Gerlach R (2019) WHO grade of intracranial meningiomas differs with respect to patient’s age, location, tumor size and peritumoral edema. J Neurooncol 145:277–286
Muley KD, Shaikh ST, Deopujari CE, Andar UB (2017) Primary intraventricular meningiomas in children-experience of two cases with review of literature. Childs Nerv Syst 33:1589–1594
Liu J, Zhao K, Wang J, Shu K (2021) Clinical features and long-term outcomes of pediatric meningiomas. Childs Nerv Syst 37:3041–3047
Solomon DA, Pekmezci M (2020) Pathology of meningiomas. Handb Clin Neurol 169:87–99
Mrachek EK, Davis D, Kleinschmidt-DeMasters BK (2015) Dual use of E-cadherin and D2–40 immunostaining in unusual meningioma subtypes. Am J Clin Pathol 144:923–934
Maiuri F, Mariniello G, de Divitiis O et al (2021) Progesterone receptor expression in meningiomas: pathological and prognostic implications. Front Oncol 11:611218
Tollefsen SE, Jarmund AH, Ytterhus B, Salvesen O, Mjones P, Torp SH (2021) Somatostatin receptors in human meningiomas-clinicopathological aspects. Cancers (Basel) 13:5704
Baxter DS, Orrego A, Rosenfeld JV, Mathiesen T (2014) An audit of immunohistochemical marker patterns in meningioma. J Clin Neurosci 21:421–426
Sahab-Negah S, Gorji A (2020) Meningioma tumor microenvironment. Adv Exp Med Biol 1296:33–48
Toland A, McNulty SN, Pekmezci M et al (2020) Pediatric meningioma: a clinicopathologic and molecular study with potential grading implications. Brain Pathol 30:1134–1143
Kerr K, Qualmann K, Esquenazi Y, Hagan J, Kim DH (2018) Familial syndromes involving meningiomas provide mechanistic insight into sporadic disease. Neurosurgery 83:1107–1118
Perry A, Giannini C, Raghavan R et al (2001) Aggressive phenotypic and genotypic features in pediatric and NF2-associated meningiomas: a clinicopathologic study of 53 cases. J Neuropathol Exp Neurol 60:994–1003
Yuzawa S, Nishihara H, Tanaka S (2016) Genetic landscape of meningioma. Brain Tumor Pathol 33:237–247
Jungwirth G, Warta R, Beynon C et al (2019) Intraventricular meningiomas frequently harbor NF2 mutations but lack common genetic alterations in TRAF7, AKT1, SMO, KLF4, PIK3CA, and TERT. Acta Neuropathol Commun 7:140
Sievers P, Chiang J, Schrimpf D et al (2020) YAP1-fusions in pediatric NF2-wildtype meningioma. Acta Neuropathol 139:215–218
Apra C, Peyre M, Kalamarides M (2018) Current treatment options for meningioma. Expert Rev Neurother 18:241–249
Acknowledgements
We thank Haibo Zhang for revision of the manuscript prior to submission, and also thank all the other participants for their involvement in the study.
Funding
This study received funding from the Shanghai Xin Hua Hospital (JZPI201701 to JM), Shanghai Science and Technology Committee (17411951800 to JM).
Author information
Authors and Affiliations
Contributions
Yufan Chen and Shuaiwei Tian contributed equally to this work (analyzed the data and drafted the manuscript). Yang Zhao and Jie Ma conceived the idea and directed the whole project. Zhuangzhuang Liang, Jiajia Wang, and Baocheng Wang contributed to review and technical assistance. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine. Informed consent was obtained from the patients’ parents or guardians.
Consent for publication
We confirm that we have read the Journal’s position on issues involved in the publication and affirm that this report is consistent with those guidelines.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, Y., Tian, S., Wang, J. et al. Lateral ventricle meningiomas in children: clinicopathological and neuroradiological features. Childs Nerv Syst 39, 151–158 (2023). https://doi.org/10.1007/s00381-022-05680-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-022-05680-8