Abstract
The efficacy of tumor removal via craniotomies on preoperative hydrocephalus (HC) in adult patients with intracranial tumors is largely unknown. Therefore, we sought to evaluate the effect of tumor resection in patients with preoperative HC and identify the incidence and risk factors for postoperative VP shunt dependency. All craniotomies for intracranial tumors at Oslo University Hospital in patients ≥ 18 years old during a 10-year period (2004–2013) were reviewed. Patients with radiologically confirmed HC requiring surgery and subsequent development of shunt dependency were identified by cross-linking our prospectively collected tumor database to surgical procedure codes for hydrocephalus treatment (AAF). Patients with preexisting ventriculoperitoneal (VP) shunts (N = 41) were excluded. From 4774 craniotomies performed on 4204 patients, a total of 373 patients (7.8%) with HC preoperatively were identified. Median age was 54.4 years (range 18.1–83.9 years). None were lost to follow-up. Of these, 10.5% (39/373) required permanent CSF shunting due to persisting postoperative HC. The risk of becoming VP shunt dependent in patients with preexisting HC was 7.0% (26/373) within 30 days and 8.9% (33/373) within 90 days. Only secondary (repeat) surgery was a significant risk factor for VP shunt dependency. In this large, contemporary, single-institution consecutive series, 10.5% of intracranial tumor patients with preoperative HC became shunt-dependent post-craniotomy, yielding a surgical cure rate for HC of 89.5%. To the best of our knowledge, this is the first and largest study regarding postoperative shunt dependency after craniotomies for intracranial tumors, and can serve as benchmark for future studies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Intracranial brain tumors may give rise to severe neurological impairment if left untreated, and the primary treatment is by tumor resection via craniotomy, which has been established in numerous studies in the past and proven to prolong life in patients harboring these potentially deadly diseases [11, 24, 33, 44, 45, 65]. Nonetheless, apart from increasing overall survival and improved quality of life [4, 29, 49], potential unwanted risks of surgery such as infections [39, 41], postoperative hematomas [22, 32, 39], neurological sequelae [18, 34, 60], postoperative altered CSF dynamics [27, 38], or surgical morbidity/mortality [4, 5, 18, 39] do not warrant definite cure for brain tumors.
Studies on perioperative hydrocephalus (HC) related to intracranial tumor surgery have been reported in the past, but these have been limited to specific tumor histologies and/or tumor locations [8, 19, 47, 50]. Analyses of risk factors of de novo postoperative HC with subsequent VP shunt dependency in brain tumor patients have been reported as well [26], and risk factors for developing postoperative HC in the pediatric population are reported to be younger age [12, 54], surgical approach, tumor histology and infections [12, 27, 40], among others. However, in contemporary literature, there is gap in knowledge concerning incidence of preexisting HC and their surgical success rate of tumor resection. Furthermore, studies on risk factors leading to permanent CSF diversion after surgery for intracranial tumors are scarce, which is the primary end-point of this study.
Based on our prospectively collected database of all adult patients who underwent craniotomies for intracranial tumors in a 10-year period, we wanted to identify the incidence of preexisting HC, and risk factors for shunt dependency after craniotomies for intracranial tumors in adult patients.
Materials and methods
Data collection
A prospectively kept single-center database of consecutive patients with CNS tumors was reviewed over a 10-year period (2004–2013).
The following data were recorded: age, sex, preoperative HC, tumor location, tumor volume (cm3), primary or secondary (repeated) resection, histology (WHO classification), ventricular entry (yes/no), post-craniotomy VP shunt (yes/no), radiotherapy (yes/no), and days from craniotomy to shunt implantation.
To identify patients who had persisting postoperative VP shunt-dependent hydrocephalus (HC), we then cross-linked the tumor database with our surgical procedure code database using the Nordic Medico-Statistical Committee Classification of Surgical Procedures (NCSP) codes for CSF-related procedures (operation codes AAF).
Preoperative images (CT and/or MRI) were reviewed and evaluated for the presence/absence of preoperative HC, defined as radiological signs of ventricular enlargement. An experienced neuroradiologist and neurosurgeon assessed the preoperative images at time of surgery, and later retrospectively reviewed by the first author (SAMH). Prominent temporal horns on CT or MRI were identified as HC, as well as a transverse diameter > 5 mm, ballooning of frontal horn with periventricular hypodensity on CT or T2-weighted/FLAIR MRI. The ventricular Evans index was used in instances of doubt. Tumor location was dichotomized into supratentorial and infratentorial.
First craniotomy in a specific location was defined as primary craniotomy, while all subsequent craniotomies in the same location were defined as secondary. Hence, a patient could have had more than one primary craniotomy, if operated on different/multiple locations.
For postoperative VP shunting, the patients were verified to have undergone only one craniotomy in the final analysis.
With respect to postoperative radiotherapy, time to VP shunt surgery was dichotomized into within 90 days and more than 90 days post-craniotomy for comparison.
Postoperative VP shunt timing was reviewed in order to see how many patients became shunt dependent within 30 days, ≤ 60 days, ≤ 90 days, and > 90 days post-craniotomy.
Statistical analysis
Chi-square (X2) test and Fisher’s exact test were used for comparison between categorical variables. Analysis of variance and Student’s t test were used for continuous variables. An analysis of means of proportions was used to detect tumor histologies associated with significantly higher risk of VP shunt dependency. The Kaplan-Meier method was used for time to VP shunt surgery and the log-rank test was conducted to compare VP shunt dependency to different variables. A univariate Cox regression analysis was subsequently run on clinical and pathological criteria. Independent prognostic factors with a p value < 0.20 were assessed with a multivariate stepwise Cox model. Box-plot was used supplementary to visualize and compare radiotherapy to VP shunt dependency. A p value < 0.05 was considered statistically significant. For all statistical analysis, the statistical software program JMP (version 9.03, SAS Institute Inc.) was used.
Ethical considerations
The study has been approved by the institutional ethics committee (Personvernombudets tilrådning 2013/14574).
Results
Population data
A total of 4815 craniotomies for intracranial tumors were performed on 4204 patients (Fig. 1). Patients with preexisting VP shunts were excluded, leaving a total of 4774 cases (Table 1), of which 373 patients had preoperative HC (7.8%) for further analysis. None were lost to follow-up.
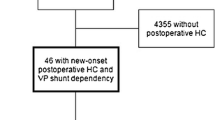
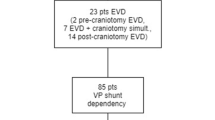
Flow chart demonstrating pre- and postoperative hydrocephalus (HC) in all patients whom underwent craniotomy for intracranial tumors, with subsequent VP shunt dependency. a: patients with de novo postoperative HC and shunt dependency, adopted from Hosainey et al. [26]
Median patient age at time of craniotomy for intracranial tumor surgery was 54.4 years (mean 52.7 years, range 18.1–83.9 years). There were 196 males (52.5%) and 177 females (47.5%) (Table 1).
All patients had preoperative MRI scans. The tumors were located supratentorially in 186 cases (49.9%), while 187 of the tumors (50.1%) were located infratentorially (Table 1).
At time of craniotomy for tumor resection, mean overall tumor volume was 38.7 cm3 (range 1–247.5 cm3). Tumors located supratentorially had a mean tumor volume of 53.3 cm3 (range 1–247.5 cm3), while infratentorially mean tumor volume was 24.2 cm3 (range 2–115 cm3).
Primary craniotomies were performed in 332 cases (89.0%), while 41 (11.0%) were secondary craniotomies (Table 1).
Of the 373 patients with preoperative HC, 148 (39.7%) had ventricular entry during tumor resection.
Persisting postoperative HC and curative surgical treatment
From the total of 4774 craniotomies, 85 patients developed postoperative HC and became VP shunt dependent (Fig. 1). Of these, 39 patients had persisting postoperative HC from the 373 patients (10.5%) with preoperative HC (Table 1), while the rest of the 46 patients developed de novo postoperative HC (Fig. 1).
In patients with persisting postoperative HC (n = 39), the final ratio between craniotomies and patients was 1:1, and median patient age at VP shunt surgery was 54.8 years (mean 55 years, range 23.1–78.6) (Table 1). Neither age (p = 0.34) nor sex (p = .61) was significantly associated with postoperative VP shunt dependency for patients with preoperative HC. Curative treatment of HC by tumor resection alone was achieved in the rest of the 334 patients (89.5%) with preoperative HC.
Tumor location
Out of the 39 patients with postoperative VP shunt dependency, 24 patients (61.5%) had tumors located in the supratentorial compartment, while 15 had infratentorial tumors (38.5%) (Table 1). In both uni- and multivariate analysis, there were no significantly associated risk factors with regard to VP shunt dependency and tumor location (p = .12).
Tumor volume
Mean tumor volume was 31.7 cm3 (range 3.2–115 cm3) in all 39 patients whom became VP shunt dependent. Tumor volume was not a significant risk factor for VP shunt dependency (p = .24) in neither univariate nor multivariate analysis.
Primary vs secondary surgery
Thirty patients (77%) underwent primary craniotomy, while 9 patients (23%) had secondary (repeat) surgery. Secondary (repeat) surgery was a statistically significant risk factor for VP shunt dependency in the univariate analysis only (OR 2.8, CI [1.2–6.5], p < .05).
Histology
Out of the 39 patients with postoperative VP shunt dependency, the tumor histologies were as follows in descending order: 10 high-grade gliomas (25.6%), 9 meningiomas (23.0%), 5 metastatic tumors (12.8%), 4 ependymomas (10.3%), 4 other tumors (10.3%), 2 low-grade gliomas (5.1%), 2 craniopharyngiomas (5.1%), 1 choroid plexus tumor (2.6%), 1 pituitary adenoma (2.6%), and 1 schwannoma (2.6%) (Table 1). Histology was not significantly associated with VP shunt dependency in neither uni- nor multivariate analysis (Fig. 2).
Analysis of means of proportions for histology and VP shunt dependency. The shaded area represents the 95% confidence interval for the proportion of VP shunt dependency of each histological type. Green dots indicate non-significance. HGG, high-grade glioma; LGG, low-grade glioma; PNET, primitive neuroectodermal tumor
Ventricular entry
Twenty-one patients (53.8%) had ventricular entry during tumor resection, while 18 patients (46.2%) did not. Ventricular entry did not reach statistical significance in neither the univariate (p = .056) nor multivariate analysis.
Radiotherapy
From the 39 patients with persisting postoperative HC, 19 patients (48.7%) had postoperative radiotherapy. Postoperative radiotherapy was not significantly associated with VP shunt dependency (p = .88) in neither univariate nor multivariate analysis (Fig. 3).
Timeline
The patients with persisting postoperative HC received VP shunts after a median of 21 days (mean 41.2 days, range 6–198 days) (Fig. 4). The cumulative risk of becoming VP shunt dependent in patients with preexisting HC was 7.0% (26/373) within 30 days and 8.9% (33/373) within 90 days. Six patients had very late VP shunt implantation (> 90 days postoperatively) (Table 1).
Discussion
The main surgical treatment modality of brain tumor-associated HC is achieved by careful planning of tumor resection via craniotomy when indicated and in cases of persistent HC despite tumor removal, alternative surgical treatments for evident relief of HC include permanent CSF diversion with endoscopic third ventriculostomy (ETV) and/or VP shunt placement, for which their values and efficacies have been discussed in numerous studies [8, 15, 36, 43, 53, 54, 58]. Recently, we reported on the incidence and risk factors for postoperative VP shunt dependency in brain tumor patients without preoperative HC and observed that 0.5% of adult patients developed de novo postoperative HC requiring permanent CSF diversion within 90 days after surgery [26]. With the primary aim of studying persisting HC post-craniotomy requiring surgical intervention, in this current study, we focused on adult patients with preexisting HC prior to brain tumor surgery and found that only 7.8% of the patients had preoperative HC (373/4774). This is a much lower overall incidence in adults than observed in pediatric populations (32.5%) [27]. Other studies have reported incidences of preoperative HC ranging from 2.9 to 83.0% [8, 12, 20, 21, 43, 50, 52, 58], but these have been limited by patient group, tumor location, and histology (Table 2).
We observed that the cumulative risk of developing persistent VP shunt-dependent HC was 7.0% (26/373) within 30 days and 8.9% (33/373) within 90 days. In our study, neither age, sex nor tumor location was significantly associated with a risk of persistent HC requiring VP shunts postoperatively. In contrast, younger age and tumor location have been demonstrated to be a significant risk factor for postoperative HC in pediatric patients [27, 66]. Considering the Monroe-Kelly doctrine, a possible theory for persisting postoperative HC might be related to the craniotomy causing a vicious cycle with obstruction of subarachnoid CSF pathways causing impaired venous drainage [55], which will further cause cell death and axonal damage [14, 16, 25], brain elasticity alterations [31], edema [6], and ultimately progression to permanent changes to CSF dynamics and cerebral autoregulation.
In our study, 39/373 patients (10.5%) required permanent CSF diversion, while the remaining 334 were successfully treated by craniotomy, yielding a cure rate for HC of 89.5% with tumor resection alone. A similar surgical success rate was reported by in the abovementioned study by Hosainey et al. in pediatric patients with intracranial tumors, where tumor resection cured patients with preexisting HC in 86.6% of the total [27]. Pirouzmand et al. reported that only tumor removal was required without any further treatment of their preoperative HC in 18/27 patients (67%) in their series of patients with cerebellopontine angle tumors, although limited to vestibular schwannomas [50]. Culley et al. reported 97 out of 117 (83%) pediatric patients with preoperative HC due to posterior fossa tumors that 35% required postoperative shunting, but they did not find preoperative HC as a significant risk factor for postoperative shunting [12]. Greganov et al. reported that 53 out of 400 patients (13.3%) presented with HC at admission, of whom 48 did not require additional HC treatment (87.5%) [21]. Although HC was present at admission, this study was restricted to vestibular schwannomas only, similar to other studies [46, 50]. Likewise, Nanda et al. reported in their study of complications of petroclival meningiomas that 8 out of 50 patients (16%) developed HC and required VP shunt placement, but whether HC was present before surgery or not has not been specifically stated [48] (Table 2).
Tumor volume was not significantly associated with shunt dependency in our study. Riva-Cambrin et al. also reported in their pediatric series of posterior fossa tumors that tumor volume was not significantly associated with postoperative HC [54]. In a study by Fischer et al. on risk factors of HC after resection and adjuvant radiochemotherapy in GBM patients, average tumor volume was 58.6 cm3 in 11 patients whom developed communicating HC after tumor resection, compared to 35.1 cm3 in the remaining 140 patients without HC [19]. They found an insignificant trend towards frontal tumor location and large tumor volume (p = .115), but this study was restricted to glioblastoma patients only. In comparison, with regard to volume as a space occupying lesion (compare brain tumors as space occyping lesions), this is in contrast to intracranial hemorrhagic lesions as reported by Hsu et al. of patients with spontaneous cerebellar hemorrhage [28], where initial hydrocephalus at admission and hematoma volume was demonstrated to be independent significant risk factors for VP shunt dependency. This discrepancy might be explained by the difference in the natural course of rather slowly growing intracranial tumors resulting in gradual adaptation to changes in CSF dynamics compared to hemorrhagic lesions that may increase in size rather rapidly and eventually cause obstruction by hemorrhaging intraventricularly from the brain parenchyma.
Interestingly, secondary (repeat) surgery was significantly associated with persisting postoperative HC and subsequent VP shunt dependency (p < .05), as opposed to in the pediatric population where it was not associated with persisting postoperative HC [27]. Likewise, for development of de novo postoperative HC, secondary (repeat) surgery has not been shown to be a significant risk factor for VP shunt dependency [26]. There were 9 out of 39 patients whom underwent secondary (repeat) surgery in our study, which has been shown to prolong survival in patients with HGGs in numerous studies [3, 5, 9, 10, 23, 56, 61, 64, 68], metastatic tumors [2, 62], and LGGs [1, 51], among others. Nonetheless, surgical complications after reoperation such as decline in KPS [42], increased rate of morbidity and mortality [23, 42, 67], CSF leak and delayed wound healing [35, 37], infections [13, 17, 35, 41], and neurological worsening [10] have also been reported. Montano et al. reported that ventricular opening was a risk factor for communicating hydrocephalus when the analysis was restricted to patients undergoing ≥ 2 operations for tumor removal [47].
Interestingly, histology was not shown to result in higher risk of VP shunt dependency in this study, whose effect we expected to reach statistical significance. This is in contrast to patients with de novo postoperative HC where choroid plexus tumor and craniopharyngiomas increase the risk of postoperative VP shunt dependency [26]. Ependymomas had the highest proportion of risk for postoperative VP shunt dependency (25%), although non-significant (Fig. 3). Conversely, significant correlation between tumor type and subsequent need for shunt has been found with ependymomas and medulloblastomas [27, 54, 59]. These tumors either line the ventricular wall or extend into ventricular compartments, and craniotomies for tumor resection with ventricular entry have been reported to be associated with increased risk of postoperative HC [19, 30, 47]. Some studies have reported an association between histology and postoperative shunting [7, 54, 58], while others have not [12, 50]. However, these studies are limited by patient group, tumor location, and histology.
Twenty-one out of the 39 patients with persisting postoperative HC had ventricular entry during tumor resection. Ventricular entry was not a significant risk factor for shunt dependency in our study, albeit noteworthy in the univariate analysis as a risk factor (p = .56). Nevertheless, ventricular entry has been reported to be associated with increased risk of postoperative HC with subsequent VP shunt implantation in previous reports [19, 30, 47].
Ten patients out of 82 with HGG, 9/70 patients with meningioma and 5/90 patients with metastasis became VP shunt dependent (Table 1). Six patients (1 HGG, 3 meningioma, 1 metastasis, and 1 pituitary adenoma) had VP shunt surgery > 90 days post-craniotomy. The patients with malignant tumors received chemotherapy and/or radiotherapy postoperatively as routine follow-up treatment for malignancy. Therefore, these patients are not considered to have become VP shunt dependent due to surgery per se. Also, 19 out of the 39 patients with persisting postoperative HC underwent postoperative radiotherapy. Loss of brain tissue and elevated CSF pressure resulting in HC due to fibrosis of the arachnoid granulations reducing CSF outflow after radiotherapy has been reported [63], but we did not find radiotherapy as a significant risk factor for postoperative VP shunt dependency in this study (Fig. 4), as also indicated by a similar study on patients with development of de novo HC after craniotomy for intracranial tumors [26]. In their study of HC after tumor resection of glioblastoma patients by Fischer et al. [19], 11 out of 151 patients (7.28%) developed communicating HC and required VP shunt implantation. Of these, 4 patients were shunted before radiotherapy, while the remaining 7 patients received implantation after radiotherapy. However, they emphasized that since all 151 patients received radiotherapy, radiotherapy-induced fibrosis could not be considered a risk factor for the development of communicating HC. To elucidate this issue, a separate analysis of intracranial tumor patients and their respective timing of postoperative radiation therapy is warranted.
In our series, 26 out of 373 patients (7.0%) with preoperative HC became VP shunt dependent within 30 days postoperatively and cumulatively, 33 patients (8.9%) required VP shunt within the first 90 days (Fig. 1). Comparatively, in the abovementioned study by Fischer et al., 4 of 11 glioblastoma patients with postoperative HC had VP implantation < 30 days, while the remaining were shunted 66–728 days postoperatively (median 110 days) [19]. Similarly, Roth et al. reported that 16/530 patients (3%) received VP shunt placement from 3 weeks to 10 months postoperatively, with improvement of cognitive function in 11 patients [57]. Our overall low rate of shunt dependency can be explained by our inclusion of all craniotomies with intracranial tumors regardless of location or histology.
Strength of the study
Oslo University Hospital, consisting of two hospitals (Rikshospitalet and Ullevål), is a centralized health care center and has a population-based referral of patients from a well-defined region of Norway with approximately 2.8 million inhabitants. Hence, the total number of patients and data restriction to one center reduces possible confounding effects of differences in access to health care services. Also, we have avoided the selection bias inherently present in large multicenter studies, as there is only one neurosurgical unit performing these surgeries in a well-defined geographical area. Additionally, its design, setting, and clinical follow-up contribute greatly to its exclusivity. We did not find any other large-scale studies with primary outcome of shunt dependency post-craniotomy by including all intracranial tumors regardless of patient group, histology or tumor locations to compare our study with, which demonstrates the uniqueness of our study. There is no selection bias as the study includes all craniotomies performed for a histologically verifiable intracranial tumor with the study period. Our study design is a retrospective analysis of a prospectively collected database. Additionally, by cross-linking our tumor database with the registry for neurosurgical procedures, we have included all craniotomies targeting the primary outcome of shunt dependency. Lastly, we obtained complete follow-up and to the extent of our knowledge, this is the largest study with regard to postoperative shunt dependency after craniotomies for patients with intracranial tumors in adults.
Limitations of the study
Its retrospective design is the foremost limitation of this study. Perioperative infections as potential risk for VP shunt dependency were not accounted for in the analyses. Another limitation is that our study included only craniotomies for brain tumors and did not include endonasal surgeries for removal of tumors located in the pituitary area as potential risk factor for VP shunt dependency. Postoperative hemorrhage after tumor surgery was not included in the analysis of our study. Also, temporary drainage of CSF such as lumbar drainage, insertion of external ventricular drainage prior to permanent shunting was not included in the risk factor analyses of our study, which might have affected the outcome with respect to postoperative permanent CSF shunting. Image analysis was not performed in an automatized manner, due to lack of comparability across the different imaging modalities in absence of age-adjusted normal values and due to lack of comparability across the different imaging modalities. Despite availability of CT and/or MRI for all patients included in the study, identification of the presence or absence of preoperative/postoperative hydrocephalus may have been limited by human error. Although being a large study, the number of patients may be so low in the final analyses, that statistical type II error may arise, thus failing to identify a true prognostic factor. Direct comparisons to our study were difficult as most reported studies are biased with limitations to certain patient groups, tumor histologies and/or locations.
Conclusions
In this large, contemporary, single-institution consecutive series of all adult patients with intracranial tumors requiring surgery, the incidence of preoperative HC was 7.8%. Of the total, tumor resection alone yielded a surgical successful cure rate for HC of 89.5%. The risk of shunt dependency in patients with persisting postoperative HC was only 7.0% (26/373) within 30 days and 8.9% (33/373) within 90 days, where secondary (repeat) surgery was the only statistically significant risk factor. This report can serve as benchmark for future studies.
References
Ahmadi R, Dictus C, Hartmann C, Zurn O, Edler L, Hartmann M, Combs S, Herold-Mende C, Wirtz CR, Unterberg A (2009) Long-term outcome and survival of surgically treated supratentorial low-grade glioma in adult patients. Acta Neurochir 151:1359–1365. https://doi.org/10.1007/s00701-009-0435-x
Al-Zabin M, Ullrich WO, Brawanski A, Proescholdt MA (2010) Recurrent brain metastases from lung cancer: the impact of reoperation. Acta Neurochir 152:1887–1892. https://doi.org/10.1007/s00701-010-0721-7
Ammirati M, Galicich JH, Arbit E, Liao Y (1987) Reoperation in the treatment of recurrent intracranial malignant gliomas. Neurosurgery 21:607–614
Barker FG 2nd (2004) Craniotomy for the resection of metastatic brain tumors in the U.S., 1988-2000: decreasing mortality and the effect of provider caseload. Cancer 100:999–1007. https://doi.org/10.1002/cncr.20058
Barker FG 2nd, Chang SM, Gutin PH, Malec MK, McDermott MW, Prados MD, Wilson CB (1998) Survival and functional status after resection of recurrent glioblastoma multiforme. Neurosurgery 42:709–720 discussion 720-703
Bloch O, Auguste KI, Manley GT, Verkman AS (2006) Accelerated progression of kaolin-induced hydrocephalus in aquaporin-4-deficient mice. J Cereb Blood Flow Metab 26:1527–1537. https://doi.org/10.1038/sj.jcbfm.9600306
Bognar L, Borgulya G, Benke P, Madarassy G (2003) Analysis of CSF shunting procedure requirement in children with posterior fossa tumors. Childs Nerv Syst 19:332–336. https://doi.org/10.1007/s00381-003-0745-x
Castro BA, Imber BS, Chen R, McDermott MW, Aghi MK (2017) Ventriculoperitoneal shunting for glioblastoma: risk factors, indications, and efficacy. Neurosurgery 80:421–430. https://doi.org/10.1227/NEU.0000000000001263
Chaichana KL, Zadnik P, Weingart JD, Olivi A, Gallia GL, Blakeley J, Lim M, Brem H, Quinones-Hinojosa A (2013) Multiple resections for patients with glioblastoma: prolonging survival. J Neurosurg 118:812–820. https://doi.org/10.3171/2012.9.JNS1277
Chang SM, Parney IF, McDermott M, Barker FG 2nd, Schmidt MH, Huang W, Laws ER Jr, Lillehei KO, Bernstein M, Brem H, Sloan AE, Berger M, Glioma Outcomes I (2003) Perioperative complications and neurological outcomes of first and second craniotomies among patients enrolled in the glioma outcome project. J Neurosurg 98:1175–1181. https://doi.org/10.3171/jns.2003.98.6.1175
Claus EB, Bondy ML, Schildkraut JM, Wiemels JL, Wrensch M, Black PM (2005) Epidemiology of intracranial meningioma. Neurosurgery 57:1088–1095 discussion 1088-1095
Culley DJ, Berger MS, Shaw D, Geyer R (1994) An analysis of factors determining the need for ventriculoperitoneal shunts after posterior fossa tumor surgery in children. Neurosurgery 34:402–407 discussion 407-408
Dashti SR, Baharvahdat H, Spetzler RF, Sauvageau E, Chang SW, Stiefel MF, Park MS, Bambakidis NC (2008) Operative intracranial infection following craniotomy. Neurosurg Focus 24:E10. https://doi.org/10.3171/FOC/2008/24/6/E10
Del Bigio MR, Zhang YW (1998) Cell death, axonal damage, and cell birth in the immature rat brain following induction of hydrocephalus. Exp Neurol 154:157–169. https://doi.org/10.1006/exnr.1998.6922
Dewan MC, Lim J, Shannon CN, Wellons JC 3rd (2017) The durability of endoscopic third ventriculostomy and ventriculoperitoneal shunts in children with hydrocephalus following posterior fossa tumor resection: a systematic review and time-to-failure analysis. J Neurosurg Pediatr 19:578–584. https://doi.org/10.3171/2017.1.PEDS16536
Ding Y, McAllister JP 2nd, Yao B, Yan N, Canady AI (2001) Neuron tolerance during hydrocephalus. Neuroscience 106:659–667
Dirks P, Bernstein M, Muller PJ, Tucker WS (1993) The value of reoperation for recurrent glioblastoma. Can J Surg 36:271–275
Fadul C, Wood J, Thaler H, Galicich J, Patterson RH Jr, Posner JB (1988) Morbidity and mortality of craniotomy for excision of supratentorial gliomas. Neurology 38:1374–1379
Fischer CM, Neidert MC, Peus D, Ulrich NH, Regli L, Krayenbuhl N, Woernle CM (2014) Hydrocephalus after resection and adjuvant radiochemotherapy in patients with glioblastoma. Clin Neurol Neurosurg 120:27–31. https://doi.org/10.1016/j.clineuro.2014.02.012
Fukuda M, Oishi M, Kawaguchi T, Watanabe M, Takao T, Tanaka R, Fujii Y (2007) Etiopathological factors related to hydrocephalus associated with vestibular schwannoma. Neurosurgery 61:1186–1192; discussion 1192-1183. https://doi.org/10.1227/01.neu.0000306096.61012.22
Gerganov VM, Pirayesh A, Nouri M, Hore N, Luedemann WO, Oi S, Samii A, Samii M (2011) Hydrocephalus associated with vestibular schwannomas: management options and factors predicting the outcome. J Neurosurg 114:1209–1215. https://doi.org/10.3171/2010.10.JNS1029
Gerlach R, Raabe A, Scharrer I, Meixensberger J, Seifert V (2004) Post-operative hematoma after surgery for intracranial meningiomas: causes, avoidable risk factors and clinical outcome. Neurol Res 26:61–66. https://doi.org/10.1179/016164104773026543
Harsh GR, Levin VA, Gutin PH, Seager M, Silver P, Wilson CB (1987) Reoperation for recurrent glioblastoma and anaplastic astrocytoma. Neurosurgery 21:615–621
Hart MG, Grant R, Walker M, Dickinson H (2005) Surgical resection and whole brain radiation therapy versus whole brain radiation therapy alone for single brain metastases. Cochrane Database Syst Rev 1:CD003292. https://doi.org/10.1002/14651858.CD003292.pub2
Hirayama A (1980) Histopathological study of congenital and acquired experimental hydrocephalus. Brain Dev 2:171–189
Hosainey SAM, Lassen B, Hald JK, Helseth E, Meling TR (2018) Risk factors for new-onset shunt-dependency after craniotomies for intracranial tumors in adult patients. Neurosurg Rev 41:465–472. https://doi.org/10.1007/s10143-017-0869-1
Hosainey SA, Lassen B, Helseth E, Meling TR (2014) Cerebrospinal fluid disturbances after 381 consecutive craniotomies for intracranial tumors in pediatric patients. J Neurosurg Pediatr 14:604–614. https://doi.org/10.3171/2014.8.peds13585
Hsu S-Y, Chang H-H, Shih F-Y, Lin Y-J, Lin W-C, Cheng B-C, Su Y-J, Kung C-T, Ho Y-N, Tsai N-W, Lu C-H, Wang H-C (2017) Risk factors for ventriculoperitoneal shunt dependency in patients with spontaneous cerebellar hemorrhage. World Neurosurg 105:63–68. https://doi.org/10.1016/j.wneu.2017.05.119
Jakola AS, Myrmel KS, Kloster R, Torp SH, Lindal S, Unsgard G, Solheim O (2012) Comparison of a strategy favoring early surgical resection vs a strategy favoring watchful waiting in low-grade gliomas. JAMA 308:1881–1888. https://doi.org/10.1001/jama.2012.12807
John JK, Robin AM, Pabaney AH, Rammo RA, Schultz LR, Sadry NS, Lee IY (2016) Complications of ventricular entry during craniotomy for brain tumor resection. J Neurosurg 127:1–7. https://doi.org/10.3171/2016.7.JNS16340
Juge L, Pong AC, Bongers A, Sinkus R, Bilston LE, Cheng S (2016) Changes in rat brain tissue microstructure and stiffness during the development of experimental obstructive hydrocephalus. PLoS One 11:e0148652. https://doi.org/10.1371/journal.pone.0148652
Kalfas IH, Little JR (1988) Postoperative hemorrhage: a survey of 4992 intracranial procedures. Neurosurgery 23:343–347
Keles GE, Chang EF, Lamborn KR, Tihan T, Chang CJ, Chang SM, Berger MS (2006) Volumetric extent of resection and residual contrast enhancement on initial surgery as predictors of outcome in adult patients with hemispheric anaplastic astrocytoma. J Neurosurg 105:34–40. https://doi.org/10.3171/jns.2006.105.1.34
Kim SS, McCutcheon IE, Suki D, Weinberg JS, Sawaya R, Lang FF, Ferson D, Heimberger AB, DeMonte F, Prabhu SS (2009) Awake craniotomy for brain tumors near eloquent cortex: correlation of intraoperative cortical mapping with neurological outcomes in 309 consecutive patients. Neurosurgery 64:836–845; discussion 345-836. https://doi.org/10.1227/01.NEU.0000342405.80881.81
Korinek AM (1997) Risk factors for neurosurgical site infections after craniotomy: a prospective multicenter study of 2944 patients. The French Study Group of Neurosurgical Infections, the SEHP, and the C-CLIN Paris-Nord. Service Epidemiologie Hygiene et Prevention. Neurosurgery 41:1073–1079 discussion 1079-1081
Kulkarni AV, Drake JM, Kestle JR, Mallucci CL, Sgouros S, Constantini S, Canadian Pediatric Neurosurgery Study G (2010) Endoscopic third ventriculostomy vs cerebrospinal fluid shunt in the treatment of hydrocephalus in children: a propensity score-adjusted analysis. Neurosurgery 67:588–593. https://doi.org/10.1227/01.NEU.0000373199.79462.21
Landy HJ, Feun L, Schwade JG, Snodgrass S, Lu Y, Gutman F (1994) Retreatment of intracranial gliomas. South Med J 87:211–214
Lassen B, Helseth E, Egge A, Due-Tonnessen BJ, Ronning P, Meling TR (2012) Surgical mortality and selected complications in 273 consecutive craniotomies for intracranial tumors in pediatric patients. Neurosurgery 70:936–943; discussion 943. https://doi.org/10.1227/NEU.0b013e31823bcc61
Lassen B, Helseth E, Ronning P, Scheie D, Johannesen TB, Maehlen J, Langmoen IA, Meling TR (2011) Surgical mortality at 30 days and complications leading to recraniotomy in 2630 consecutive craniotomies for intracranial tumors. Neurosurgery 68:1259–1268; discussion 1268-1259. https://doi.org/10.1227/NEU.0b013e31820c0441
Lee M, Wisoff JH, Abbott R, Freed D, Epstein FJ (1994) Management of hydrocephalus in children with medulloblastoma: prognostic factors for shunting. Pediatr Neurosurg 20:240–247
Lietard C, Thebaud V, Besson G, Lejeune B (2008) Risk factors for neurosurgical site infections: an 18-month prospective survey. J Neurosurg 109:729–734. https://doi.org/10.3171/JNS/2008/109/10/0729
Mandl ES, Dirven CM, Buis DR, Postma TJ, Vandertop WP (2008) Repeated surgery for glioblastoma multiforme: only in combination with other salvage therapy. Surg Neurol 69:506–509; discussion 509. https://doi.org/10.1016/j.surneu.2007.03.043
Marx S, Reinfelder M, Matthes M, Schroeder HWS, Baldauf J (2018) Frequency and treatment of hydrocephalus prior to and after posterior fossa tumor surgery in adult patients. Acta Neurochir 160:1063–1071. https://doi.org/10.1007/s00701-018-3496-x
McGirt MJ, Chaichana KL, Gathinji M, Attenello FJ, Than K, Olivi A, Weingart JD, Brem H, Quinones-Hinojosa AR (2009) Independent association of extent of resection with survival in patients with malignant brain astrocytoma. J Neurosurg 110:156–162. https://doi.org/10.3171/2008.4.17536
Mirimanoff RO, Dosoretz DE, Linggood RM, Ojemann RG, Martuza RL (1985) Meningioma: analysis of recurrence and progression following neurosurgical resection. J Neurosurg 62:18–24. https://doi.org/10.3171/jns.1985.62.1.0018
Miyakoshi A, Kohno M, Nagata O, Sora S, Sato H (2013) Hydrocephalus associated with vestibular schwannomas: perioperative changes in cerebrospinal fluid. Acta Neurochir 155:1271–1276. https://doi.org/10.1007/s00701-013-1742-9
Montano N, D'Alessandris QG, Bianchi F, Lauretti L, Doglietto F, Fernandez E, Maira G, Pallini R (2011) Communicating hydrocephalus following surgery and adjuvant radiochemotherapy for glioblastoma. J Neurosurg 115:1126–1130. https://doi.org/10.3171/2011.8.JNS11738
Nanda A, Javalkar V, Banerjee AD (2011) Petroclival meningiomas: study on outcomes, complications and recurrence rates. J Neurosurg 114:1268–1277. https://doi.org/10.3171/2010.11.JNS10326
Nitta T, Sato K (1995) Prognostic implications of the extent of surgical resection in patients with intracranial malignant gliomas. Cancer 75:2727–2731
Pirouzmand F, Tator CH, Rutka J (2001) Management of hydrocephalus associated with vestibular schwannoma and other cerebellopontine angle tumors. Neurosurgery 48:1246–1253 discussion 1253-1244
Ramakrishna R, Hebb A, Barber J, Rostomily R, Silbergeld D (2015) Outcomes in reoperated low-grade gliomas. Neurosurgery 77:175–184; discussion 184. https://doi.org/10.1227/NEU.0000000000000753
Reddy GK, Bollam P, Caldito G, Willis B, Guthikonda B, Nanda A (2011) Ventriculoperitoneal shunt complications in hydrocephalus patients with intracranial tumors: an analysis of relevant risk factors. J Neuro-Oncol 103:333–342. https://doi.org/10.1007/s11060-010-0393-4
Rinaldo L, Brown D, Lanzino G, Parney IF (2017) Outcomes following cerebrospinal fluid shunting in high-grade glioma patients. J Neurosurg 1–13. https://doi.org/10.3171/2017.6.JNS17859. https://www.ncbi.nlm.nih.gov/pubmed/29271709. Accessed 18 June 2018
Riva-Cambrin J, Detsky AS, Lamberti-Pasculli M, Sargent MA, Armstrong D, Moineddin R, Cochrane DD, Drake JM (2009) Predicting postresection hydrocephalus in pediatric patients with posterior fossa tumors. J Neurosurg Pediatr 3:378–385. https://doi.org/10.3171/2009.1.PEDS08298
Rossitti S (2013) Pathophysiology of increased cerebrospinal fluid pressure associated to brain arteriovenous malformations: the hydraulic hypothesis. Surg Neurol Int 4:42. https://doi.org/10.4103/2152-7806.109657
Rostomily RC, Spence AM, Duong D, McCormick K, Bland M, Berger MS (1994) Multimodality management of recurrent adult malignant gliomas: results of a phase II multiagent chemotherapy study and analysis of cytoreductive surgery. Neurosurgery 35:378–388 discussion 388
Roth J, Constantini S, Blumenthal DT, Ram Z (2008) The value of ventriculo-peritoneal shunting in patients with glioblastoma multiforme and ventriculomegaly. Acta Neurochir 150:41–46; discussion 46-47. https://doi.org/10.1007/s00701-007-1454-0
Sainte-Rose C, Cinalli G, Roux FE, Maixner R, Chumas PD, Mansour M, Carpentier A, Bourgeois M, Zerah M, Pierre-Kahn A, Renier D (2001) Management of hydrocephalus in pediatric patients with posterior fossa tumors: the role of endoscopic third ventriculostomy. J Neurosurg 95:791–797. https://doi.org/10.3171/jns.2001.95.5.0791
Santos de Oliveira R, Barros Juca CE, Valera ET, Machado HR (2008) Hydrocephalus in posterior fossa tumors in children. Are there factors that determine a need for permanent cerebrospinal fluid diversion? Childs Nerv Syst 24:1397–1403. https://doi.org/10.1007/s00381-008-0649-x
Sawaya R, Hammoud M, Schoppa D, Hess KR, Wu SZ, Shi WM, Wildrick DM (1998) Neurosurgical outcomes in a modern series of 400 craniotomies for treatment of parenchymal tumors. Neurosurgery 42:1044–1055 discussion 1055-1046
Suchorska B, Weller M, Tabatabai G, Senft C, Hau P, Sabel MC, Herrlinger U, Ketter R, Schlegel U, Marosi C, Reifenberger G, Wick W, Tonn JC, Wirsching HG (2016) Complete resection of contrast-enhancing tumor volume is associated with improved survival in recurrent glioblastoma-results from the DIRECTOR trial. Neuro-Oncology 18:549–556. https://doi.org/10.1093/neuonc/nov326
Sundaresan N, Sachdev VP, DiGiacinto GV, Hughes JE (1988) Reoperation for brain metastases. J Clin Oncol 6:1625–1629. https://doi.org/10.1200/JCO.1988.6.10.1625
Thiessen B, DeAngelis LM (1998) Hydrocephalus in radiation leukoencephalopathy: results of ventriculoperitoneal shunting. Arch Neurol 55:705–710
Tully PA, Gogos AJ, Love C, Liew D, Drummond KJ, Morokoff AP (2016) Reoperation for recurrent glioblastoma and its association with survival benefit. Neurosurgery 79:678–689. https://doi.org/10.1227/NEU.0000000000001338
Vecht CJ, Avezaat CJ, van Putten WL, Eijkenboom WM, Stefanko SZ (1990) The influence of the extent of surgery on the neurological function and survival in malignant glioma. A retrospective analysis in 243 patients. J Neurol Neurosurg Psychiatry 53:466–471
von Lehe M, Kim HJ, Schramm J, Simon M (2013) A comprehensive analysis of early outcomes and complication rates after 769 craniotomies in pediatric patients. Childs Nerv Syst 29:781–790. https://doi.org/10.1007/s00381-012-2006-3
Wallner KE, Galicich JH, Malkin MG, Arbit E, Krol G, Rosenblum MK (1989) Inability of computed tomography appearance of recurrent malignant astrocytoma to predict survival following reoperation. J Clin Oncol 7:1492–1496. https://doi.org/10.1200/JCO.1989.7.10.1492
Yong RL, Wu T, Mihatov N, Shen MJ, Brown MA, Zaghloul KA, Park GE, Park JK (2014) Residual tumor volume and patient survival following reoperation for recurrent glioblastoma. J Neurosurg 121:802–809. https://doi.org/10.3171/2014.6.JNS132038
Funding
There are no financial disclosures or financial interests for the work reported in this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical approval
The study has been approved by the institutional ethics committee (Personvernombudets tilrådning 2013/14574).
Informed consent
Informed consent was waived in accordance with the institutional regulations of the institutional ethics committee.
Rights and permissions
About this article
Cite this article
Hosainey, S.A.M., Lassen, B., Hald, J.K. et al. The effect of tumor removal via craniotomies on preoperative hydrocephalus in adult patients with intracranial tumors. Neurosurg Rev 43, 141–151 (2020). https://doi.org/10.1007/s10143-018-1021-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-018-1021-6