Abstract
The risk of developing a de novo shunt-dependent hydrocephalus (HC) after undergoing a craniotomy for brain tumor in adult patients is largely unknown. All craniotomies for intracranial tumors at Oslo University Hospital in adult patients ≥18 years of age during a 10-year period (2004–2013) were included. None were lost to follow-up. Patients who developed a shunt-dependent HC were identified by cross-linking our prospectively collected tumor database to patients with a NCSP surgical procedure code of hydrocephalus (AAF). Patients with pre-existing HC or ventriculoperitoneal (VP) shunts were excluded from the study. A total of 4401 craniotomies were performed. Of these, 46 patients (1.0%) developed de novo postoperative HC requiring a VP shunt after a median of 93 days (mean 115 days, range 6–442). Median age was 62.0 years (mean 58.9 years, range 27.3–80.9) at time of VP shunt surgery. Patients without pre-existing HC had a 0.2% (n = 8/4401) risk of becoming VP shunt dependent within 30 days and 0.5% (n = 22/4401) within 90 days. Age, sex, tumor location, primary/secondary surgery, and radiotherapy were not associated with VP shunt dependency. Choroid plexus tumors and craniopharyngiomas had increased risk of VP shunt dependency. In this large, contemporary, single-institution consecutive series, the risk of postoperative shunt-dependency after craniotomies for brain tumors without pre-existing HC was very low. This is the largest study with regards to de novo postoperative shunt-dependency after craniotomies for patients with intracranial tumors and can serve as a benchmark for future studies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Neurosurgical treatment of brain tumors aims at symptom relief, improved quality of life, reduction of tumor burden prior to adjuvant therapy, and improved survival, but also to establish an exact tissue diagnosis [1,2,3,4,5,6,7]. However, craniotomies are not without inherent risks, be they surgical mortality [8,9,10], postoperative hematomas [10,11,12], infections [10, 13], postoperative infarctions and neurological deficits [14], or post-craniotomy CSF disturbances that lead to permanent changes to CSF circulation dynamics [14, 15].
Hydrocephalus (HC) after craniotomies for intracranial tumors have been reported in the past, but these have been limited to specific tumor histologies and/or tumor locations [16,17,18]. Studies on risk factors leading to permanent CSF–diversion after intracranial tumors are scarce, but in the pediatric population risk factors reported include younger age [19, 20], surgical approach [15], tumor histology, and infections [15, 19, 21]. However, there is a lack of knowledge in contemporary literature regarding the incidence of new-onset HC development after undergoing a craniotomy in adult patients without preoperative HC. In this study, based on our prospectively collected database of all adult patients who underwent craniotomies for intracranial tumors in a 10-year period, we wanted to identify the incidence of, and risk factors for, de novo shunt-dependency after craniotomies for intracranial tumors in adult patients.
Materials and methods
Data collection
By reviewing our prospectively kept single-center database of consecutive patients with CNS tumors, we identified adult patients treated over a 10-year period (2004–2013).
The following data were recorded: age, sex, preoperative HC, tumor location, primary or secondary (repeated) resection, tumor histology, de novo postoperative HC, post-craniotomy VP-shunt (yes/no), days from craniotomy to shunt implantation, and postoperative radiation-therapy (yes/no).
The preoperative images (CT and/or MRI) of the cases were reviewed and evaluated by an experienced neuroradiologist and neurosurgeon at time of surgery and reviewed retrospectively by the first authors (SAMH and BL) for the presence/absence of preoperative HC, defined as radiological signs of ventricular enlargement requiring surgical intervention. HC was identified on CT or MRI as prominent temporal horns, a transverse diameter of third ventricle >5 mm, ballooning of frontal horn with periventricular hypodensity on CT or T2 W MRI, and in instances of doubt, the ventricular Evans index was used. Tumor location was dichotomized into supratentorial and infratentorial.
Primary craniotomy was defined as the first craniotomy in a specific location, while all subsequent craniotomies in the same location were defined as secondary. Hence, a patient could have had more than one primary craniotomy, if operated on multiple locations.
To identify patients who developed new-onset VP-shunt dependent HC, we cross-linked the tumor database with our surgical procedure codes database using the Nordic Medico-Statistical Committee Classification of Surgical Procedures (NCSP) codes for CSF-related procedures (codes AAF).
Tumor histology was retrieved from our tumor database and grouped accordingly with respect craniotomies without and with new-onset postoperative HC.
With regards to postoperative radiotherapy, time to VP shunt surgery was dichotomized into within 90 days and more than 90 days post-craniotomy for comparison.
Statistical analysis
Comparison between categorical variables were performed with Chi-square (X 2) test and Fisher’s exact test. Analysis of variance or independent samples t test were used for continuous variables. An analysis of means of proportions was conducted to detect tumor histologies associated with significantly higher risk of VP shunt dependency. The Kaplan-Meier method was used for time to VP shunt surgery, and the log-rank test was conducted to compare VP shunt dependency to different variables. Box-plot was used supplementary to visualize and compare radiotherapy to VP shunt dependency. A p value <0.05 was considered statistically significant. For all statistical analysis, the software program JMP (version 9.03, SAS Institute Inc.) was used.
Results
Population data
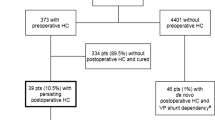
In this study, 4815 craniotomies for intracranial tumors were performed in a total of 4204 adult patients. Excluded from further analysis were craniotomies in patients with pre-existing VP-shunts or pre-existing HC, leaving 4401 craniotomies (Fig. 1, Table 1). No patients were lost to follow-up.
Median patient age at time of craniotomy for intracranial tumor resection was 58.2 years (mean 56.3 years, range 18.0–92.4). There were 2161 males (49.1%) and 2240 females (50.9%).
Preoperative MRI was available for 3645 craniotomies (82.8%), while 756 (17.2%) had only preoperative CT scans.
The tumors were located supratentorially in 3938 of the craniotomies (89.5%) performed, while 463 of the tumors (10.5%) were located infratentorially.
Primary craniotomies were performed in 3628 cases (82.4%), while 773 (17.6%) were secondary craniotomies.
New-onset postoperative HC
Of the 4401 craniotomies performed in patients without preoperative HC, 46 patients (1%) needed a permanent CSF shunt due to de novo postoperative HC (Fig. 1, Table 2). The final ratio between craniotomies and patients was 1:1. Median age at time of VP shunt surgery was 62.0 years (mean 58.9 years, range 27.3–80.9). Neither age (p = 0.2) nor sex (p = 0.6) were significantly associated with postoperative VP shunt-dependency (Table 1).
Tumor location
Of the 46 patients with postoperative VP shunt-dependency, 44 patients (95.6%) had tumors located in the supratentorial compartment, while 2 had infratentorial tumors (4.4%) (Table 1). There was no significant association between VP shunt-dependency and tumor location (p = 0.1).
Primary vs secondary surgery
Thirty-four patients (73.9%) underwent primary craniotomy, while 12 had secondary surgery (26.1%) (Table 1). Undergoing a secondary craniotomy was not significantly associated with postoperative VP shunt-dependency (p = 0.2).
Tumor histology
Out of the 46 patients with postoperative VP shunt-dependency, the tumor histologies were 13 metastatic tumors (28.3%), 12 meningiomas (26.1%), 11 high grade gliomas (23.9%), 4 other tumors (8.7%), 2 craniopharyngiomas (4.3%), 2 schwannomas (4.3%), 1 choroid plexus tumors (2.2%), and 1 pituitary adenoma (2.2%) (Table 1).
Radiotherapy
Twenty-two out of 46 (47.8%) patients with de novo postoperative HC underwent postoperative radiotherapy. Postoperative radiotherapy was not significantly associated with VP shunt-dependency (p = 0.2) (Table 1).
With respect to timing of VP-shunting relative to radiotherapy, 14/22 patients had VP shunt implantation more than 90 days after surgery, while 8/22 patients had within 90 days post-craniotomy (Table 2). This difference was not statistically significant (Fig. 4).
Timeline
The patients with de novo postoperative HC received VP shunts after a median of 93 days (mean 115 days, range 6–442) (Fig. 2). The cumulative risk of becoming VP shunt dependent in patients without pre-existing HC was 0.2% (n = 8/4401) within 30 days and 0.5% (n = 22/4401) within 90 days. Twenty-four out of 46 patients (0.5%) had very late VP shunt implantation (>90 days postoperatively) (Table 1).
Discussion
During the study period, a total of 4815 craniotomies for intracranial tumors were performed in adult patients. Patients with pre-existing VP-shunts and patients with pre-existing HC were excluded from further analysis, as their inclusion would have overestimated the true risk of developing a de novo postoperative HC due to the craniotomy per se.
The cumulative risk of developing a de novo shunt-dependent HC postoperatively was 0.2% (n = 8/4401) within 30 days and 0.5% (n = 22/4401) within 90 days. Interestingly, we did not find that age, sex, or tumor location was significantly associated with increased risk of ultimately becoming shunt-dependent, in contrast to a similar study by Hosainey et al. where younger age and infratentorial tumor location were significant risk factors for postoperative HC in pediatric patients [15]. Although limited to only benign tumors and different risk factors were considered, Bekelis et al. reported postoperative-treated HC rate of 4.2%, which was linked to surgical intervention [22]. In their study, increased atherosclerotic burden (prior stroke, coronary artery disease) and coagulopathy were associated with higher rate of HC. However, the time from surgical intervention until HC treatment was not stated. A theoretical explanation for development of new-onset postoperative HC in our study can possibly be related to the tumor burden on the brain, which may results in altered venous outflow and changes to intracerebral hemodynamics [23, 24], even after tumor resection.
In our study, repeat surgery was not significantly associated with shunt-dependent postoperative HC, in contrast to a study by Montano et al. where ≥2 craniotomies for GBM removal was performed in 45 of 124 patients (36.2%) and all patients developed communicating HC (p = 0.0006). Additionally, ventricular opening was a risk factor for communicating hydrocephalus when the analysis was restricted to patients undergoing ≥2 operations for tumor removal [17]. However, their study was restricted to patients who underwent surgery and adjuvant radio-chemotherapy for glioblastoma.
With respect to histology, the highest risk for de novo postoperative VP shunt-dependency was found in choroid plexus tumors (12.5%) and craniopharyngiomas (6.5%), both of which were significant (Fig. 3). These tumors often extend into ventricular compartments, and craniotomies with ventricular entry for tumor resection has been reported to be associated with increased risk of postoperative HC [16, 17, 25].
Analysis of means of proportions for histology and VP shunt dependency. Shaded area represents the 95% confidence interval for the proportion of VP shunt dependency for each histology type. Red dots indicate statistical significance at 5% level, green dots represent non-significance. Choroid plexus tumors and craniopharyngiomas showed significant association with VP shunt dependency. HGG high-grade glioma, LGG low-grade glioma, PNET primitive neuro-ectodermal tumor
There were only 2 of 90 patients (2.2%) with vestibular schwannomas who became shunt-dependent in our study. This is comparable and slightly higher than the rate of shunting in a study by Pirouzmand et al. [18], where only 2 of 173 patients (1%) whom underwent microsurgical removal of their tumors had new-onset postoperative HC. However, this study included only patients with vestibular schwannomas.
In our series, only 12 of 1161 patients (1.0%) with meningiomas became shunt-dependent. In the literature, both intraventricular and skull base meningiomas have been reported to be associated with shunt-dependency [26, 27]. Burkhardt et al. reported de novo postoperative HC in 35 out of 594 patients (5.9%), but limited their analysis to skull base meningiomas [26]. Some published studies have not found an association between tumor histology and postoperative shunting [18, 19], whereas others have [20, 28]. These studies however, are limited by patient group, tumor location, and specific histologies.
Patients with de novo postoperative HC received VP-shunts after a median of 93.0 days (mean 115 days, range 6–442) (Fig. 2). The majority of patients that required VP shunting more than 90 days postoperatively had either high-grade gliomas or metastatic tumors (52.2%). As they received postoperative chemotherapy and/or radiotherapy, these patients might be considered to have become shunt-dependent because of the adjuvant therapy rather than the surgical intervention. However, we did not find radiotherapy as a statistically significant risk factor for developing shunt-dependency (p = 0.2), although there was a trend towards VP shunting >90 days post-craniotomy and radiotherapy (Fig. 4). Radiation-induced HC has been reported with respect to brain tumors [29,30,31], albeit limited by patient group and histology. Furthermore, these studies have mainly been concerned with clinical outcome. In their study of 257 patients where 21 patients (8%) developed postoperative HC and all required shunting, Duong et al. reported that prior radiation therapy increased risk of developing HC, but this study was limited to cranial base surgeries for tumor resections [32]. Inamasu et al. reported that of the 50 patients with supratentorial malignant glioma patients receiving fractionated whole-brain radiotherapy, 5 patients (10%) developed communicating HC postoperatively and were treated with shunt-surgery from 2 to 15 months after surgery [33]. Nonetheless, this study assessed mainly clinical outcome, similar other studies [34, 35]. Elevated CSF pressure resulting in HC due to fibrosis of arachnoid granulations that reduce CSF outflow after radiotherapy has been reported [31]. Montano et al. reported in their study regarding communicating hydrocephalus following surgery and adjuvant radio-chemotherapy for glioblastoma patients that 7 out of 124 patients (5.6%) developed HC requiring VP-shunts [17]. Likewise, in the aforementioned study by Fischer et al., 11 out of 154 patients (7.1%) received VP shunts after surgery for glioblastoma, where 4 and 7 patients received VP shunts before and after radiation therapy, respectively [16]. Median time to VP shunt implantation after initial surgery (tumor resection) was 110 days. These studies however, were limited to glioblastoma patients only.
Timing of VP shunt and radiation therapy (black horizontal line shows mean days to VP shunt surgery, 115 days). Red box-plot shows interquartile ranges for each group with their corresponding group median (horizontal red line in the middle of the boxes). Green diamond plot represents summary measure and the 95% confidence interval for each group with their corresponding group mean (horizontal green line inside each diamond plot in the middle). Blue connecting line of the means from both groups is shown for comparison
Strength of the study
The total number of patients and restriction of data to one centralized health-care center (Oslo University Hospital), thereby reducing confounding effects of differences in medical practices and health care access, are the main strengths of this study. We have also avoided the selection bias inherently present in large multicenter studies. The neurosurgical catchment area is a well-defined geographical area and no patients were lost to follow-up. We did not find any other large-scale studies with primary outcome of determining shunt-dependency by including all intracranial tumors regardless of patient group, histology, or tumor locations to compare our study with, which demonstrates the uniqueness of our study. There is no selection bias as the study includes all craniotomies performed for a histologically verifiable intracranial tumor within the study period. Our study design is a retrospective analysis of a prospectively collected database. Lastly, by crosslinking our tumor database with the registry for neurosurgical procedures, we have included all craniotomies targeting the primary outcome of shunt-dependency.
Limitations of the study
The retrospective collection of data not included in our prospective database is the foremost limitation of this study. In absence of age-adjusted normal values and due to lack of comparability across the different imaging modalities, image analysis was not performed in an automatized manner. Even though imaging with CT and/or MRI was available for all patients included in the study, identification of the presence or absence of preoperative hydrocephalus may have been prone to differences in analyzing the images depending on the observer. Other preoperative variables such as tumor size, peritumoral edema, postoperative infections, ventricular entry, and adjuvant chemotherapy were not included in the analyses, which may impact the status of postoperative HC in patients whom underwent tumor resection. Despite being a large study, the low number of patients with postoperative HC may not be sufficient to avoid a statistical type II error, thus failing to identify true prognostic factors. Most published studies are limited to a certain patient group, tumor histologies and/or tumor locations, thereby making direct comparisons to our study difficult.
Conclusions
In this large, contemporary, single-institution consecutive series, the risk of postoperative shunt-dependency after craniotomies for brain tumors in adult patients without pre-existing HC was only 0.2% (8/4401) within 30 days and 0.5% (22/4401) within 90 days. This report can serve as a benchmark for future studies.
References
Claus EB, Bondy ML, Schildkraut JM, Wiemels JL, Wrensch M, Black PM (2005) Epidemiology of intracranial meningioma. Neurosurgery 57:1088–1095 discussion 1088-1095
Hart MG, Grant R, Walker M, Dickinson H (2005) Surgical resection and whole brain radiation therapy versus whole brain radiation therapy alone for single brain metastases. Cochrane Database Syst Rev:CD003292. doi:10.1002/14651858.CD003292.pub2
Keles GE, Chang EF, Lamborn KR, Tihan T, Chang CJ, Chang SM, Berger MS (2006) Volumetric extent of resection and residual contrast enhancement on initial surgery as predictors of outcome in adult patients with hemispheric anaplastic astrocytoma. J Neurosurg 105:34–40. doi:10.3171/jns.2006.105.1.34
McGirt MJ, Chaichana KL, Gathinji M, Attenello FJ, Than K, Olivi A, Weingart JD, Brem H, Quinones-Hinojosa AR (2009) Independent association of extent of resection with survival in patients with malignant brain astrocytoma. J Neurosurg 110:156–162. doi:10.3171/2008.4.17536
Mirimanoff RO, Dosoretz DE, Linggood RM, Ojemann RG, Martuza RL (1985) Meningioma: analysis of recurrence and progression following neurosurgical resection. J Neurosurg 62:18–24. doi:10.3171/jns.1985.62.1.0018
Nitta T, Sato K (1995) Prognostic implications of the extent of surgical resection in patients with intracranial malignant gliomas. Cancer 75:2727–2731
Vecht CJ, Avezaat CJ, van Putten WL, Eijkenboom WM, Stefanko SZ (1990) The influence of the extent of surgery on the neurological function and survival in malignant glioma. A retrospective analysis in 243 patients. J Neurol Neurosurg Psychiatry 53:466–471
Barker FG 2nd (2004) Craniotomy for the resection of metastatic brain tumors in the U.S., 1988-2000: decreasing mortality and the effect of provider caseload. Cancer 100:999–1007. doi:10.1002/cncr.20058
Fadul C, Wood J, Thaler H, Galicich J, Patterson RH Jr, Posner JB (1988) Morbidity and mortality of craniotomy for excision of supratentorial gliomas. Neurology 38:1374–1379
Lassen B, Helseth E, Ronning P, Scheie D, Johannesen TB, Maehlen J, Langmoen IA, Meling TR (2011) Surgical mortality at 30 days and complications leading to recraniotomy in 2630 consecutive craniotomies for intracranial tumors. Neurosurgery 68:1259–1268; discussion 1268-1259. doi:10.1227/NEU.0b013e31820c0441
Gerlach R, Raabe A, Scharrer I, Meixensberger J, Seifert V (2004) Post-operative hematoma after surgery for intracranial meningiomas: causes, avoidable risk factors and clinical outcome. Neurol Res 26:61–66. doi:10.1179/016164104773026543
Kalfas IH, Little JR (1988) Postoperative hemorrhage: a survey of 4992 intracranial procedures. Neurosurgery 23:343–347
Mahaley MS Jr, Mettlin C, Natarajan N, Laws ER Jr, Peace BB (1989) National survey of patterns of care for brain-tumor patients. J Neurosurg 71:826–836. doi:10.3171/jns.1989.71.6.0826
Lassen B, Helseth E, Egge A, Due-Tonnessen BJ, Ronning P, Meling TR (2012) Surgical mortality and selected complications in 273 consecutive craniotomies for intracranial tumors in pediatric patients. Neurosurgery 70:936–943; discussion 943. doi:10.1227/NEU.0b013e31823bcc61
Hosainey SA, Lassen B, Helseth E, Meling TR (2014) Cerebrospinal fluid disturbances after 381 consecutive craniotomies for intracranial tumors in pediatric patients. J Neurosurg Pediatr 14:604–614. doi:10.3171/2014.8.peds13585
Fischer CM, Neidert MC, Peus D, Ulrich NH, Regli L, Krayenbuhl N, Woernle CM (2014) Hydrocephalus after resection and adjuvant radiochemotherapy in patients with glioblastoma. Clin Neurol Neurosurg 120:27–31. doi:10.1016/j.clineuro.2014.02.012
Montano N, D'Alessandris QG, Bianchi F, Lauretti L, Doglietto F, Fernandez E, Maira G, Pallini R (2011) Communicating hydrocephalus following surgery and adjuvant radiochemotherapy for glioblastoma. J Neurosurg 115:1126–1130. doi:10.3171/2011.8.JNS11738
Pirouzmand F, Tator CH, Rutka J (2001) Management of hydrocephalus associated with vestibular schwannoma and other cerebellopontine angle tumors. Neurosurgery 48:1246–1253 discussion 1253-1244
Culley DJ, Berger MS, Shaw D, Geyer R (1994) An analysis of factors determining the need for ventriculoperitoneal shunts after posterior fossa tumor surgery in children. Neurosurgery 34:402–407 discussion 407-408
Riva-Cambrin J, Detsky AS, Lamberti-Pasculli M, Sargent MA, Armstrong D, Moineddin R, Cochrane DD, Drake JM (2009) Predicting postresection hydrocephalus in pediatric patients with posterior fossa tumors. J Neurosurg Pediatr 3:378–385. doi:10.3171/2009.1.PEDS08298
Lee M, Wisoff JH, Abbott R, Freed D, Epstein FJ (1994) Management of hydrocephalus in children with medulloblastoma: prognostic factors for shunting. Pediatr Neurosurg 20:240–247
Bekelis K, Kalakoti P, Nanda A, Missios S (2015) A predictive model of unfavorable outcomes after benign intracranial tumor resection. World Neurosurg 84:82–89. doi:10.1016/j.wneu.2015.02.032
Rossitti S (2013) Pathophysiology of increased cerebrospinal fluid pressure associated to brain arteriovenous malformations: the hydraulic hypothesis. Surg Neurol Int 4:42. doi:10.4103/2152-7806.109657
Williams H (2008) The venous hypothesis of hydrocephalus. Med Hypotheses 70:743–747. doi:10.1016/j.mehy.2007.08.013
John JK, Robin AM, Pabaney AH, Rammo RA, Schultz LR, Sadry NS, Lee IY (2016) Complications of ventricular entry during craniotomy for brain tumor resection. J Neurosurg:1–7. doi:10.3171/2016.7.JNS16340
Burkhardt JK, Zinn PO, Graenicher M, Santillan A, Bozinov O, Kasper EM, Krayenbuhl N (2011) Predicting postoperative hydrocephalus in 227 patients with skull base meningioma. Neurosurg Focus 30:E9. doi:10.3171/2011.3.FOCUS117
Ma J, Cheng L, Wang G, Lin S (2014) Surgical management of meningioma of the trigone area of the lateral ventricle. World Neurosurg 82:757–769. doi:10.1016/j.wneu.2014.05.026
Bognar L, Borgulya G, Benke P, Madarassy G (2003) Analysis of CSF shunting procedure requirement in children with posterior fossa tumors. Childs Nerv Syst 19:332–336. doi:10.1007/s00381-003-0745-x
Abay EO 2nd, Laws ER Jr, Grado GL, Bruckman JE, Forbes GS, Gomez MR, Scott M (1981) Pineal tumors in children and adolescents. Treatment by CSF shunting and radiotherapy. J Neurosurg 55:889–895. doi:10.3171/jns.1981.55.6.0889
Shaw EG, Evans RG, Scheithauer BW, Ilstrup DM, Earle JD (1987) Postoperative radiotherapy of intracranial ependymoma in pediatric and adult patients. Int J Radiat Oncol Biol Phys 13:1457–1462
Thiessen B, DeAngelis LM (1998) Hydrocephalus in radiation leukoencephalopathy: results of ventriculoperitoneal shunting. Arch Neurol 55:705–710
Duong DH, O'Malley S, Sekhar LN, Wright DG (2000) Postoperative hydrocephalus in cranial base surgery. Skull Base Surg 10:197–200
Inamasu J, Nakamura Y, Saito R, Kuroshima Y, Mayanagi K, Orii M, Ichikizaki K (2003) Postoperative communicating hydrocephalus in patients with supratentorial malignant glioma. Clin Neurol Neurosurg 106:9–15
Marquardt G, Setzer M, Lang J, Seifert V (2002) Delayed hydrocephalus after resection of supratentorial malignant gliomas. Acta Neurochir 144:227–231; discussion 231. doi:10.1007/s007010200030
Roth J, Constantini S, Blumenthal DT, Ram Z (2008) The value of ventriculo-peritoneal shunting in patients with glioblastoma multiforme and ventriculomegaly. Acta Neurochir 150:41–46; discussion 46-47. doi:10.1007/s00701-007-1454-0
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study has been approved by the institutional ethics committee (Personvernombudets tilrådning 2013/14574).
Rights and permissions
About this article
Cite this article
Hosainey, S.A.M., Lassen, B., Hald, J.K. et al. Risk factors for new-onset shunt-dependency after craniotomies for intracranial tumors in adult patients. Neurosurg Rev 41, 465–472 (2018). https://doi.org/10.1007/s10143-017-0869-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-017-0869-1