Abstract
Traumatic injury to the spinal cord remains a catastrophic event that has lifelong consequences. While decades of research have elucidated much of the pathophysiology associated with spinal cord injury (SCI), there still remains no clinically approved treatments for restoring lost sensorimotor function. The traditional dogma suggests central nervous system (CNS) neurons do not regenerate after injury but active areas of research aim to overcome this biological bottleneck. One particular approach using low-level direct current electric fields (DC EFs) appears especially promising based on a rich set of experimental data. This review highlights the biological basis for EF-induced regeneration and discusses the pre-clinical and clinical trials using the oscillating field stimulator (OFS)—a medical device designed to deliver DC EFs in vivo. I further report ongoing developments in our laboratory that refreshes the OFS concept with the hope of renewing interest in conducting additional clinical trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spinal cord injuries (SCI) remain a devastating pathology with significant physical, emotional, and psychological consequences. It is estimated that about 17,500 new spinal cord injuries occur each year, adding to a pool of ~300,000 patients with existing injuries (National SCI Statistical Center, available at: https://www.nscisc.uab.edu/Public/Facts%202015.pdf). Besides the multi-modal toll on the body and mind, it has been estimated that lifetime costs associated with SCI range from $2.1 to $5.4Mil if the injury occurs at age 25 [1]. To date, there are no approved treatments to resolve the functional deficits arising from SCI. Developing therapeutics that improve quality of life remains an unsolved grand challenge in medicine. The pathophysiology of SCI is complex and evolves temporally. Cellular players involving inflammatory cells, glia, and neurons play different roles during the stages of primary (initial mechanical/chemical insult) and secondary injury (biochemical events that exacerbate the primary lesion). Not surprisingly, various treatments have been researched that target these events and several have gone through human trials. Such experimental therapies have included the use of methylprednisolone [2,3,4], gangliosides [5], and minocycline [6]. In addition, cellular transplantation trials with stem cells, [7, 8] Schwann cells [9], activated macrophages [10], and olfactory ensheathing cells [11, 12] have been investigated. A few have shown potential but none has translated into a standard of care.
In this review, I discuss the use of direct current electric fields (DC EF) as a potential therapeutic for regenerating damaged tissues post-SCI. It is useful to differentiate low-level DC fields from other forms of electrical stimulation techniques such as brain, epidural, and functional electrical stimulation. In those instances, pulsed or AC waveforms are often employed to induce neuralplasticity or to directly stimulate peripheral nerves/muscles (see [13] for an overview). In the present context, the exogenous DC currents are below the threshold for neuronal activation, are steady, and mimic the endogenous flow of currents during biologic phenomena such as embryonic development and wound healing. Unlike neuromodulatory methods, these currents are initiated during the sub-acute phase (< 18 days) of spinal cord injury and last for approximately 15 weeks. While the mechanisms associated with EF-mediated cell/tissue responses remain unclear, there is no lack of evidence that demonstrates the important role of EFs in normal physiology and after tissue injury. By highlighting the existing pre-clinical and clinical data as well as new developments from our laboratory, my goal is to stoke scientific enthusiasm to an approach that merits further clinical investigation. Indeed, in an age of molecular biology, many biophysical stimuli such as electric fields have received diminished attention.
Scientific rationale for use of electric fields in spinal cord regeneration
The existence of DC electric fields and their occurrence in cellular processes has been established for well over a hundred years [14]. The electrical currents (and associated voltage gradients) that flow within the body are not electron based but are carried by ions. The fields set up by these ions, measured as the voltage difference over the distance of current flow (mV/mm), have physiologic roles and their effects can be clearly observed in many biologic states such as dermal/corneal wound healing [15,16,17], limb regeneration [18, 19], and early embryonic development [20,21,22]. For instance, the mammalian cornea establishes a + 30 to + 40 mV internally positive trans-corneal potential via the active pumping of Na+ and K+ ions inwards and Cl− outwards across the epithelium. This disparity in charge sets up an electric field across the epithelium. Damage to the cornea induces ion flow through the breach, creating a “disturbance” DC EF. Such fields initiate downstream cellular responses that ultimately end in closure of the breach (i.e., wound healing). A similar phenomenon occurs in amphibian limb regeneration whereby an amputated limb exhibits outward flow of injury current [18]. These long-lasting “stump currents” most likely initiate the process of limb regeneration [19]. Manipulation of these injury currents via mechanical methods, application of a counter field or with pharmacologic agents, can impede normal physiologic processes. In the example of the cornea, wounding of the corneal epithelium causes the cells bordering the injury to proliferate. Addition of ouabain, an inhibitor of Na+/K+ pumps, alters cellular orientation and suppresses mitosis by collapsing the trans-corneal potential [23]. If electric field manipulations are applied to the developing embryo, severe anatomical defects (absence of anatomical structures, limbs and tail) can be induced [20, 24, 25].
Within the nervous system, neurons cultured in vitro show remarkable behaviors when exposed to DC electric fields. EFs as low as 10 mV/mm can cause axonal growth cones to turn towards the cathode (negative pole). Anode facing neurites either resorb into the soma or turn 180 degrees toward the cathode. EFs enhance the rate of neurite outgrowth [26, 27] and branching is promoted [28]. The direction of turning (cathodal or anodal) is dependent on neuron type, substratum charge, and whether the process is a dendrite or axon [29]. However, as a whole, decades of culture data show that under physiologic conditions and physiologic substrates (negatively charged), neurons generally extend processes towards the cathode and retract/turn neurites facing the anode [30]. Interestingly, migration of neural cells towards the cathode has been documented [20] while peripheral nerve Schwann cells are drawn to the anode [31].
The mechanism of EF transduction within the cell is unclear, but externally applied EFs result in an asymmetry of many charged cell surface proteins such as receptors and ion channels. The most likely cause is an electrophoretic effect across the cell [32]. Increase in Ca+2 may be altered from this clustering of cell surface proteins/channels, which could lead to changes along a single side of the cell. Downstream signaling pathways that trigger cytoskeletal events may further be initiated (see [33] for review). In addition to axon guidance, applied DC electric fields can inhibit axon dieback, as demonstrated in lampreys after spinal cord axotomy [34, 35]. Imposed DC fields most likely set up a counter field that reduces the entry of Ca+2 and Na+ into the transected end of the axon [36]. Ca+2 overload, from trauma such as axotomy, is well understood to initiate neurodegenerative programs within the cell [37, 38].
The phenomenon of simultaneous EF-induced cathodal attraction and reduction in axon dieback provides the foundation for its use in treating SCI. Assessment of exogenously applied DC fields to spinal injuries was detailed in a series of experiments in guinea pigs [39,40,41]. In one of these seminal studies, animals were subjected to a hemisection injury scheme and treated with an electrical implant (battery) to deliver steady DC current. The cathode electrode was placed cranial to the hemisection while the anode was caudal to the injury. After treatment for 50–60 days at up to 40 μV/mm, the authors found that cut axons reached the transection plane and some were found growing near or around the astroglial scar, albeit not through it. The projection of these axons was in the ascending direction (towards the cathode). In comparison, no spontaneous axon growth around the lesion was observed and axons actually retracted from the transection plane in the controls. Follow-up studies aimed to decipher the effects of electrode placement in mediating regeneration were conducted in another cohort of guinea pigs [41]. Animals were subjected to a right lateral hemisection and the cutaneous trunci muscle reflex (CTM) and freefall righting reflex were used to assess ascending or descending pathways, respectively. The investigators noted the CTM was lost after injury but was recovered in 13% of the animals having the cathode cranial to the cut plane. Interestingly, no such return of the CTM was found in any of the animals with a caudal cathode or in the shams. This suggests that ascending interneuron spinal fibers, which are part of the CTM circuit, may have crossed the lesion. Further, animals with a caudal cathode installation recovered the free-fall righting response. However, most animals regained this reflex spontaneously. The researchers noted regeneration could have occurred in this group but the effects were masked by the natural recovery of the righting reflex. Nonetheless, the experimental results are consistent with the hypothesis that the cathode seems to attract axon growth.
The group of Tator also conducted comparable studies in rats and showed a 14 μA DC current (cathode distal to compressive clip injury) resulted in functional and anatomical improvements [42]. Metrics included higher inclined plane scores and amplitude of the motor evoked potentials, and a greater number of labeled cells in the red nucleus, raphé nuclei, and vestibular nuclei. Further experiments from the same group confirmed the initial findings and suggested electrode placement (either caudal or cranial to the injury) may also have differing outcomes [43].
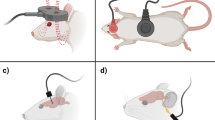
These early in vivo investigations revealed a peculiar anatomical caveat. For ideal sensory and motor recovery, injured spinal axons must extend both cranially and caudally across the lesion to synapse with functional, healthy neurons. This two-way projection poses an interesting problem since DC electric field–induced axonal growth tends to be biased towards the cathode. However, this enigma was resolved through on an observation that axon growth towards the cathode is faster than retraction from the anode [26]. That is, there is a biologic window (~ 30–60-min duration) in which reversal of the field does not completely undo axon growth towards the cathode. This biologic asymmetry to the EF response led to the concept of using a reversing or oscillating electric field stimulation (OFS) for inducing bilateral spinal cord regeneration. Similar to the initially implanted battery in rodents, oscillating field stimulation uses an implantable device that produces a steady DC current/electric field across the lesion (Fig. 1). However, at every 15-min intervals, the electrode polarity is switched (i.e., oscillates). This polarity reversal accomplishes two key goals: (1) It encourages bidirectional axon growth across the damaged cord region and (2) polarity reversal neutralizes the electrochemical byproducts that accumulate at the electrodes. The latter point is quite relevant as local pH changes near the electrodes may be cytotoxic [43, 44]. Polarity reversal minimizes this effect and permits longer sustained DC stimulation in vivo.
The bidirectional dilemma. A distally placed negative pole (cathode) tends to attract growth of descending axons (a). b If the cathode is placed cranial to the injury, ascending axons will be stimulated. c By oscillating the polarity of the DC fields every 15 min, both ascending and descending projections can be encouraged to cross the lesion. d Schematic representation of the OFS device and electrode placements
Pre-clinical results with OFS
Initial trials exploiting the OFS concept were first conducted in dogs by the group of Borgens et al. [45]. In these randomized studies, canines diagnosed with thoracolumbar intervertebral disk herniation (Hansen type 1 injury) and categorized as having complete paraplegia received OFS with electrodes sutured a few millimeters above the cord at ends of the laminectomy site. Stimulators delivered 200 μA of current for a period of either 3, 6, or 15 weeks. Functional metrics included both neurological and electrophysiological exams at 6 weeks and 6 months post-implantation. Compared to shams, dogs receiving the active stimulators showed a statistically significant improvement at 6 weeks and 6 months in the aggregate neurologic score, which consisted of equal weighting of deep pain sensation, locomotion response, superficial pain, and proprioceptive placing. Return of some somatosensory evoked potentials (SSEPs) was found in four of the 12 implanted animals while none of the 11 sham dogs recovered electrophysiologically. Few complications were found, and they were unrelated to the electrical therapy.
A second blinded study was conducted using very similar criteria in which paraplegic dogs were treated with revised OFS units suitable for human implantation [46]. Enrolled dogs received standard decompression surgery, spinal stabilization, and administration of methylprednisolone sodium succinate. With the OFS group, the stimulation units provided an estimated field strength of about 500–600 μV/mm for a period of 15 weeks. Both radiologic and neurologic exams were conducted, with neurologic testing consisting of reflex assessment, urologic and urodynamic tests, superficial and deep pain sensation, proprioception, ambulation, and SSEPs. Longitudinal assessment was again made at 6 weeks and 6 months post-operation. Trial results showed that in some individual metrics, statistical significance was not attained. However, the total neurologic score was again higher for OFS-treated dogs (n = 20) vs sham animals (n = 14) at 6 months. SSEP recordings were found in 7 of 17 OFS-treated dogs at 6 months while only 2 of 14 shams had any return of nerve conduction. Comparable to the first study, no side effects related to the OFS were found.
From these canine cohorts, it became clear that the OFS electronic devices implanted for an extended period had no detrimental effects and provided improvements in electrophysiology and in the combined neurologic assessment. While some degree of spontaneous recovery can be expected in animals following decompressive surgery [47], these results showed that OFS stimulation still fared better than the animals which received a sham implant. Moreover, time to ambulation after decompressive spinal surgery peaks at around 11–13 days in dogs [48, 49] and in this study, the authors waited up to 18 days to verify the animals remained paraplegic prior to device implant. Thus, the likelihood that these animals would have recovered naturally without the OFS implant was low. The cumulative in vivo data in rodents and dogs provided the justification for use in subsequent human trials.
Clinical results with OFS
First units of the OFS device were implanted into ten human patients with neurological complete acute SCI [50]. The trial was led by the Indiana School of Medicine using the same implants designs as in the canine study (Fig. 2). The entry criteria for the trial were very strict and shown in Table 1. Nine out of ten patients received some sort of surgical intervention, which included decompression or internal fixation. All patients were also given the same methylprednisolone dose, as was common protocol. Sterile OFS devices were subsequently implanted into the patients within the first 18 days of the initial injury, via a second surgery.
Image of implanted OFS implant per clinical trials outlined in [50]. Implants were encased in a silicone matrix for biocompatibility
A set of three electrodes were placed one segment above the injury, while the opposing set of three electrodes were placed one segment below the injury, permitting bidirectional stimulation of spared peri-lesion tissue. Electrodes were coiled platinum iridium wires, designed to optimize the current density output. Electrode placement was extradural with one electrode sutured to the spinous process and two others to the lateral musculature. Each electrode emitted 200 μA, for a total of 600 μA. Polarity of the electrodes was reversed every 15 min. Device explants were made at 15 weeks. Patient status was measured via several metrics, including the American Spinal Injury Association (ASIA) scoring system, visual analog scale (VAS), and SSEPs. Patient status was observed at baseline (before implantation) 6 weeks, 6 months, and 1 year after implantation. Summary results from the initial ten patients are shown in Table 2.
Throughout the study, patients recovered more with the OFS as all of the tested metrics were in favor of the treatment cohort. The motor recovery at 1 year was 6.3 above the baseline (0–100 scale). The change in pinprick score was + 20.4 (19 revised) while the light touch mean was 25.5 (23.9 revised) higher after 1 year (0–112 scales). SSEP recovery was observed in four out of five cervical patients and one out of five thoracic injuries. However, SSEPs have not been tracked longitudinally in SCI patients so it is unclear what the SSEP may reveal. Improvement in upper extremity use was observed in a few patients. One patient recovered sexual function [51]. The positive outcomes of this first trial convinced the FDA to approve a follow-up study with another 10 patients. However, only four new patients received the implant prior to the ending of the trial by the corporate sponsors. Detailed results from those four patients are described by [51]. The aggregate patient data of the 14 patients receiving implants showed a net gain of 6.9 points in motor scores, 25.9 point increase in light touch, and 15.2 increase in pinprick sensation. No regression in neurologic function was found. It is interesting to note that with OFS treatment, sensory recovery in the human patients was much higher vs motor function—a phenomenon that is still not well understood. The collective data showed the OFS implants were safe; 13 of the 14 units were still operational with one device failing sometime after week 14. No side effects were experienced by this patient and the failure was attributed to a fragile circuit connector. The frequency of wound infection (one patient) was within the bounds of typical surgical procedures.
Since this initial phase 1 trial aimed to determine the safety of the OFS device, a placebo group was not part of the experimental design. However, historical controls of similarly categorized patients from the National Acute Spinal Cord Injury Studies (NASCIS II and IIIFootnote 1*) can be used as a reference. These trials investigated the use of methylprednisolone steroid (MP) after SCI. In NASCIS II, a very small increase in motor recovery was observed in a small subset of neurologically complete patients at 6 months and 1 year, but no differences in pin prick or light touch were detected vs placebo [4, 52]. Moreover, there was a trend towards worsening motor and sensory scores in neurologically incomplete patients at 1 year vs placebo [52]. In NASCIS III, the post treatment change in motor scores ranged from 1.9 to 6.1 points higher (depending on timing of dosage) at 6 months post-injury for neurologically complete patients, with virtually identical results after 1 year [53]. Statistical significance was not reached. No MP regime increased either pin prick or light touch scores by more than 3.8 points after 1 year and again, the results were not statistically significant. While direct value comparisons between OFS and NASCIS II/III data are difficult due to slight variations in the scoring and patient classification, the results show that versus placebo, MP produced marginal to no changes in motor and sensory scores. While the OFS trials also used MP per NASCIS III guidelines, the degree of functional recovery observed was higher than those in the NASCIS III trials. Thus, the results indicate a benefit from the OFS.
In another clinical trial using GM-1 ganglioside (Sygen), the measured endpoints and ASIA metrics were similar to the OFS trials. Compared to the matched placebos from this group, the OFS was superior in pinprick and light touch for cervical injuries and significantly better in pinprick scores for thoracic injuries (https://www.sec.gov/Archives/edgar/data/1180253/000114420407037653/v081531_sb2a1.htm). The placebo group also saw a deterioration in pinprick and light touch responses in thoracic injuries while no such deficits were observed with OFS.
In light of these historical controls, the results with OFS indicate moderate efficacy as it is clear the outcomes cannot be solely explained by spontaneous recovery or the use of MP. The improvement in sensory scores and the lack of neuropathic pain without any motor ability are encouraging findings. Others have noted that efficacy review panels and even scientists who conduct animal experiments tend to heavily favor motor recovery, but clinicians and especially patients realize the importance of even modest gains in sensory performance [56, 57]. Any return of function translates into a significant improvement in quality of life, especially in higher level cervical injuries. The OFS device is not intended as a “cure” per se, as the pathophysiology of SCI is complex and multi-dimensional. Indeed, it is unlikely that any single therapy will completely resolve SCI. But having a surgical option to potentially restore some level of function from catastrophic SCI holds a significant value to the SCI community. Even a small return of sensation, bladder/bowel control, or sexual function is a leap forward in ameliorating the debilitating consequences of SCI.
Follow-up work
Following the initial dog and human trials conducted by the team of Borgens and Shapiro, the OFS technology has remained relatively dormant. This was due to several factors, primarily from the demise of the biotech company that licensed the OFS technology. The OFS patent has now also expired, thus reducing the financial incentive that another company may take this technology to market. However, new in vitro findings have resurrected our interest in the use of DC fields in spinal cord regeneration. Borgens had previously shown that astrocyte numbers were greatly reduced at the lesion with DC field stimulation [58]. Scarring by dysfunctional or hyper-reactive astrocytes create a physical and biochemical border, which has been implicated as a potential barrier to regeneration ([59, 60] Reduction in astrocyte numbers may be beneficial for facilitating axon penetration through the lesion.
The Borgens’ group postulated that other cell types intimately involved in SCI pathophysiology may be responsive to DC fields. Recently, the lab of Rajnicek observed that monocytes migrated cathodally when subjected to a 300 mV/mm DC field [61]. In contrast, differentiated macrophages migrated anodally. Field stimulated macrophages also increased their phagocytic activity to both cellular and inorganic targets. Macrophages that infiltrate from the hemorrhagic lesion post-SCI are key players in the subsequent inflammatory process. These cells are responsible for debridement of cellular debris, can secrete both pro-inflammatory and anti-inflammatory cytokines, and facilitate the repair process [62]. The dysfunctional transition from pro-inflammatory to pro-healing macrophage phenotypes after SCI has been implicated as a barrier to repair [62]. The data by Rajnicek et al. suggest blood-derived macrophages (and possibly microglia, the macrophages of the CNS) may be activated and modulated with electric fields. Further work will need to be done to fully characterize macrophage phenotypic changes, but these preliminary results offer new insight for potential recruitment and polarization of macrophages.
Another widely researched area in SCI therapeutics is the concept of cellular transplantation. Briefly, this approach uses the patient’s own cells (i.e., stem cells, Schwann cells) that are harvested post-SCI, expanded/modified in vitro, and injected back into the damaged site. The rationale assumes these re-engineered cells can survive and integrate with the host to restore synaptic connections or perform other vital functions such as remyelination of damaged axons. Recent work with electric fields has demonstrated very intriguing outcomes. For instance, Liu showed that neural stem cell–derived oligodendrocyte precursor cells isolated from fetal mice migrated towards the cathode. This bias was proportional to the field strength [63]. Embryonic human–sourced neural stem cells also were guided by electric fields as small as 16 mV/mm [64], whereas peripheral nerve Schwann cells migrated within a field of 50–200 mV/mm [65]. Other experiments with organotypic spinal cord slices also showed transplanted neural progenitor cells migrated towards the cathode. These findings suggest EFs can be potentially used to enhance recruitment of transplanted precursor/stem cells to the lesion.
The emerging in vitro work with DC electric fields provides added motivation to continue development of the technology, especially in relation to immuno-modulation and cellular transplantation. The original OFS electronics are now outdated, while new miniaturized wireless semiconductor technologies have become readily available. To this end, our laboratory has refreshed the OFS design and implemented modern microelectronics as well as improved functionality. We are also re-evaluating electrode geometries as well as in situ placements to optimize field intensities. Our hope is that these modifications will improve the therapeutic efficacy of the OFS implants and catalyze the next phase of human trials.
Limitations of the OFS and ongoing research
The OFS used in dogs and humans was state of the art at the time of development. Nonetheless, there were several technological limitations to these early studies. First, the devices were passive in nature and could not transmit information back to the clinicians. The verification of battery life could only be made during device retrieval (one unit had an electronic failure found only during the explant). This deficiency made it difficult to determine if the device was performing to specifications in vivo. Secondly, the electric fields generated within the cord parenchyma were unknown and the in vivo field intensity and distribution of EFs may be quite different. Variations of the EFs in the cord may potentially explain the disparate outcomes in sensory vs motor function in the phase I FDA trials. To remedy these unknowns, our center has made considerable progress in several areas.
Development of wireless telemetry
In the current generation of the OFS device, we have incorporated a wireless communications system based on the Bluetooth protocol. Integrating telemetry microelectronics into an updated low-power circuit, we are not only able to generate the same magnitude of current (200 μA per electrode) but also simultaneously monitor the current output throughout the implantation period. The wireless system transmits data to an iOS-based smartphone and via a simple free App (Purdue OFS), the user can interface with the medical device (Fig. 3). All communications are password encrypted and encoded. The control user interface has a variety of functions, providing information on battery power, individual lead current output, oscillation time, and ability to shut off individual leads or the entire unit (Fig. 3). Users can further forward the stored data via e-mail for offline analysis for performance and safety assessment. Our laboratory is also building in other features such as ability to measure the voltage drop between electrodes. This measurement will permit quantitation of temporal local resistivity changes and could provide insights about fibrosis/scarring at the electrode-host tissue interface. Such data could allow for real-time adjustment of current flow to optimize therapeutic effect.
Left: Image of a prototype OFS implant with a 3-D printed polymer casing. The size of the implant is comparable to that of a camera battery. The OFS communicates with the Apple-based iOS devices wirelessly via Bluetooth. Right: Data screen of the control interface of the App. The App can be downloaded from the App store as “Purdue OFS”
A critique of the original OFS trial was that implantation of the device occurred several days after surgical decompression, necessitating a second surgery that may pose additional risks to the patient [66]. A suggestion was made to implant the OFS during the first surgery. This was not possible at the time as the OFS was turned on just prior to implantation. However, the revised OFS units can be implanted during the initial decompressive surgery and powered on after some delay/stabilization period. The ability to control/monitor device output further permits correlation of clinical outcomes to the device performance history. For instance, if a patient recovers poorly, one can evaluate the device log to look for anomalies that could explain the outcomes. And conversely, the stimulation regime that best induces patient recovery can be used as a template for additional refinement. The new technological advancements ultimately minimize device-related variability to deliver a more consistent therapeutic effect.
Computational mapping of electric fields
The electric field strengths of 500–600 μV/mm reported in prior clinical trials were extrapolated based on rudimentary measurements initially made in guinea pigs and dogs. However, actual in vivo field intensity and distribution of EFs may be quite different [67, 68]. Thus, a second area of investigation in our center is the mapping of electric fields within the spinal cord using computational methods. For these studies, finite element analysis (FEA) coupled with human MRI data are being used to construct anatomically accurate computational models for electromagnetic simulations. FEA is a common tool in biological modeling and has been employed in our prior studies to estimate the stress-strain distributions in the spinal cord during crush injury [69].
Characterization of the intracord field intensity has several important implications. First, we hypothesize that spatial differences in field strengths may explain the observed functional outcomes in the FDA patient trials. It was reported that sensory recovery was significantly improved, but motor function plateaued to levels just above the baseline. The proximity of the electrodes towards the dorsal aspect of the cord (location of major sensory tracts) may contribute to a ventral-dorsal asymmetry in field intensities that result in a discrepancy in sensorimotor recovery. We are currently exploring these EF variations as it pertains to clinical outcomes.
Secondly, mapping of these fields provides insight on optimization strategies for increasing therapeutic effect. For instance, the clinical electrodes were originally coiled in shape to maximize the surface area and subsequently, current output, without incurring tissue damage. We are determining whether such geometries can be modified to deliver even more current. These parameters along with the effects of electrode placement in vivo and changes to post-SCI tissue electrical properties are being studied. A pivotal component is collaboration with neurosurgeons to best assess surgical approaches (both dorsal and ventral) and implant dimensions that can be feasibility installed in the patient. With FEA, we are able to systemically evaluate these variables and make proposed changes for increasing the intracord field levels. We intend to validate these simulations in canine trials prior to testing in humans.
Conclusions
Oscillating field stimulation remains a promising technology for treating acute SCI. OFS has shown to be safe in early pre-clinical and clinical studies with no known side effects. The limited number of patients receiving OFS implants recovered modest sensory function and did not experience any deterioration in motor function. A few patients experienced remarkable outcomes, including return of bladder and sexual function. Based on historical SCI data, such positive results cannot be attributed to spontaneous recovery. The lack of continued research in humans was halted due to funding shortages from the sponsoring company. However, these early successes set a clinical framework for large-scale randomized placebo-controlled trials and also provide motivation to enhance the existing technology. Our center is committed to refining the core OFS devices and has already made new implants that are more user-friendly and improve data gathering/analysis, and we are investigating ways to optimize the therapeutic ratio. The surgical implantation process is straightforward and it is possible to combine the OFS modality with emerging therapies. Nonetheless, we envision that these improvements to the OFS platform will evolve into a novel intellectual property and catalyze a renewed interest for further human testing.
Notes
The NASCIS trials were a series of clinical trials beginning in 1984 that investigated the use of high-dose methylprednisolone to mitigate cellular oxidative stress and inflammation after acute SCI [52, 53]. The NASCIS II and III trials claimed some modest benefit of using MP following spinal injury. In retrospect, these studies have been widely criticized for poor experimental design, data reporting and suspect post-hoc statistical analysis [54, 55]. Nonetheless, the trials were the first of its kind and reported data that can be used as historical controls, especially in assessing the effects of spontaneous recovery
References
Cao Y, Chen Y, DeVivo M (2011) Lifetime direct costs after spinal cord injury. Top Spinal Cord Inj Rehabil 16(4):10–16
Bracken MB, Collins WF, Freeman DF, Shepard MJ, Wagner FW, Silten RM, Hellenbrand KG, Ransohoff J, Hunt WE, Perot PL Jr (1984) Efficacy of methylprednisolone in acute spinal cord injury. JAMA 251(1):45–52
Bracken MB, Shepard MJ, Holford TR, Leo-Summers L, Aldrich EF, Fazl M, Fehlings MG, Herr DL, Hitchon PW, Marshall LF, Nockels RP, Pascale V, Perot PL Jr, Piepmeier J, Sonntag VK, Wagner F, Wilberger JE, Winn HR, Young W (1998) Methylprednisolone and neurological function 1 year after spinal cord injury. Results of the National Acute Spinal Cord Injury Study. J Neurosurg 89(5):699–706
Bracken MB, Shepard MJ, Collins WF, Holford TR, Young W, Baskin DS, Eisenberg HM, Flamm E, Leo-Summers L, Maroon J, Marshall LF, Perot PL Jr, Piepmeier J, Sonntag VKH, Wagner FC, Wilberger JE, Winn HR (1990) A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the Second National Acute Spinal Cord Injury Study. N Engl J Med 322(20):1405–1411
Geisler FH, Dorsey FC, Coleman WP (1991) Recovery of motor function after spinal-cord injury--a randomized, placebo-controlled trial with GM-1 ganglioside. N Engl J Med 324(26):1829–1838
Casha S, Zygun D, McGowan MD, Bains I, Yong VW, John Hurlbert R (2012) Results of a phase II placebo-controlled randomized trial of minocycline in acute spinal cord injury. Brain 135(Pt 4):1224–1236
Santamaria AJ et al (2018) Clinical and neurophysiological changes after targeted intrathecal injections of bone marrow stem cells in a C3 tetraplegic subject. J Neurotrauma
Servick K (2017) Failed spinal cord trial offers cautionary tale. Science 355(6326):679
Bunge MB et al (2017) From transplanting Schwann cells in experimental rat spinal cord injury to their transplantation into human injured spinal cord in clinical trials. Brain Prog Res 231:107–133
Knoller N, Auerbach G, Fulga V, Zelig G, Attias J, Bakimer R, Marder JB, Yoles E, Belkin M, Schwartz M, Hadani M (2005) Clinical experience using incubated autologous macrophages as a treatment for complete spinal cord injury: phase I study results. J Neurosurg Spine 3(3):173–181
Feron F et al (2005) Autologous olfactory ensheathing cell transplantation in human spinal cord injury. Brain 128(Pt 12):2951–2960
Mackay-Sim A, Feron F, Cochrane J, Bassingthwaighte L, Bayliss C, Davies W, Fronek P, Gray C, Kerr G, Licina P, Nowitzke A, Perry C, Silburn PAS, Urquhart S, Geraghty T (2008) Autologous olfactory ensheathing cell transplantation in human paraplegia: a 3-year clinical trial. Brain 131(Pt 9):2376–2386
James ND, McMahon SB, Field-Fote EC, Bradbury EJ (2018) Neuromodulation in the restoration of function after spinal cord injury. Lancet Neurol 17(10):905–917
McCaig CD, Rajnicek AM, Song B, Zhao M (2005) Controlling cell behavior electrically: current views and future potential. Physiol Rev 85(3):943–978
Sta Iglesia DD, Vanable JW Jr (1998) Endogenous lateral electric fields around bovine corneal lesions are necessary for and can enhance normal rates of wound healing. Wound Repair Regen 6(6):531–542
Zhao M, Song B, Pu J, Wada T, Reid B, Tai G, Wang F, Guo A, Walczysko P, Gu Y, Sasaki T, Suzuki A, Forrester JV, Bourne HR, Devreotes PN, McCaig CD, Penninger JM (2006) Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-gamma and PTEN. Nature 442(7101):457–460
Chiang M, Robinson KR, Vanable JW Jr (1992) Electrical fields in the vicinity of epithelial wounds in the isolated bovine eye. Exp Eye Res 54(6):999–1003
Borgens RB, Vanable JW Jr, Jaffe LF (1977) Bioelectricity and regeneration: large currents leave the stumps of regenerating newt limbs. Proc Natl Acad Sci U S A 74(10):4528–4532
Borgens RB, Vanable JW Jr, Jaffe LF (1979) Role of subdermal current shunts in the failure of frogs to regenerate. J Exp Zool 209(1):49–56
Shi R, Borgens RB (1994) Embryonic neuroepithelial sodium transport, the resulting physiological potential, and cranial development. Dev Biol 165(1):105–116
Hotary KB, Robinson KR (1992) Evidence of a role for endogenous electrical fields in chick embryo development. Development 114(4):985–996
Shi R, Borgens RB (1995) Three-dimensional gradients of voltage during development of the nervous system as invisible coordinates for the establishment of embryonic pattern. Dev Dyn 202(2):101–114
Song B, Zhao M, Forrester JV, McCaig CD (2002) Electrical cues regulate the orientation and frequency of cell division and the rate of wound healing in vivo. Proc Natl Acad Sci U S A 99(21):13577–13582
Metcalf MEM, Borgens RB (1994) Weak applied voltages interfere with amphibian morphogenesis and pattern. J Exp Zool 268(4):323–338
Hotary KB, Robinson KR (1994) Endogenous electrical currents and voltage gradients in Xenopus embryos and the consequences of their disruption. Dev Biol 166(2):789–800
McCaig CD (1987) Spinal neurite reabsorption and regrowth in vitro depend on the polarity of an applied electric field. Development 100(1):31–41
Jaffe LF, Poo MM (1979) Neurites grow faster towards the cathode than the anode in a steady-field. J Exp Zool 209(1):115–127
Mccaig CD (1990) Nerve branching is induced and oriented by a small applied electric-field. J Cell Sci 95:605–615
Rajnicek AM, Robinson KR, McCaig CD (1998) The direction of neurite growth in a weak DC electric field depends on the substratum: contributions of adhesivity and net surface charge. Dev Biol 203(2):412–423
Borgens R (2003) Restoring function to the injured human spinal cord. Springer-Verlag, Berlin
McKasson MJ, Huang L, Robinson KR (2008) Chick embryonic Schwann cells migrate anodally in small electrical fields. Exp Neurol 211(2):585–587
Jaffe LF (1977) Electrophoresis along cell membranes. Nature 265(5595):600–602
Yao L, Li Y (2016) The role of direct current electric field-guided stem cell migration in neural regeneration. Stem Cell Rev 12(3):365–375
Borgens RB, Jaffe LF, Cohen MJ (1980) Large and persistent electrical currents enter the transected lamprey spinal cord. Proc Natl Acad Sci U S A 77(2):1209–1213
Roederer E, Goldberg NH, Cohen MJ (1983) Modification of retrograde degeneration in transected spinal axons of the lamprey by applied DC current. J Neurosci 3(1):153–160
Strautman AF, Cork RJ, Robinson KR (1990) The distribution of free calcium in transected spinal axons and its modulation by applied electrical fields. J Neurosci 10(11):3564–3575
Berliocchi L, Bano D, Nicotera P (2005) Ca2+ signals and death programmes in neurons. Philos Trans R Soc Lond Ser B Biol Sci 360(1464):2255–2258
Marambaud P, Dreses-Werringloer U, Vingtdeux V (2009) Calcium signaling in neurodegeneration. Mol Neurodegener 4:20
Borgens RB, Blight AR, McGinnis ME (1987) Behavioral recovery induced by applied electric fields after spinal cord hemisection in guinea pig. Science 238(4825):366–369
Borgens RB, Mourey ME, Blight AR (1993) Delayed application of direct current electric fields in experimental spinal cord injuries. Restor Neurol Neurosci 5(3):173–179
Borgens RB, Blight AR, McGinnis ME (1990) Functional recovery after spinal cord hemisection in guinea pigs: the effects of applied electric fields. J Comp Neurol 296(4):634–653
Fehlings MG, Tator CH, Linden RD (1988) The effect of direct-current field on recovery from experimental spinal cord injury. J Neurosurg 68(5):781–792
Fehlings MG, Tator CH (1992) The effect of direct current field polarity on recovery after acute experimental spinal cord injury. Brain Res 579(1):32–42
McGinnis ME, Murphy DJ (1992) The lack of an effect of applied d.c. electric fields on peripheral nerve regeneration in the guinea pig. Neuroscience 51(1):231–244
Borgens RB, Toombs JP, Blight AR, McGinnis M, Bauer MS, Widmer WR, Cook JR Jr (1993) Effects of applied electric fields on clinical cases of complete paraplegia in dogs. Restor Neurol Neurosci 5(5):305–322
Borgens RB et al (1999) An imposed oscillating electrical field improves the recovery of function in neurologically complete paraplegic dogs. J Neurotrauma 16(7):639–657
Ruddle TL, Allen DA, Schertel ER, Barnhart MD, Wilson ER, Lineberger JA, Klocke NW, Lehenbauer TW (2006) Outcome and prognostic factors in non-ambulatory Hansen type I intervertebral disc extrusions: 308 cases. Vet Comp Orthop Traumatol 19(1):29–34
Ferreira AJ, Correia JH, Jaggy A (2002) Thoracolumbar disc disease in 71 paraplegic dogs: influence of rate of onset and duration of clinical signs on treatment results. J Small Anim Pract 43(4):158–163
Davis GJ, Brown DC (2002) Prognostic indicators for time to ambulation after surgical decompression in nonambulatory dogs with acute thoracolumbar disk extrusions: 112 cases. Vet Surg 31(6):513–518
Shapiro S, Borgens R, Pascuzzi R, Roos K, Groff M, Purvines S, Rodgers RB, Hagy S, Nelson P (2005) Oscillating field stimulation for complete spinal cord injury in humans: a phase 1 trial. J Neurosurg Spine 2(1):3–10
Shapiro S (2014) A review of oscillating field stimulation to treat human spinal cord injury. World Neurosurg 81(5–6):830–835
Bracken MB et al (1992) Methylprednisolone or naloxone treatment after acute spinal cord injury: 1-year follow-up data. Results of the second National Acute Spinal Cord Injury Study. J Neurosurg 76(1):23–31
Bracken MB, Shepard MJ, Holford TR, Leo-Summers L, Aldrich EF, Fazl M, Fehlings MG, Herr DL, Hitchon PW, Marshall LF, Nockels RP, Pascale V, Perot PL Jr, Piepmeier J, Sonntag VK, Wagner F, Wilberger JE, Winn HR, Young W (1998) Methylprednisolone or tirilazad mesylate administration after acute spinal cord injury: 1-year follow up. Results of the third National Acute Spinal Cord Injury randomized controlled trial. J Neurosurg 89(5):699–706
Coleman WP, Benzel E, Cahill DW, Ducker T, Geisler F, Green B, Gropper MR, Goffin J, Madsen PW III, Maiman DJ, Ondra SL, Rosner M, Sasso RC, Trost GR, Zeidman S (2000) A critical appraisal of the reporting of the National Acute Spinal Cord Injury Studies (II and III) of methylprednisolone in acute spinal cord injury. J Spinal Disord 13(3):185–199
Nesathurai S (1998) Steroids and spinal cord injury: revisiting the NASCIS 2 and NASCIS 3 trials. J Trauma 45(6):1088–1093
Walters BC (2010) Oscillating field stimulation in the treatment of spinal cord injury. PM R 2(12):S286–S291
Anderson KD (2004) Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma 21(10):1371–1383
Moriarty LJ, Borgens RB (2001) An oscillating extracellular voltage gradient reduces the density and influences the orientation of astrocytes in injured mammalian spinal cord. J Neurocytol 30(1):45–57
Silver J, Miller JH (2004) Regeneration beyond the glial scar. Nat Rev Neurosci 5(2):146–156
Sofroniew MV (2009) Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci 32(12):638–647
Hoare JI, Rajnicek AM, McCaig CD, Barker RN, Wilson HM (2016) Electric fields are novel determinants of human macrophage functions. J Leukoc Biol 99(6):1141–1151
Gensel JC, Zhang B (2015) Macrophage activation and its role in repair and pathology after spinal cord injury. Brain Res 1619:1–11
Li YC et al (2015) ARP2/3 complex is required for directional migration of neural stem cell-derived oligodendrocyte precursors in electric fields. Stem Cell Res Ther 6:41
Feng JF, Liu J, Zhang XZ, Zhang L, Jiang JY, Nolta J, Zhao M (2012) Guided migration of neural stem cells derived from human embryonic stem cells by an electric field. Stem Cells 30(2):349–355
Yao L, Li Y, Knapp J, Smith P (2015) Exploration of molecular pathways mediating electric field-directed Schwann cell migration by RNA-seq. J Cell Physiol 230(7):1515–1524
Tator CH (2005) Phase 1 trial of oscillating field stimulation for complete spinal cord injury in humans. J Neurosurg Spine 2(1):1 discussion 1-2
Greenebaum B (2015) Calculated spinal cord electric fields and current densities for possible neurite regrowth from quasi-DC electrical stimulation. Bioelectromagnetics 36(8):564–575
Hernandez-Labrado GR et al (2011) Spinal cord direct current stimulation: finite element analysis of the electric field and current density. Med Biol Eng Comput 49(4):417–429
Ouyang H, Sun W, Fu Y, Li J, Cheng JX, Nauman E, Shi R (2010) Compression induces acute demyelination and potassium channel exposure in spinal cord. J Neurotrauma 27(6):1109–1120
Acknowledgements
I would like to thank Michel Schweinsberg and Megan Saenger for the illustrations and Dr. Richard Borgens for thoughtful discussions.
Funding
This work was financially funded by the State of Indiana.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
No human subjects were involved, and no informed consent was required for this review.
Rights and permissions
About this article
Cite this article
Li, J. Weak direct current (DC) electric fields as a therapy for spinal cord injuries: review and advancement of the oscillating field stimulator (OFS). Neurosurg Rev 42, 825–834 (2019). https://doi.org/10.1007/s10143-018-01068-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-018-01068-y