Abstract
Purpose of Review
To discuss the potential use of transcranial direct current stimulation (tDCS) to improve motor behavior after brain injury.
Recent Findings
Despite evidence that tDCS can improve motor function following brain injury, meta-analysis studies have largely failed to find conclusive support for tDCS as a viable treatment. In part, these inconsistencies arise from widespread variability in individuals’ responsiveness to tDCS because of biological and experimental factors.
Summary
Properly designed smart clinical studies are still needed to determine the optimal stimulation parameters and combinations of tDCS. However, some patterns of “best practice” have begun to emerge: (1) pairing tDCS concurrently with high-intensity motor training as opposed to before, after, or in the absence of physical practice, (2) repeating sessions of stimulation in close succession over a single administration, (3) administering stimulation during more acute periods of recovery over chronic states, and (4) utilizing modeling techniques based on individual anatomy to tailor electrode placement and optimize current flow.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There has been a recent rise in popularity of using transcranial direct current stimulation (tDCS) to modulate cortical excitability in hopes of improving behavior. Here, we will discuss the potential use of tDCS for neurorehabilitation to improve motor behavior after brain injuries. We begin with a review of how tDCS induces neuroplastic changes. Next, we summarize how neurophysiological changes associated with motor recovery following brain injury and motor learning can inform treatment. Finally, we coalesce the successes and failures that have been documented using tDCS to treat brain-injured patients into recommendations for practical application.
Mechanisms of tDCS
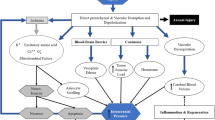
Evidence suggests tDCS is a form of non-invasive brain stimulation (NIBS) that can elicit functional and morphological changes of the underlying cells it targets. It is thought to slightly depolarize/hyperpolarize cell membranes depending on the polarity of the stimulation and can affect cellular excitability from minutes to hours [1,2,3,4, 5•, 6]. In vitro and in vivo animal studies using direct current stimulation (DCS)–induced changes in synaptic plasticity such as modulations in spontaneous neuronal activity [7•, 8,9,10], neuronal evoked potentials [11••, 12, 13, 14•], and neuronal paired-pulse plasticity [12]. DCS also increased expression of BDNF, Ca2+, and other genes that play a role in long-term potentiation (LTP)/long-term depression (LTD) [15,16,17]. DCS-induced effects rely on calcium-dependent mechanisms [14,15,16], NMDA receptors [18], and BDNF and TrkB receptor pathways [11, 19], and are modulated by other types of LTP-inducing protocols [20]. Other cell types besides neurons are affected by DCS; DCS increased Ca2+ surges in astrocytes, cells with a crucial role in NMDA-dependent plasticity [14•].
In addition to functional changes, DCS elicits morphological changes. DCS can influence orientation of neuronal processes and their growth direction [21], increase spine density [22•], and increase oligodendrocyte-specific progenitor cells [23]. Individual cells can be affected by orientation of the electrical field, distance from the current, and their own morphology. Hence, the orientation of the electrical field influences different cell types and different compartments within the same cell [24••].
In humans, there is similar evidence that tDCS modulates cortical excitability. tDCS can modulate motor evoked potential (MEP) amplitudes and cerebello-brain inhibition (CBI) as assessed by transcranial magnetic stimulation (TMS), [2, 5•, 25,26,27,28,29] spectra changes at different frequency bands in electroencephalogram (EEG) [30], blood oxygenation level–dependent (BOLD) changes assessed by functional magnetic resonance imaging (fMRI) [31,32,33], and GABA and glutamatergic concentrations assessed by magnetic resonance spectroscopy (MRS) [34, 35]. Pharmacologically, NMDA receptors [36] and Ca2+ are underlying mechanisms for the lasting effects of tDCS [1, 2, 5•].
Overall, the literature suggests tDCS uses direct electrical currents to modulate both the functional strength and morphology of neuronal and non-neuronal synapses. Substantial evidence links these effects to LTP-like and LTD-like mechanisms. Generally, anodal tDCS is believed to enhance cortical excitability of the targeted brain area whereas cathodal tDCS diminishes it. However, it is important to note that this is an oversimplification of what happens with an electrical current in a complex structure as the human brain.
Neurophysiological Changes in Motor Recovery and Motor Learning
The motivation behind using tDCS in neurorehabilitation largely rises from two bodies of literature: first, research on the neurophysiological changes associated with motor recovery after brain injury and second, neurophysiological changes associated with learning new motor tasks. A basic understanding of any overlapping mechanisms that drive recovery and learning can guide tDCS strategies for neurorehabilitation.
Neurophysiological studies of upper limb motor recovery in chronic (> 6 months post injury) stroke patients using TMS have found higher motor thresholds and reduced MEP amplitudes in ipsilesional motor cortex (iM1) as compared to contralesional M1 (cM1) and healthy controls, and are often correlated with measures of hand dexterity and function [37, 38]. In healthy individuals, the two motor cortices exert mutual inhibition at rest and prior to movement (i.e., interhemispheric inhibition—IHI). However, chronic stroke patients have stronger IHI imbalances (from cM1 to iM1) both at rest and prior to movement onset that are correlated with more severe motor impairments [39•, 40, 41]. Interhemispheric imbalances are also present in other motor areas such as the dorsal premotor (dPM) cortex where more unbalanced contralesional PMd (cPMd) influence on iM1 is present in patients with greater clinical impairment [42,43,44]. Similarly, stroke patients with right hemisphere neglect have shown pathologically exaggerated intrahemispheric influence from left contralesional posterior parietal cortex (cPPC) to left cM1 that was related to severity of neglect [45].
In studies following stroke recovery in acute stroke patients (< 3 months after injury), iM1 thresholds and corticomotor excitability of the upper limb were initially reduced but over time increased with improved upper limb impairment and function [46•, 47,48,49]. Interestingly, in acute stroke patients, premovement IHI was initially normal following injury and later became abnormal. This emergence of the abnormal pattern from acute to chronic recovery was inversely correlated with motor recovery suggesting that interhemispheric imbalances might be a consequence of other underlying recovery processes rather than the cause of poor motor recovery [50].
Neurophysiological changes following motor recovery in traumatic brain injury show a similar profile to stroke. Moderate to severe chronic TBI patients show higher motor thresholds [51, 52], higher MEP variability [52], and reduced MEP amplitudes in the paretic upper limb as compared to the non-paretic side, changes that were found to be related to the severity of diffuse axonal injury (DAI) and motor impairment [51, 53•]. In acute cases of mild to moderate TBI, patients had initially higher motor thresholds, higher MEP variability, and reduced MEP amplitude that then showed a trend to return to normal levels in the chronic state [52, 54].
Overall, both stroke and TBI patients show neurophysiological evidence of cortical reorganization after injury and normalization of these corticomotor changes with recovery. This suggests motor and functional recovery in brain injury patients might be linked to the restoration of normal inter- and intracorticomotor patterns. Based on this theory of restoring normal corticomotor patterns after brain injury, several strategies for neurorehabilitation have emerged to either upregulate excitability of ipsilesional motor areas (e.g., M1, dPM, PPC), downregulate excitability of contralesional motor areas, or do both simultaneously to ameliorate the paretic side motor dysfunction. However, it is important not to assume that all behaviors follow the same recovery pattern as the upper limb. For example, in contrast to upper limb motor recovery post stroke, in swallowing motor recovery, corticomotor excitability of laryngeal cM1 in acute stroke patients was initially reduced, but over time increased along with improved swallowing impairment and function [55]. Moreover, an important caveat to this body of work is that it is limited to stroke and TBI patients for whom it is possible to obtain MEPs (i.e., mild to moderate motor deficits). Thus, it remains unclear what neurophysiological recovery may look like for patients with more severe motor deficits lacking MEPs.
Similar neurophysiological changes happen with motor learning as seen with motor recovery following brain injury. Motor learning in neurorehabilitation is to either relearn to perform a task the same way as it was done before injury (recovery) or to implement a different strategy from that used before injury (compensation). Although recovery and compensation likely involve different pathophysiological mechanisms, both are associated with an increase in corticomotor excitability. Changes in the plasticity of synaptic connections of M1 are widely believed to play an essential role in learning and memory [56•]. In both animals and humans, there is evidence of increased M1 excitability following motor learning [57,58,59,60,61,62,63,64], as well as functional and structural changes to a distributed brain network that connects M1 with the dPM, PPC, cerebellum, somatosensory cortex, supplementary motor area (SMA), and other motor areas [65,66,67].
Based on studies demonstrating increased excitability of motor areas following motor learning, a body of research has emerged attempting to use tDCS to modulate M1 excitability and/or its connection with other motor areas to augment motor learning and retention. Notably, several studies have demonstrated application of tDCS during motor learning can boost performance in healthy humans: sequential visual isometric pinch task [11••, 27, 68•], finger sequencing [2, 69,70,71], reaching adaptation [29, 72, 73], walking adaptation [74], balance [75], and even functional motor tasks such as the Jebsen-Taylor Hand Function Test (JTT) [76•].
Utilizing tDCS to Augment Motor Recovery in Neurorehabilitation
Converging evidence that motor recovery and learning engage overlapping neurophysiological mechanisms and tDCS can improve motor learning in healthy individuals has made tDCS an appealing strategy for augmenting motor recovery in brain-injured patients. Here, we identify some successes and failures associated with using tDCS over various motor areas to treat motor syndromes such as hemiparesis, hemineglect, and dysphagia.
Hemiparesis
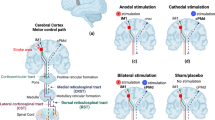
The most studied application of tDCS in brain injury patients is hemiparesis of both the upper and lower limbs. The most commonly studied electrode montages are anodal tDCS applied over ipsilesional M1 (anodal-iM1), cathodal tDCS applied over contralesional M1 (cathodal-cM1), and bihemispheric-M1 (i.e., anode and cathode are placed simultaneously over iM1 and cM1, respectively).
In hemiparesis of the upper limb, tDCS has been used to improve arm and hand function. In some studies of chronic stroke patients, even a single session of anodal-iM1, cathodal-cM1, or bihemispheric-M1 stimulation during motor training improved upper motor limb function measures such as JTT, pinch force, and reaction time [77,78,79,80,81] and was associated with enhanced cortical excitability and reduced intracortical inhibition of iM1 [77, 78]. Studies with multiple sessions (ranging from 5 to 20) of tDCS over M1, applied prior to or concurrently with motor training, have elicited immediate and long-lasting behavioral improvements of JTT, Upper Extremity Fugl-Meyer (UE-FM) Scale, Action Research Arm Test (ARAT), Wolf Motor Function Test (WMFT), Modified Ashworth Scale (MAS), Stroke Impact Scale (SIS), Medical Research Council (MRC) Scale, Barthel Index (BI), National Institutes of Health Stroke Scale (NIHSS), and muscle strength with effects ranging from weeks up to a year in acute and chronic stroke patients [82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97]. Some of these behavioral changes were also accompanied and/or correlated with increased iM1 cortical excitability [96, 98••], reduced IHI imbalance [98••], increased fMRI activation in ipsilesional and decreased in contralesional motor areas [85, 91], and increased fractional anisotropy of descending motor tracts of iM1 [94]. Though largely less examined, studies targeting other motor areas like ipsilesional PM (iPM) have also shown that repeated anodal stimulation over iPM with concurrent motor training can improve UE-FM Scale, MAS, MRC Scale, and BI, [99,100,101] behavioral changes that were accompanied with increased iM1 cortical excitability [99]. Surprisingly, only one study has explored the effects of tDCS to treat upper limb hemiparesis in TBI. Pairing multiple sessions of bihemispheric-M1 with concurrent motor training improved UE-FM Scale, SIS, Box and Block Test, and Purdue Pegboard Test in chronic TBI patients, changes that were largely sustained 6 months post intervention [102].
In contrast to these promising findings, other studies have presented contradictory results. In a single session of bihemispheric-M1 applied concurrently with motor training, although chronic stroke patients showed reduced IHI imbalance, there was no improvement in UE-FM Scale [103]. In other studies where anodal-iM1, cathodal-cM1, or bihemispheric tDCS was administered for multiple sessions either in the absence of [104], prior to [105], or concurrently with motor training [106,107,108,109], tDCS failed to elicit improvements in UE-FM Scale, MAS, Motricity Index (MI), NIHSS, and Box and Block Test beyond sham stimulation, and in some patients even worsened performance [109].
Meta-analysis studies have largely failed to validate the effectiveness of tDCS for improving upper limb function following a stroke. [110] This may be due to the vast variability in patient demographics and experimental protocols, as well as a lack of large robust randomized controlled trials. However, some patterns supporting improved effectiveness have emerged. Namely, multiple stimulation sessions in close succession with concurrent motor training are suggestive of better outcomes [111]. Additionally, efficacy is influenced by dose-related parameters relating to electrode size, charge density, and current density [112].
In hemiparesis of the lower limb, tDCS studies have focused on improvement of gait and balance. In acute and chronic stroke patients, a single session of anodal-M1 or bihemispheric-M1 tDCS, with concurrent motor training, has acutely enhanced paretic ankle control, Five Times Sit-to-Stand (FTSTS) Test, Timed Up and Go (TUG) Test, lower limb force production, postural stability, and walking speed [113,114,115,116,117] and simultaneously increased iM1 and decreased cM1 cortical excitability [118]. Another single-session study, targeting ipsilesional SMA during motor training, showed improved 10-Meter Walk Test (10MWT) and TUG Test [119]. Similarly, studies with multiple sessions (ranging from 10 to 14) of anodal-iM1 tDCS applied concurrently with motor training have elicited immediate and long-lasting behavioral improvements of Lower Extremity Fugl-Meyer (LE-FM) Scale, Lower Extremity Motricity Index (LE-MI), Functional Ambulatory Category (FAC), 6-min Walking Test (6MWT), 10MWT, TUG Test, and SIS [120•, 121, 122], and increased iM1 cortical excitability [120•].
In contrast, some single-session studies of either anodal-iM1 or bihemispheric-M1 applied before [123], after [124], or concurrently with motor training [125, 126] have failed to show behavioral improvements in spatial-temporal gait parameters and MAS nor elicit cortical excitability changes in iM1 or cM1. Additionally, one study with repeated sessions of anodal-iM1 with concurrent motor training failed to improve 6MWT or 10MWT [127].
However, in spite of some conflicting evidence, a systemic review with meta-analysis supports tDCS in improving gait speed after stroke, with multiple sessions being more effective than single sessions to improve functional outcomes [128].
Hemineglect
In hemineglect, tDCS montages are based off a similar concept as hemiparesis studies with either increasing activity of a hypoactive cortical region affected by the stroke (i.e., the right hemisphere in the case of neglect) or reducing cortical hyperactivity of the corresponding cortical region in the contralateral left hemisphere. Hence, tDCS studies on neglect have exclusively studied montages of either anodal applied over ipsilesional right posterior parietal cortex (anodal-iPPC), cathodal applied over contralesional left PPC (cathodal-cPPC), or simultaneously placed anode and cathode over iPPC and cPPC, respectively (bihemispheric-PPC).
In acute and chronic stroke patients, a single session of tDCS of anodal-iPPC, cathodal-cPPC, or bihemispheric-PPC, even without therapy, has shown significant improvement of Line Bisection Test (LBT), Star Cancellation Test (SCT), and Neglect Subtest of Test Battery for Attentional Performance (TAP) [129,130,131]. In other studies where multiple sessions (ranging from 10 to 15) of tDCS were applied either prior to or concurrently with motor training, both anodal-iPPC and cathodal-cPPC improved performance on the Motor-Free Visual Perception Test (MVPT), LBT, SCT, Modified BI, and Behavioral Inattention Test (BIT) [132, 133•]. In contrast, one study applying bihemispheric-PPC for multiple sessions (unpaired with therapy) failed to show improvements in BIT [134].
Though no meta-analysis study has focused solely on the efficacy of tDCS in the treatment of hemineglect, other meta-analyses including tDCS and other NIBS protocols have found moderate-quality evidence supporting the effectiveness of NIBS techniques for the treatment of neglect especially when combined with therapy [135, 136].
Dysphagia
Unlike upper and lower limb muscles, cortical representations of swallowing muscles have more bilateral cortical innervations [137]. Though cortical representations of swallowing muscles are asymmetrical (showing a swallowing dominant side unrelated to handedness) [137, 138], research studies have exclusively explored stimulating either iM1 or cM1. Currently, only four studies have specifically investigated the effects of tDCS in post stroke dysphagia.
In each of these studies of either acute, subacute, or chronic stroke patients, repeated sessions (ranging from 5 to 10) of anodal tDCS over iM1 or cM1, paired with concurrent motor training, were found to improve Functional Dysphagia Score (FDS) and Dysphagia and Outcome Severity Scale (DOSS) either immediately following treatment [139, 140•, 141] or at a 1–3-month follow-up [140, 142].
Although no meta-analysis studies have focused solely on the efficacy anodal tDCS in the treatment of dysphagia, other meta-analyses including tDCS and other NIBS protocols have found a small but significant effect of NIBS on post stroke dysphagia with slightly better effect size for stimulation of cM1 over iM1 [143, 144].
Limited and Conflicting Evidence for tDCS in Neurorehabilitation
Some consistent patterns have emerged implicating a common thread of “best practice” across syndromes: Pairing tDCS concurrently with motor training, repeated sessions of stimulation in close succession, and early administration during more acute periods of recovery show the best efficacy. A noteworthy limitation in the tDCS literature on the treatment of brain injury is the bias toward the study of stroke recovery. Few studies have examined the use of tDCS as clinical intervention for motor recovery in TBI patients.
Additionally, despite promising evidence that tDCS can serve as an adjunct to maximize motor recovery in brain injury patients, there have been some studies showing inconsistent and even contradictory effects such that meta-analysis studies have failed to find conclusive support for the use of tDCS as a viable treatment [110]. Factors relating to both biological and experimental variability are major culprits that underlie some of these inconsistencies across the literature [145••].
Biological Factors
Even in healthy participants, there is a fair amount of heterogeneity in responsiveness to tDCS due to a variety of biological factors. These biological factors can be subdivided into factors that affect inter- (across) and intra- (within) subject variability. Inter-subject variability is affected by factors that are due to an individual’s constant traits, such as age [146], gender [147], anatomical variability [148, 149], and genetics [11••, 150, 151]. Intra-subject variability is affected by factors that are due to an individual’s current state, such as hormonal cycles (menstrual cycle and circadian rhythms) [152, 153], prior history of brain activation [154], sleep deprivation [155], attentional focus [156, 157], alcohol/drug use [158], and fluctuations in resting brain activity [159].
In brain injury patients, these same biological factors are further complicated by additional heterogeneity introduced by the brain lesion itself. For example, brain injury patients may have structural brain changes related to the underlying pathology (e.g., brain atrophy) that can alter the current distribution [160]. In a tDCS modeling study based on individual MRI anatomy, patients with skull damage (after TBI) were found to have significant changes in their current distribution which altered the efficacy of tDCS application and in some cases even lead to unfavorable neurophysiologic changes [161, 162]. Brain injury patients are also likely to be on concurrent medications affecting the nervous system (e.g., antidepressants, antipsychotics, anxiolytics, analgesics, anticonvulsants) and have other comorbidities that may alter their neurochemistry. Overall, these trait and state biological factors introduce significant challenges in standardizing current flow and intensity across individuals.
Experimental Factors
In addition to individual variability introduced by biological factors, there are several other experimental factors relating to the administration of tDCS that influence its efficacy: electrode montage and type, dosage, timing, and endpoint measures.
Electrode montage, skin preparation, and type of electrodes are all critical for the spatial distribution and direction of the electric current. The diversity of tDCS montages (unilateral or bilateral, and over various motor areas), type (bipolar or high-definition tDCS [163]), and polarity (anodal or cathodal) can induce varied effects on the brain. Even when targeting a particular motor region, neighboring and connected areas can be affected as well. Not surprisingly, research has shown that different montages have distinct effects on a motor behavior—i.e., targeting M1 preferentially influenced speed and retention of motor learning [68•], whereas targeting the cerebellum promoted accuracy and acquisition [164].
A second experimental factor is the dosage of stimulation, including both intensity, duration, and number of sessions and their relative spacing—all of which influence the longevity, magnitude, and even direction of the effects of tDCS [5]. To a certain extent, longer bouts of stimulation elicit longer-lasting aftereffects [6], increasing stimulation intensity can increase the magnitude and improve the consistency of aftereffects [2, 28], and consecutively repeated sessions of stimulation can have cumulative effects [83, 165,166,167]. However, linearly increasing each of these parameters does not necessarily increase their effects in a linear way. In some cases, increasing intensity, duration, and number of sessions and session spacing can engage homeostatic mechanisms that shift or even reverse the direction of their effects [28, 167, 168], mechanisms that may even further interact with biological factors as well.
A third critical factor is the timing of stimulation and time of stimulation relative to the time of injury. Studies have varied application of tDCS, and here timing will pertain to two independent dimensions: timing of stimulation relative to motor training and timing of stimulation relative to acuity of injury. With motor training, studies have varied application of tDCS to either occur prior to motor training (as a priming effect) [34, 35, 89, 93, 105, 169, 170], immediately following motor training (to target consolidation) [171], concurrently [2, 31, 68, 164, 172,173,174], or in the absence of any motor training [104, 175]. However, evidence in humans indicates that timely co-application of tDCS with motor training is associated with the largest and most consistent behavioral gains [104, 176]. Similarly, in animal studies, applying DCS over M1 with concurrent activity was crucial to induce LTP [11••], and a lack of concurrent activity resulted in no aftereffects. With regard to the time of injury, most studies have focused on studying brain injury patients in a chronic state of recovery, though there is evidence to suggest that tDCS applied during more acute stages of injury may be more effective for recovery [92, 120, 177,178,179]. Special caution is needed to avoid use of tDCS too early in the recovery process as there is the possibility it could aggravate the injury. In an animal model of stroke, tDCS applied 1 week following the injury was associated with better recovery than when applied only 1 day after injury [180].
A final critical experimental factor is the variability of endpoint measures across the literature which limits direct comparisons across studies. To efficiently move the field forward, a consensus on specific and appropriate endpoint measures that span the model of disability (including metrics assessing recovery at the level of body function, activity, and participation) is needed. Having a comprehensive combination of appropriate endpoint measures will help identify which experimental factors are most relevant for neurorehabilitation and what biological factors may preclude certain individuals from benefiting from tDCS as a treatment.
Modeling techniques that use a patient’s MRI to simulate current flow offer one effective approach to improve and individualize the use of tDCS in neurorehabilitation. For one, modeling techniques can be used to optimize electrode placement and intensity to improve uniformity of current flow across individuals [181, 182]. Second, current distribution models can be applied to past data sets and can identify study confounds and distribution patterns that are associated with better behavioral outcomes.
Conclusions
Substantial evidence supports that tDCS can induce both functional and structural changes of the cells it targets depending on their orientation and type, and these effects are linked to LTP-like and LTD-like mechanisms. Similar to motor learning, motor recovery is associated with increased corticomotor excitability and other types of cortical reorganization, mechanisms that can enable functional restoration following brain injury. In some cases, neurophysiological and behavioral restoration following motor training has been boosted with concurrent application of tDCS. Despite promising evidence that tDCS can improve motor syndromes following brain injury (e.g., hemiparesis, hemineglect, and dysphagia), meta-analysis studies have largely failed to find conclusive support for tDCS as a viable treatment. Variability in biological and experimental factors between studies explains, in part, these inconsistencies in the literature. Nonetheless, some patterns of “best practice” have begun to emerge: (1) pairing tDCS concurrently with high-intensity motor training, (2) repeating sessions of stimulation in close succession over a single administration, (3) administration during more acute periods of recovery, and (4) tailoring electrode placement with modeling techniques to optimize electrical distribution patterns. Properly designed smart clinical studies are needed to determine the optimal stimulation parameters and combinations (i.e., with medications, behavior, etc.) of tDCS to verify its true efficacy in clinical settings.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Liebetanz D, Nitsche MA, Tergau F, Paulus W. Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability. Brain J Neurol. 2002;125(Pt 10):2238–47. https://doi.org/10.1093/brain/awf238.
Nitsche MA, Nitsche MS, Klein CC, Tergau F, Rothwell JC, Paulus W. Level of action of cathodal DC polarisation induced inhibition of the human motor cortex. Clin Neurophysiol Off J Int Fed Clin Neurophysiol. 2003;114(4):600–4. https://doi.org/10.1016/s1388-2457(02)00412-1.
Nitsche MA, Grundey J, Liebetanz D, Lang N, Tergau F, Paulus W. Catecholaminergic consolidation of motor cortical neuroplasticity in humans. Cereb Cortex N Y N 1991. 2004;14(11):1240–5. https://doi.org/10.1093/cercor/bhh085.
Nitsche MA, Jaussi W, Liebetanz D, Lang N, Tergau F, Paulus W. Consolidation of human motor cortical neuroplasticity by D-cycloserine. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2004;29(8):1573–8. https://doi.org/10.1038/sj.npp.1300517.
• Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527(Pt 3):633–9. https://doi.org/10.1111/j.1469-7793.2000.t01-1-00633One of the first experiments to demonstrate that tDCS can modulate corticomotor excitability in humans.
Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 2001;57(10):1899–901. https://doi.org/10.1212/wnl.57.10.1899.
• Purpura DP, Shofer RJ, Housepian EM, Noback CR. Comparative ontogenesis of structure-function relations in cerebral and cerebellar cortex. In: Purpura DP, Schadé JP, editors. Progress in brain research. Vol 4. Growth and maturation of the brain: Elsevier; 1964. p. 187–221. https://doi.org/10.1016/S0079-6123(08)61277-7. Demonstrated that DCS can affect spontaneous neural activity.
Bindman LJ, Lippold OCJ, Redfearn JWT. Long-lasting changes in the level of the electrical activity of the cerebral cortex produced by polarizing currents. Nature. 1962;196(4854):584–5. https://doi.org/10.1038/196584a0.
Bindman LJ, Lippold OC, Redfearn JW. The action of brief polarizing currents on the cerebral cortex of the rat (1) during current flow and (2) in the production of long-lasting after-effects. J Physiol. 1964;172:369–82. https://doi.org/10.1113/jphysiol.1964.sp007425.
Creutzfeldt OD, Fromm GH, Kapp H. Influence of transcortical d-c currents on cortical neuronal activity. Exp Neurol. 1962;5(6):436–52. https://doi.org/10.1016/0014-4886(62)90056-0.
•• Fritsch B, Reis J, Martinowich K, et al. Direct current stimulation promotes BDNF-dependent synaptic plasticity: potential implications for motor learning. Neuron. 2010;66(2):198–204. https://doi.org/10.1016/j.neuron.2010.03.035Demonstrated that DCS affects neuronal evoked potentials and is dependent on expression of BDNF to induce plasticity changes.
Márquez-Ruiz J, Leal-Campanario R, Sánchez-Campusano R, et al. Transcranial direct-current stimulation modulates synaptic mechanisms involved in associative learning in behaving rabbits. Proc Natl Acad Sci. 2012;109(17):6710–5. https://doi.org/10.1073/pnas.1121147109.
Cambiaghi M, Velikova S, Gonzalez-Rosa JJ, Cursi M, Comi G, Leocani L. Brain transcranial direct current stimulation modulates motor excitability in mice. Eur J Neurosci. 2010;31(4):704–9. https://doi.org/10.1111/j.1460-9568.2010.07092.x.
• Monai H, Ohkura M, Tanka M, et al. Calcium imaging reveals glial involvement in transcranial direct current stimulation-induced plasticity in mouse brain. - PubMed - NCBI. https://www.ncbi.nlm.nih.gov/pubmed/?term=Calcium+imaging+reveals+glial+involvement+in+transcranial+direct+current+stimulation-induced+plasticity+in+mouse+brain. Accessed Dec 5, 2019.-Demonstrates that other non-neuronal cell types such as astrocytes are affected by DCS.
Islam N, Moriwaki A, Hattori Y, Hori Y. Anodal polarization induces protein kinase C gamma (PKC gamma)-like immunoreactivity in the rat cerebral cortex. Neurosci Res. 1994;21(2):169–72. https://doi.org/10.1016/0168-0102(94)90159-7.
Islam N, Aftabuddin M, Moriwaki A, Hattori Y, Hori Y. Increase in the calcium level following anodal polarization in the rat brain. Brain Res. 1995;684(2):206–8. https://doi.org/10.1016/0006-8993(95)00434-r.
Moriwaki A, Hattori Y, Hayashi Y, Lu YF, Islam N, Hori Y. Repeated application of anodal direct current produces regional dominance in histamine-elicited cyclic AMP accumulation in rabbit cerebral cortex. Acta Med Okayama. 1994;48(6):323–6. https://doi.org/10.18926/AMO/31097.
Rohan JG, Carhuatanta KA, McInturf SM, Miklasevich MK, Jankord R. Modulating hippocampal plasticity with in vivo brain stimulation. J Neurosci. 2015;35(37):12824–32. https://doi.org/10.1523/JNEUROSCI.2376-15.2015.
Podda MV, Cocco S, Mastrodonato A, et al. Anodal transcranial direct current stimulation boosts synaptic plasticity and memory in mice via epigenetic regulation of BDNF expression. Sci Rep. 2016;6:22180. https://doi.org/10.1038/srep22180.
Ranieri F, Podda MV, Riccardi E, et al. Modulation of LTP at rat hippocampal CA3-CA1 synapses by direct current stimulation. J Neurophysiol. 2012;107(7):1868–80. https://doi.org/10.1152/jn.00319.2011.
Alexander JK, Fuss B, Colello RJ. Electric field-induced astrocyte alignment directs neurite outgrowth. Neuron Glia Biol. 2006;2(2):93–103. https://doi.org/10.1017/S1740925X0600010X.
• Gellner A-K, Reis J, Holtick C, Schubert C, Fritsch B. Direct current stimulation-induced synaptic plasticity in the sensorimotor cortex: structure follows function. Brain Stimulat. 2020;13(1):80–8. https://doi.org/10.1016/j.brs.2019.07.026DCS elicits morphological changes such as affecting spine density.
Li Q, Brus-Ramer M, Martin JH, McDonald JW. Electrical stimulation of the medullary pyramid promotes proliferation and differentiation of oligodendrocyte progenitor cells in the corticospinal tract of the adult rat. Neurosci Lett. 2010;479(2):128–33. https://doi.org/10.1016/j.neulet.2010.05.043.
•• Rahman A, Reato D, Arlotti M, et al. Cellular effects of acute direct current stimulation: somatic and synaptic terminal effects. J Physiol. 2013;591(Pt 10):2563–78. https://doi.org/10.1113/jphysiol.2012.247171Direction of electrical current flow influences different cell types and different compartments within a cell.
Nitsche MA, Seeber A, Frommann K, et al. Modulating parameters of excitability during and after transcranial direct current stimulation of the human motor cortex. J Physiol. 2005;568(1):291–303. https://doi.org/10.1113/jphysiol.2005.092429.
Cantarero G, Tang B, O’Malley R, Salas R, Celnik P. Motor learning interference is proportional to occlusion of LTP-like plasticity. J Neurosci. 2013;33(11):4634–41. https://doi.org/10.1523/JNEUROSCI.4706-12.2013.
Cantarero G, Lloyd A, Celnik P. Reversal of long-term potentiation-like plasticity processes after motor learning disrupts skill retention. J Neurosci. 2013;33(31):12862–9. https://doi.org/10.1523/JNEUROSCI.1399-13.2013.
Ammann C, Lindquist MA, Celnik PA. Response variability of different anodal transcranial direct current stimulation intensities across multiple sessions. Brain Stimulat. 2017;10(4):757–63. https://doi.org/10.1016/j.brs.2017.04.003.
Galea JM, Jayaram G, Ajagbe L, Celnik P. Modulation of cerebellar excitability by polarity-specific noninvasive direct current stimulation. J Neurosci. 2009;29(28):9115–22. https://doi.org/10.1523/JNEUROSCI.2184-09.2009.
Boonstra TW, Nikolin S, Meisener A-C, Martin DM, Loo CK. Change in mean frequency of resting-state electroencephalography after transcranial direct current stimulation. Front Hum Neurosci. 2016;10:270. https://doi.org/10.3389/fnhum.2016.00270.
Antal A, Polania R, Schmidt-Samoa C, Dechent P, Paulus W. Transcranial direct current stimulation over the primary motor cortex during fMRI. NeuroImage. 2011;55(2):590–6. https://doi.org/10.1016/j.neuroimage.2010.11.085.
Venkatakrishnan A, Sandrini M. Combining transcranial direct current stimulation and neuroimaging: novel insights in understanding neuroplasticity. J Neurophysiol. 2012;107(1):1–4. https://doi.org/10.1152/jn.00557.2011.
Sehm B, Kipping J, Schäfer A, Villringer A, Ragert P. A comparison between uni- and bilateral tDCS effects on functional connectivity of the human motor cortex. Front Hum Neurosci. 2013;7. https://doi.org/10.3389/fnhum.2013.00183.
Stagg CJ, Best JG, Stephenson MC, et al. Polarity-sensitive modulation of cortical neurotransmitters by transcranial stimulation. J Neurosci. 2009;29(16):5202–6. https://doi.org/10.1523/JNEUROSCI.4432-08.2009.
Stagg CJ, Wylezinska M, Matthews PM, et al. Neurochemical effects of theta burst stimulation as assessed by magnetic resonance spectroscopy. J Neurophysiol. 2009;101(6):2872–7. https://doi.org/10.1152/jn.91060.2008.
Medeiros LF, de Souza ICC, Vidor LP, et al. Neurobiological effects of transcranial direct current stimulation: a review. Front Psychiatry. 2012;3. https://doi.org/10.3389/fpsyt.2012.00110.
Rosso C, Lamy J-C. Does resting motor threshold predict motor hand recovery after stroke? Front Neurol. 2018;9. https://doi.org/10.3389/fneur.2018.01020.
Cakar E, Akyuz G, Durmus O, et al. The relationships of motor-evoked potentials to hand dexterity, motor function, and spasticity in chronic stroke patients: a transcranial magnetic stimulation study. Acta Neurol Belg. 2016;116(4):481–7. https://doi.org/10.1007/s13760-016-0633-2.
• Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol. 2004;55(3):400–9. https://doi.org/10.1002/ana.10848Demonstrated presence of interhemispheric imbalances after stroke that correlate with upper limb impairment.
Duque J, Hummel F, Celnik P, Murase N, Mazzocchio R, Cohen LG. Transcallosal inhibition in chronic subcortical stroke. NeuroImage. 2005;28(4):940–6. https://doi.org/10.1016/j.neuroimage.2005.06.033.
Duque J, Murase N, Celnik P, et al. Intermanual differences in movement-related interhemispheric inhibition. J Cogn Neurosci. 2007;19(2):204–13. https://doi.org/10.1162/jocn.2007.19.2.204.
Bestmann S, Swayne O, Blankenburg F, et al. The role of contralesional dorsal premotor cortex after stroke as studied with concurrent TMS-fMRI. J Neurosci. 2010;30(36):11926–37. https://doi.org/10.1523/JNEUROSCI.5642-09.2010.
Ward NS, Brown MM, Thompson AJ, Frackowiak RSJ. Neural correlates of outcome after stroke: a cross-sectional fMRI study. Brain J Neurol. 2003;126(Pt 6):1430–48. https://doi.org/10.1093/brain/awg145.
Ward NS, Newton JM, Swayne OBC, et al. Motor system activation after subcortical stroke depends on corticospinal system integrity. Brain J Neurol. 2006;129(Pt 3):809–19. https://doi.org/10.1093/brain/awl002.
Koch G, Oliveri M, Cheeran B, et al. Hyperexcitability of parietal-motor functional connections for the intact left-hemisphere in neglect patients. Brain J Neurol. 2008;131(Pt 12):3147–55. https://doi.org/10.1093/brain/awn273.
• Stinear CM, Petoe MA, Byblow WD. Primary motor cortex excitability during recovery after stroke: implications for neuromodulation. Brain Stimulat. 2015;8(6):1183–90. https://doi.org/10.1016/j.brs.2015.06.015Corticomotor excitability and limb function improve with stroke recovery.
Shimizu T, Hosaki A, Hino T, et al. Motor cortical disinhibition in the unaffected hemisphere after unilateral cortical stroke. Brain J Neurol. 2002;125(Pt 8):1896–907. https://doi.org/10.1093/brain/awf183.
Cicinelli P, Traversa R, Rossini PM. Post-stroke reorganization of brain motor output to the hand: a 2-4 month follow-up with focal magnetic transcranial stimulation. Electroencephalogr Clin Neurophysiol. 1997;105(6):438–50. https://doi.org/10.1016/s0924-980x(97)00052-0.
Bütefisch CM, Wessling M, Netz J, Seitz RJ, Hömberg V. Relationship between interhemispheric inhibition and motor cortex excitability in subacute stroke patients. Neurorehabil Neural Repair. 2008;22(1):4–21. https://doi.org/10.1177/1545968307301769.
Xu J, Branscheidt M, Schambra H, et al. Rethinking interhemispheric imbalance as a target for stroke neurorehabilitation. Ann Neurol. 2019;85(4):502–13. https://doi.org/10.1002/ana.25452.
Chistyakov AV, Soustiel JF, Hafner H, Elron M, Feinsod M. Altered excitability of the motor cortex after minor head injury revealed by transcranial magnetic stimulation. Acta Neurochir. 1998;140(5):467–72. https://doi.org/10.1007/s007010050126.
Chistyakov AV, Hafner H, Soustiel JF, Trubnik M, Levy G, Feinsod M. Dissociation of somatosensory and motor evoked potentials in non-comatose patients after head injury. Clin Neurophysiol Off J Int Fed Clin Neurophysiol. 1999;110(6):1080–9. https://doi.org/10.1016/s1388-2457(99)00029-2.
• Bernabeu M, Demirtas-Tatlidede A, Opisso E, Lopez R, Tormos JM, Pascual-Leone A. Abnormal corticospinal excitability in traumatic diffuse axonal brain injury. J Neurotrauma. 2009;26(12):2185–93. https://doi.org/10.1089/neu.2008.0859Reduced MEP amplitude in paretic limb is related to severity of DAI and motor impairment.
Crossley M, Shiel A, Wilson B, et al. Monitoring emergence from coma following severe brain injury in an octogenarian using behavioural indicators, electrophysiological measures and metabolic studies: a demonstration of the potential for good recovery in older adults. Brain Inj. 2005;19(9):729–37. https://doi.org/10.1080/02699050400013733.
Hamdy S, Aziz Q, Rothwell JC, et al. Recovery of swallowing after dysphagic stroke relates to functional reorganization in the intact motor cortex. Gastroenterology. 1998;115(5):1104–12. https://doi.org/10.1016/s0016-5085(98)70081-2.
• Martin SJ, Grimwood PD, Morris RG. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci. 2000;23:649–711. https://doi.org/10.1146/annurev.neuro.23.1.649Review article on the role of synaptic plasticity in M1 and motor learning.
Rioult-Pedotti M-S, Friedman D, Hess G, Donoghue JP. Strengthening of horizontal cortical connections following skill learning. Nat Neurosci. 1998;1(3):230–4. https://doi.org/10.1038/678.
Monfils M-H, Teskey GC. Skilled-learning-induced potentiation in rat sensorimotor cortex: a transient form of behavioural long-term potentiation. Neuroscience. 2004;125(2):329–36. https://doi.org/10.1016/j.neuroscience.2004.01.048.
Hodgson RA, Ji Z, Standish S, Boyd-Hodgson TE, Henderson AK, Racine RJ. Training-induced and electrically induced potentiation in the neocortex. Neurobiol Learn Mem. 2005;83(1):22–32. https://doi.org/10.1016/j.nlm.2004.07.001.
Liepert J, Miltner WH, Bauder H, et al. Motor cortex plasticity during constraint-induced movement therapy in stroke patients. Neurosci Lett. 1998;250(1):5–8. https://doi.org/10.1016/s0304-3940(98)00386-3.
Muellbacher W, Ziemann U, Boroojerdi B, Cohen L, Hallett M. Role of the human motor cortex in rapid motor learning. Exp Brain Res. 2001;136(4):431–8. https://doi.org/10.1007/s002210000614.
Perez MA, Lungholt BKS, Nyborg K, Nielsen JB. Motor skill training induces changes in the excitability of the leg cortical area in healthy humans. Exp Brain Res. 2004;159(2):197–205. https://doi.org/10.1007/s00221-004-1947-5.
Rosenkranz K, Kacar A, Rothwell JC. Differential modulation of motor cortical plasticity and excitability in early and late phases of human motor learning. J Neurosci. 2007;27(44):12058–66. https://doi.org/10.1523/JNEUROSCI.2663-07.2007.
Ziemann U, Ilić TV, Iliać TV, Pauli C, Meintzschel F, Ruge D. Learning modifies subsequent induction of long-term potentiation-like and long-term depression-like plasticity in human motor cortex. J Neurosci. 2004;24(7):1666–72. https://doi.org/10.1523/JNEUROSCI.5016-03.2004.
Pascual-Leone A, Grafman J, Hallett M. Modulation of cortical motor output maps during development of implicit and explicit knowledge. Science. 1994;263(5151):1287–9. https://doi.org/10.1126/science.8122113.
Scholz J, Klein MC, Behrens TEJ, Johansen-Berg H. Training induces changes in white matter architecture. Nat Neurosci. 2009;12(11):1370–1. https://doi.org/10.1038/nn.2412.
Landi SM, Baguear F, Della-Maggiore V. One week of motor adaptation induces structural changes in primary motor cortex that predict long-term memory one year later. J Neurosci. 2011;31(33):11808–13. https://doi.org/10.1523/JNEUROSCI.2253-11.2011.
• Reis J, Schambra HM, Cohen LG, et al. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc Natl Acad Sci. 2009;106(5):1590–5. https://doi.org/10.1073/pnas.0805413106Demonstrated that performance on a skill task in healthy individuals improved with anodal tDCS applied over M1.
Vines BW, Cerruti C, Schlaug G. Dual-hemisphere tDCS facilitates greater improvements for healthy subjects’ non-dominant hand compared to uni-hemisphere stimulation. BMC Neurosci. 2008;9:103. https://doi.org/10.1186/1471-2202-9-103.
Tecchio F, Zappasodi F, Assenza G, et al. Anodal transcranial direct current stimulation enhances procedural consolidation. J Neurophysiol. 2010;104(2):1134–40. https://doi.org/10.1152/jn.00661.2009.
Zimerman M, Heise KF, Hoppe J, Cohen LG, Gerloff C, Hummel FC. Modulation of training by single-session transcranial direct current stimulation to the intact motor cortex enhances motor skill acquisition of the paretic hand. Stroke. 2012;43(8):2185–91. https://doi.org/10.1161/STROKEAHA.111.645382.
Herzfeld DJ, Vaswani PA, Marko MK, Shadmehr R. A memory of errors in sensorimotor learning. Science. 2014;345(6202):1349–53. https://doi.org/10.1126/science.1253138.
Hunter T, Sacco P, Nitsche MA, Turner DL. Modulation of internal model formation during force field-induced motor learning by anodal transcranial direct current stimulation of primary motor cortex. J Physiol. 2009;587(Pt 12):2949–61. https://doi.org/10.1113/jphysiol.2009.169284.
Jayaram G, Tang B, Pallegadda R, Vasudevan EVL, Celnik P, Bastian A. Modulating locomotor adaptation with cerebellar stimulation. J Neurophysiol. 2012;107(11):2950–7. https://doi.org/10.1152/jn.00645.2011.
Kaminski E, Hoff M, Rjosk V, et al. Anodal transcranial direct current stimulation does not facilitate dynamic balance task learning in healthy old adults. Front Hum Neurosci. 2017;11. https://doi.org/10.3389/fnhum.2017.00016.
• Boggio PS, Castro LO, Savagim EA, et al. Enhancement of non-dominant hand motor function by anodal transcranial direct current stimulation. Neurosci Lett. 2006;404(1–2):232–6. https://doi.org/10.1016/j.neulet.2006.05.051Demonstrated that performance on JTT improved with anodal tDCS over M1.
Hummel F, Cohen LG. Improvement of motor function with noninvasive cortical stimulation in a patient with chronic stroke. Neurorehabil Neural Repair. 2005;19(1):14–9. https://doi.org/10.1177/1545968304272698.
Hummel F, Celnik P, Giraux P, et al. Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain J Neurol. 2005;128(Pt 3):490–9. https://doi.org/10.1093/brain/awh369.
Fregni F, Boggio PS, Mansur CG, et al. Transcranial direct current stimulation of the unaffected hemisphere in stroke patients. Neuroreport. 2005;16(14):1551–5. https://doi.org/10.1097/01.wnr.0000177010.44602.5e.
Hummel FC, Cohen LG. Non-invasive brain stimulation: a new strategy to improve neurorehabilitation after stroke? Lancet Neurol. 2006;5(8):708–12. https://doi.org/10.1016/S1474-4422(06)70525-7.
Lefebvre S, Thonnard J-L, Laloux P, Peeters A, Jamart J, Vandermeeren Y. Single session of dual-tDCS transiently improves precision grip and dexterity of the paretic hand after stroke. Neurorehabil Neural Repair. 2014;28(2):100–10. https://doi.org/10.1177/1545968313478485.
Khedr EM, Shawky OA, El-Hammady DH, et al. Effect of anodal versus cathodal transcranial direct current stimulation on stroke rehabilitation: a pilot randomized controlled trial. Neurorehabil Neural Repair. 2013;27(7):592–601. https://doi.org/10.1177/1545968313484808.
Boggio PS, Nunes A, Rigonatti SP, Nitsche MA, Pascual-Leone A, Fregni F. Repeated sessions of noninvasive brain DC stimulation is associated with motor function improvement in stroke patients. Restor Neurol Neurosci. 2007;25(2):123–9.
Rocha S, Silva E, Foerster Á, et al. The impact of transcranial direct current stimulation (tDCS) combined with modified constraint-induced movement therapy (mCIMT) on upper limb function in chronic stroke: a double-blind randomized controlled trial. Disabil Rehabil. 2016;38(7):653–60. https://doi.org/10.3109/09638288.2015.1055382.
Allman C, Amadi U, Winkler AM, et al. Ipsilesional anodal tDCS enhances the functional benefits of rehabilitation in patients after stroke. Sci Transl Med. 2016;8(330):330re1. https://doi.org/10.1126/scitranslmed.aad5651.
Mortensen J, Figlewski K, Andersen H. Combined transcranial direct current stimulation and home-based occupational therapy for upper limb motor impairment following intracerebral hemorrhage: a double-blind randomized controlled trial. Disabil Rehabil. 2016;38(7):637–43. https://doi.org/10.3109/09638288.2015.1055379.
Bornheim S, Croisier J-L, Maquet P, Kaux J-F. Proposal of a new transcranial direct current stimulation safety screening tool. Am J Phys Med Rehabil. 2019;98(7):e77–8. https://doi.org/10.1097/PHM.0000000000001096.
Ochi M, Saeki S, Oda T, Matsushima Y, Hachisuka K. Effects of anodal and cathodal transcranial direct current stimulation combined with robotic therapy on severely affected arms in chronic stroke patients. J Rehabil Med. 2013;45(2):137–40. https://doi.org/10.2340/16501977-1099.
Ilić NV, Dubljanin-Raspopović E, Nedeljković U, et al. Effects of anodal tDCS and occupational therapy on fine motor skill deficits in patients with chronic stroke. Restor Neurol Neurosci. 2016;34(6):935–45. https://doi.org/10.3233/RNN-160668.
Kim D-Y, Lim J-Y, Kang EK, et al. Effect of transcranial direct current stimulation on motor recovery in patients with subacute stroke. Am J Phys Med Rehabil. 2010;89(11):879–86. https://doi.org/10.1097/PHM.0b013e3181f70aa7.
Nair DG, Renga V, Lindenberg R, Zhu L, Schlaug G. Optimizing recovery potential through simultaneous occupational therapy and non-invasive brain-stimulation using tDCS. Restor Neurol Neurosci. 2011;29(6):411–20. https://doi.org/10.3233/RNN-2011-0612.
Lee SJ, Chun MH. Combination transcranial direct current stimulation and virtual reality therapy for upper extremity training in patients with subacute stroke. Arch Phys Med Rehabil. 2014;95(3):431–8. https://doi.org/10.1016/j.apmr.2013.10.027.
Rabadi MH, Aston CE. Effect of transcranial direct current stimulation on severely affected arm-hand motor function in patients after an acute ischemic stroke: a pilot randomized control trial. Am J Phys Med Rehabil. 2017;96(10 Suppl 1):S178–84. https://doi.org/10.1097/PHM.0000000000000823.
Zheng C-J, Liao W-J, Xia W-G. Effect of combined low-frequency repetitive transcranial magnetic stimulation and virtual reality training on upper limb function in subacute stroke: a double-blind randomized controlled trial. J Huazhong Univ Sci Technol Med Sci Hua Zhong Ke Ji Xue Xue Bao Yi Xue Ying Wen Ban Huazhong Keji Daxue Xuebao Yixue Yingdewen Ban. 2015;35(2):248–54. https://doi.org/10.1007/s11596-015-1419-0.
Takebayashi T, Takahashi K, Moriwaki M, Sakamoto T, Domen K. Improvement of upper extremity deficit after constraint-induced movement therapy combined with and without preconditioning stimulation using dual-hemisphere transcranial direct current stimulation and peripheral neuromuscular stimulation in chronic stroke patients: a pilot randomized controlled trial. Front Neurol. 2017;8:568. https://doi.org/10.3389/fneur.2017.00568.
Lindenberg R, Renga V, Zhu LL, Nair D, Schlaug G. Bihemispheric brain stimulation facilitates motor recovery in chronic stroke patients. Neurology. 2010;75(24):2176–84. https://doi.org/10.1212/WNL.0b013e318202013a.
Alisar DC, Ozen S, Sozay S. Effects of bihemispheric transcranial direct current stimulation on upper extremity function in stroke patients: a randomized double-blind sham-controlled study. J Stroke Cerebrovasc Dis Off J Natl Stroke Assoc. 2019;104454. https://doi.org/10.1016/j.jstrokecerebrovasdis.2019.104454.
•• Bolognini N, Vallar G, Casati C, et al. Neurophysiological and behavioral effects of tDCS combined with constraint-induced movement therapy in poststroke patients. Neurorehabil Neural Repair. 2011;25(9):819–29. https://doi.org/10.1177/1545968311411056After chronic stroke, anodal tDCS over M1 paired with upper limb training improved motor performance and reduced transcallosal inhibition from the intact hemisphere.
Cunningham DA, Varnerin N, Machado A, et al. Stimulation targeting higher motor areas in stroke rehabilitation: a proof-of-concept, randomized, double-blinded placebo-controlled study of effectiveness and underlying mechanisms. Restor Neurol Neurosci. 2015;33(6):911–26. https://doi.org/10.3233/RNN-150574.
Andrade SM, Batista LM, Nogueira LLRF, et al. Constraint-induced movement therapy combined with transcranial direct current stimulation over premotor cortex improves motor function in severe stroke: a pilot randomized controlled trial. Rehabil Res Pract. 2017;2017:6842549. https://doi.org/10.1155/2017/6842549.
Plow EB, Cunningham DA, Beall E, et al. Effectiveness and neural mechanisms associated with tDCS delivered to premotor cortex in stroke rehabilitation: study protocol for a randomized controlled trial. Trials. 2013;14:331. https://doi.org/10.1186/1745-6215-14-331.
Middleton A, Fritz SL, Liuzzo DM, Newman-Norlund R, Herter TM. Using clinical and robotic assessment tools to examine the feasibility of pairing tDCS with upper extremity physical therapy in patients with stroke and TBI: a consideration-of-concept pilot study. NeuroRehabilitation. 2014;35(4):741–54. https://doi.org/10.3233/NRE-141178.
Di Lazzaro V, Capone F, Di Pino G, et al. Combining robotic training and non-invasive brain stimulation in severe upper limb-impaired chronic stroke patients. Front Neurosci. 2016;10. https://doi.org/10.3389/fnins.2016.00088.
Rossi C, Sallustio F, Di Legge S, Stanzione P, Koch G. Transcranial direct current stimulation of the affected hemisphere does not accelerate recovery of acute stroke patients. Eur J Neurol. 2013;20(1):202–4. https://doi.org/10.1111/j.1468-1331.2012.03703.x.
Fusco A, Assenza F, Iosa M, et al. The ineffective role of cathodal tDCS in enhancing the functional motor outcomes in early phase of stroke rehabilitation: an experimental trial. Biomed Res Int. 2014;2014:547290. https://doi.org/10.1155/2014/547290.
Hesse S, Werner C, Schonhardt EM, Bardeleben A, Jenrich W, Kirker SGB. Combined transcranial direct current stimulation and robot-assisted arm training in subacute stroke patients: a pilot study. Restor Neurol Neurosci. 2007;25(1):9–15.
Mazzoleni S, Tran V-D, Dario P, Posteraro F. Effects of transcranial direct current stimulation (tDCS) combined with wrist robot-assisted rehabilitation on motor recovery in subacute stroke patients: a randomized controlled trial. IEEE Trans Neural Syst Rehabil Eng Publ IEEE Eng Med Biol Soc. 2019;27(7):1458–66. https://doi.org/10.1109/TNSRE.2019.2920576.
Triccas LT, Burridge JH, Hughes A, Verheyden G, Desikan M, Rothwell J. A double-blinded randomised controlled trial exploring the effect of anodal transcranial direct current stimulation and uni-lateral robot therapy for the impaired upper limb in sub-acute and chronic stroke. NeuroRehabilitation. 2015;37(2):181–91. https://doi.org/10.3233/NRE-151251.
Bradnam LV, Stinear CM, Barber PA, Byblow WD. Contralesional hemisphere control of the proximal paretic upper limb following stroke. Cereb Cortex N Y N 1991. 2012;22(11):2662–71. https://doi.org/10.1093/cercor/bhr344.
Elsner B, Kwakkel G, Kugler J, Mehrholz J. Transcranial direct current stimulation (tDCS) for improving capacity in activities and arm function after stroke: a network meta-analysis of randomised controlled trials. J NeuroEng Rehabil. 2017;14(1):95. https://doi.org/10.1186/s12984-017-0301-7.
Tedesco Triccas L, Burridge JH, Hughes AM, et al. Multiple sessions of transcranial direct current stimulation and upper extremity rehabilitation in stroke: a review and meta-analysis. Clin Neurophysiol Off J Int Fed Clin Neurophysiol. 2016;127(1):946–55. https://doi.org/10.1016/j.clinph.2015.04.067.
Chhatbar PY, Ramakrishnan V, Kautz S, George MS, Adams RJ, Feng W. Transcranial direct current stimulation post-stroke upper extremity motor recovery studies exhibit a dose-response relationship. Brain Stimulat. 2016;9(1):16–26. https://doi.org/10.1016/j.brs.2015.09.002.
Madhavan S, Shah B. Enhancing motor skill learning with transcranial direct current stimulation – a concise review with applications to stroke. Front Psychiatry. 2012;3. https://doi.org/10.3389/fpsyt.2012.00066.
Klomjai W, Aneksan B, Pheungphrarattanatrai A, et al. Effect of single-session dual-tDCS before physical therapy on lower-limb performance in sub-acute stroke patients: a randomized sham-controlled crossover study. Ann Phys Rehabil Med. 2018;61(5):286–91. https://doi.org/10.1016/j.rehab.2018.04.005.
Tanaka S, Takeda K, Otaka Y, et al. Single session of transcranial direct current stimulation transiently increases knee extensor force in patients with hemiparetic stroke. Neurorehabil Neural Repair. 2011;25(6):565–9. https://doi.org/10.1177/1545968311402091.
Sohn MK, Jee SJ, Kim YW. Effect of transcranial direct current stimulation on postural stability and lower extremity strength in hemiplegic stroke patients. Ann Rehabil Med. 2013;37(6):759–65. https://doi.org/10.5535/arm.2013.37.6.759.
Tahtis V, Kaski D, Seemungal BM. The effect of single session bi-cephalic transcranial direct current stimulation on gait performance in sub-acute stroke: a pilot study. Restor Neurol Neurosci. 2014;32(4):527–32. https://doi.org/10.3233/RNN-140393.
Jayaram G, Stinear JW. The effects of transcranial stimulation on paretic lower limb motor excitability during walking. J Clin Neurophysiol Off Publ Am Electroencephalogr Soc. 2009;26(4):272–9. https://doi.org/10.1097/WNP.0b013e3181af1d41.
Manji A, Amimoto K, Matsuda T, Wada Y, Inaba A, Ko S. Effects of transcranial direct current stimulation over the supplementary motor area body weight-supported treadmill gait training in hemiparetic patients after stroke. Neurosci Lett. 2018;662:302–5. https://doi.org/10.1016/j.neulet.2017.10.049.
• Chang MC, Kim DY, Park DH. Enhancement of cortical excitability and lower limb motor function in patients with stroke by transcranial direct current stimulation. Brain Stimulat. 2015;8(3):561–6. https://doi.org/10.1016/j.brs.2015.01.411After chronic stroke, anodal tDCS paired with physical therapy improved lower limb function and increased corticomotor excitability of the affected hemisphere.
Seo JS, Yang HS, Jung S, Kang CS, Jang S, Kim DH. Effect of reducing assistance during robot-assisted gait training on step length asymmetry in patients with hemiplegic stroke: a randomized controlled pilot trial. Medicine (Baltimore). 2018;97(33):e11792. https://doi.org/10.1097/MD.0000000000011792.
Danzl MM, Chelette KC, Lee K, Lykins D, Sawaki L. Brain stimulation paired with novel locomotor training with robotic gait orthosis in chronic stroke: a feasibility study. NeuroRehabilitation. 2013;33(1):67–76. https://doi.org/10.3233/NRE-130929.
Coppens MJM, Staring WHA, Nonnekes J, Geurts ACH, Weerdesteyn V. Offline effects of transcranial direct current stimulation on reaction times of lower extremity movements in people after stroke: a pilot cross-over study. J Neuroeng Rehabil. 2019;16(1):136. https://doi.org/10.1186/s12984-019-0604-y.
van Asseldonk EHF, Boonstra TA. Transcranial direct current stimulation of the leg motor cortex enhances coordinated motor output during walking with a large inter-individual variability. Brain Stimulat. 2016;9(2):182–90. https://doi.org/10.1016/j.brs.2015.10.001.
Kindred JH, Kautz SA, Wonsetler EC, Bowden MG. Single sessions of high-definition transcranial direct current stimulation do not alter lower extremity biomechanical or corticomotor response variables post-stroke. Front Neurosci. 2019;13. https://doi.org/10.3389/fnins.2019.00286.
Cattagni T, Geiger M, Supiot A, Zory R, Pradon D, Roche N. A single session of bihemispheric transcranial direct current stimulation does not improve quadriceps muscle spasticity in people with chronic stroke. Brain Stimulat. 2019;12(5):1309–11. https://doi.org/10.1016/j.brs.2019.06.027.
Geroin C, Picelli A, Munari D, Waldner A, Tomelleri C, Smania N. Combined transcranial direct current stimulation and robot-assisted gait training in patients with chronic stroke: a preliminary comparison. Clin Rehabil. 2011;25(6):537–48. https://doi.org/10.1177/0269215510389497.
Vaz PG, Salazar APDS, Stein C, et al. Noninvasive brain stimulation combined with other therapies improves gait speed after stroke: a systematic review and meta-analysis. Top Stroke Rehabil. 2019;26(3):201–13. https://doi.org/10.1080/10749357.2019.1565696.
Ko M-H, Han S-H, Park S-H, Seo J-H, Kim Y-H. Improvement of visual scanning after DC brain polarization of parietal cortex in stroke patients with spatial neglect. Neurosci Lett. 2008;448(2):171–4. https://doi.org/10.1016/j.neulet.2008.10.050.
Sparing R, Thimm M, Hesse MD, Küst J, Karbe H, Fink GR. Bidirectional alterations of interhemispheric parietal balance by non-invasive cortical stimulation. Brain J Neurol. 2009;132(Pt 11):3011–20. https://doi.org/10.1093/brain/awp154.
Sunwoo H, Kim Y-H, Chang WH, Noh S, Kim E-J, Ko M-H. Effects of dual transcranial direct current stimulation on post-stroke unilateral visuospatial neglect. Neurosci Lett. 2013;554:94–8. https://doi.org/10.1016/j.neulet.2013.08.064.
Yi YG, Chun MH, Do KH, Sung EJ, Kwon YG, Kim DY. The effect of transcranial direct current stimulation on neglect syndrome in stroke patients. Ann Rehabil Med. 2016;40(2):223–9. https://doi.org/10.5535/arm.2016.40.2.223.
• Bang D-H, Bong S-Y. Effect of combination of transcranial direct current stimulation and feedback training on visuospatial neglect in patients with subacute stroke: a pilot randomized controlled trial. J Phys Ther Sci. 2015;27(9):2759–61. https://doi.org/10.1589/jpts.27.2759In hemineglect patients, anodal tDCS over parietal cortex during training improved symptoms of neglect.
Smit M, Schutter DJLG, Nijboer TCW, et al. Transcranial direct current stimulation to the parietal cortex in hemispatial neglect: a feasibility study. Neuropsychologia. 2015;74:152–61. https://doi.org/10.1016/j.neuropsychologia.2015.04.014.
Mylius V, Jung M, Menzler K, et al. Effects of transcranial direct current stimulation on pain perception and working memory. Eur J Pain Lond Engl. 2012;16(7):974–82. https://doi.org/10.1002/j.1532-2149.2011.00105.x.
Salazar APS, Vaz PG, Marchese RR, Stein C, Pinto C, Pagnussat AS. Noninvasive brain stimulation improves hemispatial neglect after stroke: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2018;99(2):355–366.e1. https://doi.org/10.1016/j.apmr.2017.07.009.
Hamdy S, Aziz Q, Rothwell JC, et al. The cortical topography of human swallowing musculature in health and disease. Nat Med. 1996;2(11):1217–24. https://doi.org/10.1038/nm1196-1217.
Mistry S, Verin E, Singh S, et al. Unilateral suppression of pharyngeal motor cortex to repetitive transcranial magnetic stimulation reveals functional asymmetry in the hemispheric projections to human swallowing. J Physiol. 2007;585(Pt 2):525–38. https://doi.org/10.1113/jphysiol.2007.144592.
Kumar S, Wagner CW, Frayne C, et al. Noninvasive brain stimulation may improve stroke-related dysphagia: a pilot study. Stroke. 2011;42(4):1035–40. https://doi.org/10.1161/STROKEAHA.110.602128.
• Shigematsu T, Fujishima I, Ohno K. Transcranial direct current stimulation improves swallowing function in stroke patients. Neurorehabil Neural Repair. 2013;27(4):363–9. https://doi.org/10.1177/1545968312474116After chronic stroke, anodal tDCS over M1 paired with motor training improved swallowing.
Ahn YH, Sohn H-J, Park J-S, et al. Effect of bihemispheric anodal transcranial direct current stimulation for dysphagia in chronic stroke patients: a randomized clinical trial. J Rehabil Med. 2017;49(1):30–5. https://doi.org/10.2340/16501977-2170.
Yang EJ, Baek S-R, Shin J, et al. Effects of transcranial direct current stimulation (tDCS) on post-stroke dysphagia. Restor Neurol Neurosci. 2012;30(4):303–11. https://doi.org/10.3233/RNN-2012-110213.
Pisegna JM, Kaneoka A, Pearson WG, Kumar S, Langmore SE. Effects of non-invasive brain stimulation on post-stroke dysphagia: a systematic review and meta-analysis of randomized controlled trials. Clin Neurophysiol Off J Int Fed Clin Neurophysiol. 2016;127(1):956–68. https://doi.org/10.1016/j.clinph.2015.04.069.
Yang SN, Pyun S-B, Kim HJ, Ahn HS, Rhyu BJ. Effectiveness of non-invasive brain stimulation in dysphagia subsequent to stroke: a systemic review and meta-analysis. Dysphagia. 2015;30(4):383–91. https://doi.org/10.1007/s00455-015-9619-0.
•• Polanía R, Nitsche MA, Ruff CC. Studying and modifying brain function with non-invasive brain stimulation. Nat Neurosci. 2018;21(2):174–87. https://doi.org/10.1038/s41593-017-0054-4Review over variability in individuals’ responsiveness to tDCS because of biological and experimental factors.
Fujiyama H, Hyde J, Hinder MR, et al. Delayed plastic responses to anodal tDCS in older adults. Front Aging Neurosci. 2014;6:115. https://doi.org/10.3389/fnagi.2014.00115.
Kuo M-F, Paulus W, Nitsche MA. Sex differences in cortical neuroplasticity in humans. Neuroreport. 2006;17(16):1703–7. https://doi.org/10.1097/01.wnr.0000239955.68319.c2.
Bikson M, Rahman A, Datta A. Computational models of transcranial direct current stimulation. Clin EEG Neurosci. 2012;43(3):176–83. https://doi.org/10.1177/1550059412445138.
Datta A, Truong D, Minhas P, Parra LC, Bikson M. Inter-individual variation during transcranial direct current stimulation and normalization of dose using MRI-derived computational models. Front Psychiatry. 2012;3. https://doi.org/10.3389/fpsyt.2012.00091.
Kleim JA, Chan S, Pringle E, et al. BDNF val66met polymorphism is associated with modified experience-dependent plasticity in human motor cortex. Nat Neurosci. 2006;9(6):735–7. https://doi.org/10.1038/nn1699.
Cheeran BJ, Ritter C, Rothwell JC, Siebner HR. Mapping genetic influences on the corticospinal motor system in humans. Neuroscience. 2009;164(1):156–63. https://doi.org/10.1016/j.neuroscience.2009.01.054.
Sale MV, Ridding MC, Nordstrom MA. Cortisol inhibits neuroplasticity induction in human motor cortex. J Neurosci. 2008;28(33):8285–93. https://doi.org/10.1523/JNEUROSCI.1963-08.2008.
Sale MV, Ridding MC, Nordstrom MA. Factors influencing the magnitude and reproducibility of corticomotor excitability changes induced by paired associative stimulation. Exp Brain Res. 2007;181(4):615–26. https://doi.org/10.1007/s00221-007-0960-x.
Nishida K, Koshikawa Y, Morishima Y, et al. Pre-stimulus brain activity is associated with state-anxiety changes during single-session transcranial direct current stimulation. Front Hum Neurosci. 2019;13. https://doi.org/10.3389/fnhum.2019.00266.
McIntire LK, McKinley RA, Goodyear C, Nelson J. A comparison of the effects of transcranial direct current stimulation and caffeine on vigilance and cognitive performance during extended wakefulness. Brain Stimulat. 2014;7(4):499–507. https://doi.org/10.1016/j.brs.2014.04.008.
Kiers L, Cros D, Chiappa K, Fang J. Variability of motor potentials evoked by transcranial magnetic stimulation. - PubMed - NCBI. https://www.ncbi.nlm.nih.gov/pubmed/7507428. Accessed Dec 5, 2019.
Darling WG, Wolf SL, Butler AJ. Variability of motor potentials evoked by transcranial magnetic stimulation depends on muscle activation. Exp Brain Res. 2006;174(2):376–85. https://doi.org/10.1007/s00221-006-0468-9.
McLaren ME, Nissim NR, Woods AJ. The effects of medication use in transcranial direct current stimulation: a brief review. Brain Stimulat. 2018;11(1):52–8. https://doi.org/10.1016/j.brs.2017.10.006.
Guerra A, López-Alonso V, Cheeran B, Suppa A. Variability in non-invasive brain stimulation studies: reasons and results. Neurosci Lett. 2017;133330. https://doi.org/10.1016/j.neulet.2017.12.058.
Agnew WF, McCreery DB. Considerations for safety in the use of extracranial stimulation for motor evoked potentials. Neurosurgery. 1987;20(1):143–7. https://doi.org/10.1097/00006-198701000-00030.
Datta A, Bikson M, Fregni F. Transcranial direct current stimulation in patients with skull defects and skull plates: high-resolution computational FEM study of factors altering cortical current flow. NeuroImage. 2010;52(4):1268–78. https://doi.org/10.1016/j.neuroimage.2010.04.252.
Demirtas-Tatlidede A, Vahabzadeh-Hagh AM, Bernabeu M, Tormos JM, Pascual-Leone A. Noninvasive brain stimulation in traumatic brain injury. J Head Trauma Rehabil. 2012;27(4):274–92. https://doi.org/10.1097/HTR.0b013e318217df55.
Kuo M-F, Nitsche MA. Effects of transcranial electrical stimulation on cognition. Clin EEG Neurosci. 2012;43(3):192–9. https://doi.org/10.1177/1550059412444975.
Cantarero G, Spampinato D, Reis J, et al. Cerebellar direct current stimulation enhances on-line motor skill acquisition through an effect on accuracy. J Neurosci. 2015;35(7):3285–90. https://doi.org/10.1523/JNEUROSCI.2885-14.2015.
Alonzo A, Brassil J, Taylor JL, Martin D, Loo CK. Daily transcranial direct current stimulation (tDCS) leads to greater increases in cortical excitability than second daily transcranial direct current stimulation. Brain Stimulat. 2012;5(3):208–13. https://doi.org/10.1016/j.brs.2011.04.006.
Lindenberg R, Zhu LL, Schlaug G. Combined central and peripheral stimulation to facilitate motor recovery after stroke: the effect of number of sessions on outcome. Neurorehabil Neural Repair. 2012;26(5):479–83. https://doi.org/10.1177/1545968311427568.
Monte-Silva K, Kuo M-F, Hessenthaler S, et al. Induction of late LTP-like plasticity in the human motor cortex by repeated non-invasive brain stimulation. Brain Stimulat. 2013;6(3):424–32. https://doi.org/10.1016/j.brs.2012.04.011.
Batsikadze G, Moliadze V, Paulus W, Kuo M-F, Nitsche MA. Partially non-linear stimulation intensity-dependent effects of direct current stimulation on motor cortex excitability in humans. J Physiol. 2013;591(Pt 7):1987–2000. https://doi.org/10.1113/jphysiol.2012.249730.
Amadi U, Allman C, Johansen-Berg H, Stagg CJ. The homeostatic interaction between anodal transcranial direct current stimulation and motor learning in humans is related to GABAA activity. Brain Stimulat. 2015;8(5):898–905. https://doi.org/10.1016/j.brs.2015.04.010.
Cabral ME, Baltar A, Borba R, et al. Transcranial direct current stimulation: before, during, or after motor training? Neuroreport. 2015;26(11):618–22. https://doi.org/10.1097/WNR.0000000000000397.
Rumpf J-J, Wegscheider M, Hinselmann K, et al. Enhancement of motor consolidation by post-training transcranial direct current stimulation in older people. Neurobiol Aging. 2017;49:1–8. https://doi.org/10.1016/j.neurobiolaging.2016.09.003.
Bikson M, Bestmann S, Edwards D. Neuroscience: transcranial devices are not playthings. Nature. 2013;501(7466):167. https://doi.org/10.1038/501167b.
Reis J, Fischer JT, Prichard G, Weiller C, Cohen LG, Fritsch B. Time- but not sleep-dependent consolidation of tDCS-enhanced visuomotor skills. Cereb Cortex N Y NY. 2015;25(1):109–17. https://doi.org/10.1093/cercor/bht208.
Stagg CJ, Jayaram G, Pastor D, Kincses ZT, Matthews PM, Johansen-Berg H. Polarity and timing-dependent effects of transcranial direct current stimulation in explicit motor learning. Neuropsychologia. 2011;49(5):800–4. https://doi.org/10.1016/j.neuropsychologia.2011.02.009.
Sattler V, Acket B, Raposo N, et al. Anodal tDCS combined with radial nerve stimulation promotes hand motor recovery in the acute phase after ischemic stroke. Neurorehabil Neural Repair. 2015;29(8):743–54. https://doi.org/10.1177/1545968314565465.
Reis J, Fritsch B. Modulation of motor performance and motor learning by transcranial direct current stimulation. Curr Opin Neurol. 2011;24(6):590–6. https://doi.org/10.1097/WCO.0b013e32834c3db0.
Stinear CM, Byblow WD. Predicting and accelerating motor recovery after stroke. Curr Opin Neurol. 2014;27(6):624–30. https://doi.org/10.1097/WCO.0000000000000153Stroke recovery predictions based on the presence or absence of an MEP immediately after stroke.
Wang QM, Cui H, Han SJ, et al. Combination of transcranial direct current stimulation and methylphenidate in subacute stroke. Neurosci Lett. 2014;569:6–11. https://doi.org/10.1016/j.neulet.2014.03.011.
Pavlova EL, Semenov RV, Guekht AB. Effect of tDCS on fine motor control of patients in subacute and chronic post-stroke stages. J Mot Behav. 2019:1–13. https://doi.org/10.1080/00222895.2019.1639608.
Yoon KJ, Oh B-M, Kim D-Y. Functional improvement and neuroplastic effects of anodal transcranial direct current stimulation (tDCS) delivered 1 day vs. 1 week after cerebral ischemia in rats. Brain Res. 2012;1452:61–72. https://doi.org/10.1016/j.brainres.2012.02.062.
Bestmann S, de Berker AO, Bonaiuto J. Understanding the behavioural consequences of noninvasive brain stimulation. Trends Cogn Sci. 2015;19(1):13–20. https://doi.org/10.1016/j.tics.2014.10.003.
Huang L, Deng Y, Zheng X, Liu Y. Transcranial direct current stimulation with halo sport enhances repeated sprint cycling and cognitive performance. Front Physiol. 2019;10:118. https://doi.org/10.3389/fphys.2019.00118.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Rehabilitation Technology
Rights and permissions
About this article
Cite this article
Pruski, A., Cantarero, G. Transcranial Direct Current Stimulation for Motor Recovery Following Brain Injury. Curr Phys Med Rehabil Rep 8, 268–279 (2020). https://doi.org/10.1007/s40141-020-00262-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40141-020-00262-8