Abstract
Injury to the central nervous system (CNS) often results in the loss of motor and sensory activity with a tragic impact on quality of life. The anatomic and cellular complexity of the nervous system limits its ability to repair itself, making the effects of the injury permanent. To date, the majority of attempts to restore normal function after damage to the brain or spinal cord have been unsuccessful. Recent studies have demonstrated significant improvements in voluntary motor function in patients with chronic and subacute stroke and spinal cord injury (SCI) using functional electrical stimulation (FES) therapy. In this therapy, patients are asked to perform multitudes of specific motor tasks. During each session, the therapist instructs patients to perform a specific movement at a time, and, after a few seconds of trying, highly controlled electrical stimulation is applied to facilitate that specific movement of the paralyzed limb. After completing this therapy program, individuals are often able to perform the tasks voluntarily, i.e., unassisted by the FES system. Using this approach, we have been able to assist patients with complete and incomplete spinal cord injuries, severe stroke, and pediatric stroke to recover the ability to reach, grasp, stand, and walk. In this chapter, we explain why we believe FES has achieved such extraordinary results.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Functional electrical stimulation
- Functional electrical stimulation therapy
- Stroke
- Spinal cord injury
- Rehabilitation
- Neuroplasticity
Introduction
Disruption of the neural circuitry of the central nervous system (CNS) has potential catastrophic quality of life consequences for an individual who sustained the injury, regardless of the etiology. The complexity of the anatomical organization and the microscopic diversity of neuron types that make up the nervous system pose a major fundamental challenge for self-repair and self-regeneration. The complex organization of the brain increases its sensitivity to even minor intrinsic and extrinsic perturbations. The prognosis for the recovery of functional motor and sensory loss following a CNS injury or neurodegenerative disease is frequently difficult to predict. Despite an explosion of research in restorative neurology in the recent years, we have not yet been able to successfully repair the affected parts of the CNS and restore normal functional motor and sensory activity.
However, one strategy, in particular, is receiving increasing attention because of its ability to repeatedly achieve successful restoration of voluntary upper and lower limb motor functions in severely disabled individuals. More specifically, in the recent years evidence has emerged that functional electrical stimulation (FES) therapy is capable of improving and restoring voluntary motor function in patients with chronic and subacute stroke and spinal cord injury (SCI). In this chapter, we will try to explain why FES therapy has achieved such extraordinary results to date for both stroke and SCI individuals.
Functional Electrical Stimulation
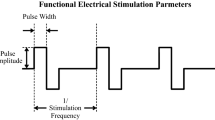
Functional electrical stimulation (FES) technology is able to to produce functional movement in paralyzed muscles after damage to the CNS including spinal cord injury and stroke [1–9]. The artificially created movement is generated by delivering electrical pulses that generate action potentials in muscle and nerve cells producing a muscle contraction [8, 9]. Careful application of highly controlled stimulation sequences makes it possible to produce complex movements such as grasping and walking (Fig. 25.1).
The stimulation can be delivered transcutaneously using electrodes placed on the skin above the nerve of the muscle to be stimulated, making the process convenient and inexpensive. However, transcutaneous stimulation may be incapable of reaching deep structures such as the nerves innervating hip flexors. This limitation can be overcome, to a certain extent, using electrodes in an array configuration, which increase stimulation selectivity by using several contacts [10–12]. It is also possible to use percutaneous and implanted electrodes to apply the stimulation. Percutaneous electrodes are thin wires inserted through the skin and suitable only for short-term FES as they are prone to infection. Implanted electrodes are placed surgically inside the body where they often stay for the rest of the person’s life. Subcutaneous (percutaneous and implanted) electrodes may have higher selectivity for stimulation and their electrical discharges can be smaller than those required with surface electrodes. An important disadvantage of implanted electrodes is that they require an invasive procedure to be introduced to the body, and, as with any surgical procedure, there is a risk of infection.
Originally, FES was envisioned to be used as an orthotic device intended to be worn permanently with users activating it whenever required. Important examples of FES-based orthoses include the Parastep [13, 14], which is designed to facilitate walking by applying electrical stimulation to the surface of the lower limbs over the quadriceps and peroneal nerve, and the Freehand system [15], which is permanently implanted in the users’ upper limb and produces grasping movements. Both systems were the first devices of their kind to receive FDA approval.
Functional Electrical Stimulation Therapy
As discussed above, most traditional FES programs require the persistent application of the electrical stimulation to provide the individual with functional motor activity. Since 2001, our group has been developing an alternative method for using FES technology. Our FES therapy program requires the individual to attend a finite number of FES therapy sessions. Upon completion of the program the individual will have recovered partial or complete voluntary motor function in their upper or lower extremity [16–27]. Our FES therapy program is designed to “retrain” the injured neuromuscular system through repetitive performance of task-specific exercises. We use FES during these “training” sessions to provide assistance with the components of the task that the individual is unable to perform independently. The assistance provided by the FES system to accomplish each task during the “training” session is determined for each individual task at each therapy session. At the completion of the FES therapy program, the individual is usually able to perform the tasks unassisted or with minimal assistance. We have successfully used our FES therapy program to assist adults with incomplete and complete SCI and severe stroke and pediatric stroke patients to recover sustained reaching and grasping motor function [16–18, 20–24]. Adults with an incomplete SCI have also enjoyed robust sustained recovery of functional standing and walking ability after completing our FES therapy program [19, 25–27] (Fig. 25.2).
In our FES therapy program, the participant must attempt to initiate or execute the specific motor task unassisted, such as pinch grasp. Once a brief (10–15 s) attempt to perform the specific task has been made, the therapist delivers an external electrical pulse to the muscles to assist the individual to complete the task. Multitudes of different reaching and grasping tasks are trained. Each task is slightly different and trained for 5 to 7 min. During the early stages of FES therapy, performance of the entire task is supported by FES. As the therapy progresses, FES assistance is slowly reduced and eventually phased out. We believe that the combination of (1) active participation of the patient during therapy, (2) the way in which FES system generates the movement, (3) the fidelity of the movement performed using FES, (4) the accuracy with which FES system mimics the natural limb movements, and (5) repetitive FES-induced movements are critical ingredients of this therapy.
A few groups around the world conduct research on the efficacy of FES therapy, focused primarily on restoration of lower and upper limb function (walking and grasping). In the next few paragraphs, we have described some of the most representative and important work in the field complementary to our work (Fig. 25.3).
FES Therapy for Lower Limb in Stroke
A common complication experienced among stroke patients is drop foot. Drop foot is the lack of ankle dorsiflexion during the swing phase of gait, resulting in foot slapping and a shortened stride length. It has been shown that a drop foot stimulator that electrically stimulates the common peroneal nerve just before a heel off phase of the gait cycle results in contraction of the muscles responsible for dorsiflexion, effectively compensating for the drop foot during the swing phase of the gait cycle. Drop foot stimulators, including the WalkAide [28] (FDA approved) and Odstock [29] surface stimulators, have been used as orthoses as well as to deliver FES therapy, in which they have repeatedly proven more effective in increasing walking speed by up to 28 % while decreasing physiological cost index (PCI) in hemiplegic stroke patients [28, 29] when compared to conventional therapy. It is important to mention that in addition to these positive results, some studies have not found improvements in walking after FES therapy [30, 31].
FES Therapy for Lower Limb in SCI
In contrast to stroke patients, SCI often results in impaired function not only of the ankle joint but also of both legs, pelvis, and the trunk. Accordingly, the FES systems used to assist walking after SCI target muscles on the whole lower limb. One of the most commonly used methods for restoring gait in individuals with paraplegia was developed by Kralj et al. [32] in which electrodes are placed bilaterally over the quadriceps muscles and peroneal nerves. Standing is produced by stimulating the quadricep muscles. Users can initiate walking using buttons placed on a walker. The swing phase starts by interrupting the stimulation to the quadriceps and stimulating the peroneal nerve on the same leg. This stimulation is applied rapidly to trigger the flexor withdrawal reflex producing hip and knee flexion and dorsiflexion. Alternate activation of the right and left legs results in gait. In addition to the drop foot stimulators mentioned above, some of the FES systems that use this strategy include Parastep [13, 14] (mentioned earlier), HAS [33, 94], and the RGO [34] which incorporate active and passive braces, and the Case Western Reserve University (CWRU)/VA neuroprosthesis [35–38], which is implanted surgically.
Bajd et al. [39] first reviewed the effect of FES therapy applied to the lower extremities of people with SCI and concluded that it has important therapeutic effects including strength training, and it benefits drop foot and plantar flexor during gait. In addition, Wieler et al. [40] found that the walking speed increased in SCI individuals by 20 % with a drop foot stimulator after FES therapy.
FET for Restoration of Upper Limb Function Following Stroke
Upper limb function is often affected after a stroke. There are many FES systems to help stroke patients compensate for lost grasping [41–52] as well as reaching and grasping functions [18, 53–56]. The effectiveness of FES therapy to improve hand function after stroke has been studied extensively. In 1996, a meta-analysis concluded that FES is effective in promoting recovery of muscle strength [57] and several studies since then, including randomized control trials, have a found positive effect in both the acute [18, 42, 46, 47, 56, 58] and chronic [17, 18, 41, 43, 45, 51, 52, 59] phases of stroke.
Important examples of FES devices used in these studies include the Freehand system [15], the NESS H200 (previously known as NESS Handmaster) [45], the Bionic Glove [46, 49, 59], the ETHZ-ParaCare neuroprosthesis for grasping [55, 60], the devices designed by Rebersek and Vodovnik [50], the Belgrade Grasping-Reaching System [53]—all of them capable of delivering stimulation with surface electrodes—and the Compex Motion neuroprosthesis developed to deliver a variety of reaching and/or grasping protocols [55]. In addition, Chae et al. [41–43] have used a percutaneous system to conduct their work. The NESS system [45, 61] and the new version of the Bionic Glove [46, 59, 62] have been tested recently for self-administering FES therapy at home instead of the usual delivery of the treatment by a therapist in a clinical environment.
FES Therapy for Restoration of Upper Limb Function following SCI
There is little existing research on the use of FES therapy for upper limb rehabilitation in the SCI population, in which an injury at a T1 level or higher affects grasping and reaching functions. The first concrete evidence of benefit from using FES as a therapy was offered in the article published by Popovic et al. (not the coauthor of this chapter) who demonstrated that using the Bionic Glove can improve voluntary upper limb function in individuals with an SCI at a C5–C7 level [48]. In 2005, Mangold et al. [63] also provided anecdotal evidence that for some individuals with SCI who used an FES system as an orthotic system resulted in the recovery of voluntary upper limb function.
Our Contributions to FES Therapy
Over the last two decades, we have developed FES therapies to promote recovery after spinal cord injury and stroke. To conduct our work, we use the Compex Motion neuroprosthesis [55], which was designed specifically for FES therapy, and, depending on what kind of therapy it is used to deliver, it could have from 4 up to 16 stimulation channels. As a result, Compex Motion may be used to produce specific and complex movements (e.g., palmar, lateral, pinch, and lumbrical grasp as well as bipedal locomotion) with a high degree of control. With this technology, we have created FES systems to restore walking in individuals who have suffered a stroke or a spinal cord injury, as well as reaching and/or grasping movements.
With respect to restoration of walking, in 2006 Thrasher et al. [19] tested the hypothesis that direct muscle stimulation would have rehabilitative potential. Five individuals with chronic, incomplete SCI, a population for whom rehabilitation is not expected to produce significant functional changes completed 12–18 weeks of training using the Compex Motion multichannel neuroprosthesis for walking [19]. All of the participants experienced significant improvements in their walking function. Four of them increased their length of stride as well as their stepping frequency resulting in greater walking speeds, while the fifth individual experienced a significant reduction in preferred assistive devices. The results suggest that the multichannel FES-based gait training regime that is directly stimulating muscles instead of using flexor withdrawal reflexes is viable for restoring voluntary gait in incomplete SCI.
More recently, Kapadia et al. [25] compared the short- and long-term effects of a multichannel FES-assisted walking program using a body weight support and treadmill system versus a non-FES exercise program on gait and balance in individuals with chronic, incomplete SCI (level C2–T12). The individuals attended the training program 3 days a week for 16 weeks in which FES was applied bilaterally to the quadriceps, hamstrings, dorsiflexors, and plantarflexors in the same sequence that they are activated in able-bodied individuals during walking. Spinal cord independent measure (SCIM) mobility subscore improved over time in the participants receiving FES therapy and all other outcomes were similar for both groups. The findings suggest that task-oriented training improves walking ability in individuals with chronic, incomplete SCI. Additional randomized controlled trials need to be conducted to verify if FES-assisted treadmill is superior to aerobic and strength training.
Our research in FES therapy for restoration of upper limb function has yielded important results. In the context of stroke rehabilitation, one of the unique aspects of our work is our focus on restoring reaching and grasping functions in individuals with severe hemiplegia (Fugl-Meyer Assessment ≤15) in which the ability to move has been greatly impaired or completely lost, and for whom recovery of motor function after rehabilitation is rare [18]. This is in contrast to the studies mentioned earlier performed by other groups, which included only participants who had reaching and/or grasping functions at least partially preserved. We recently completed randomized control trials [18, 56] to determine the effects of FES therapy for reaching and grasping in severe stroke patients (i.e., Chedoke McMaster Stages of Motor Recovery scores ≤2 or Fugl-Meyer Assessment ≤15). The findings of these studies suggest that both functions improve with FES therapy and that, in patients with severe hemiplegia, the therapy improved gross motor function but not fine motor movements of the hand. The participants in the FES therapy group experienced median improvement of 24.5 points on Fugl-Meyer Assessment, while the matched control group participants had a median improvement of 0 [18].
In 2006, we conducted the first randomized control trial to assess the impact of FES therapy on grasping after a complete and incomplete traumatic SCI (level C4–C7) [20]. The participants in the study received 40 1-h sessions of either FES therapy or conventional occupational therapy. The study provided clear evidence that participants with both complete and incomplete SCI greatly improved their grasping function following FES therapy as compared to participants who were in the control group.
In a different randomized control trial, we evaluated the effects of FES therapy for restoring grasping in incomplete, traumatic SCI (C3–C7) [21]. All of the participants of the study received conventional occupational therapy for 1 h as described in [20], followed by a 2-h break. After this pause, the subjects received an additional hour of either conventional occupational therapy or FES therapy for grasping. After 40 1-h sessions (5 days a week for 8 weeks), the results revealed a significant and function improvement in participants who received FES therapy [21]. The participants in the FES therapy group experienced median improvement of 12 points on Spinal Cord Independence Measure self-care subscore, while the matched control group participants had a median improvement of 3 points on Spinal Cord Independence Measure self-care subscore [21].
In a long-term follow up study [16], both the FES therapy and conventional therapy groups sustained or improved their hand function compared to their scores at the time of discharge, suggesting that the dramatic changes in hand function produced by FES therapy persist over time.
We recently reported preliminary evidence demonstrating the potential ability of FES therapy to restore upper limb function (reaching and grasping) in severe chronic pediatric stroke patients [17]. As with adults, rehabilitation of motor function was unsuccessful in this population. There were four participants in that study with hemiplegia, unable to use the affected arm functionally. They received one hour of FES therapy three times per week for 16 weeks (48 sessions in total). All of the participants showed considerable improvements in their upper limb function.
The results that our group achieved with FES therapy for improving reaching and grasping in stroke and SCI individuals, motivated our team to create a product that can be used to deliver this intervention. In 2014, a Canadian company MyndTec Inc. launched first FES system specially developed to deliver FES therapy for restoring upper limb function in stroke and SCI individuals. The product is called MyndMove and it incorporates all stimulation protocols and technology our team has developed in this field in the last two decades. MyndMove offers 17 FES-based interventions for stroke individuals and 13 FES-based interventions for SCI individuals.
In conclusion, there is strong evidence to support the use of FES therapy as an effective tool for retraining of walking, reaching, and grasping functions after a stroke or an SCI. In the remainder of the chapter, we will provide possible explanations to the effectiveness of FES therapy in restoring motor function.
Selected Processes in the Healthy Central Nervous System Pertinent to Neurorecovery following an Injury to the Central Nervous System
Neuroplasticity and the Healthy Adult Brain
The FES therapy program is designed to optimize the neuroplastic potential of the adult brain. It is well established that adult neural stem cells (NSCs) and neural progenitor cells (NPCs) are present throughout the CNS [64–67]. However, they are more densely distributed in two particular subregions of the adult brain, the subventricular zone and the hippocampal subgranular zone [64]. Regardless of their locations, the precursors possess the capacity for inexhaustible self-renewal and pluripotency (i.e., they can differentiate into a wide variety of neurons, astrocytes, and/or oligodendrocytes) [68]. Research has shown that adult neurogenesis is highly regulated. In 1998, Wang et al. [69] demonstrated that self-renewal and regeneration normally occur in the adult brain through highly specific and targeted neuronal apoptosis. The signals that guide the establishment and maintenance of neuronal diversity and connectivity during development also play a pivotal role during differentiation and integration of new adult-born neurons within the neurogenic niches in the adult brain [69]. Differentiation of adult-born neurons (i.e., commitment to a particular neuronal identity) is guided by endogenous and exogenous stimuli, genetic, and epigenetic factors [70]. During differentiation, the immature neurons are constantly learning and adapting to their microenvironment. These experiences and learnt lessons are unique to each immature neuron. At maturation, these neurons will become integrated (synaptogenesis) into the preexisting neural network(s). This permits the neural network to preserve old memories and store new memories. The addition of new memories to the neural network enables it to adapt to its constantly changing environment and to maintain homeostasis in a “hostile” microenvironment [71–73].
The Healthy Neurological System and Exercise
Adult neurogenesis has been shown to be acutely responsive to changes in its microenvironment. These changes may result from external or internal stimuli as well as from genetic and epigenetic factors [68]. One of the most studied influences on adult neurogenesis is the role of exercise in learning and memory creation. It is well established that exercise increases neurogenesis in the healthy brain of rodents and humans [74–78]. In 2010, Kobilo reported that adult neurogenesis in the mouse hippocampus was enhanced by voluntary exercise in a running wheel [70, 74]. The onset of the effect in the hippocampus was rapid. Running induced cell proliferation in adult mice. It peaked after 3 days of running and was significantly enhanced at 10 days. After 32 days of running, the proliferative effect returned to baseline, but the number of new neurons continued to increase. More importantly, exercise was shown to enhance the maturation of the newborn neurons. Enhancement of hippocampal neurogenesis by running is a robust phenomenon that has been replicated by many different laboratories [70, 74–78]. Exercise-induced increase in neurogenesis is associated with enhanced hippocampal synaptic plasticity, more specifically, long-term potentiation (LTP) [70]. Becker and Wojtowicz [79, 80] reported that new neurons generally lack inhibition and have superior ability to express LTP. Both of these properties make new neurons suitable for synaptic integration via spatial and temporal summation of afferent synaptic inputs [80] and ideal for creating new memories [79–81].
The Injured Neurological System, Neurogenesis, and Exercise
What blocks the brain from recovering functional motor and sensory activity following a catastrophic injury or insult? We know that regardless of age (infant, child, young adult, adult, or aging individual), the clinical outcome is related to the severity, etiology, and location of the “lesion” [82, 83]. Why are the NSCs and the NPCs unable to comprehend the severity of the damage to the brain and mount an adequate response?
What we do know is that the neural networks possess all of the necessary equipment to ultimately recover from serious injury or neurodegenerative disease. What remains a mystery is why the system appears to become terminally disabled by these insults. Varying degrees (partial) of functional motor and sensory activity are recoverable following a catastrophic CNS with the assistance of standard rehabilitation practices. Standard rehabilitation practices include a prescription of or assistance with specific exercises, manual therapy, education, manipulation, and other interventions. Rehabilitation programs are designed to optimize the benefits of exercise with respect to neurogenesis, learning, and memory. Yet, despite our valiant efforts, we are unable to achieve complete or incomplete functional motor and sensory recovery for patients who have sustained a catastrophic neurological injury or who have a neurodegenerative disease. Why?
We hypothesize that several unfavorable conditions may exist in both the injured neural tissue and in tissue affected by a neurodegenerative disease. First, the rate at which the damage occurred may result in an insufficient number of available precursor cells to bring about functional recovery. Second, the balance between neurogenesis and gliogenesis may be tipped in favor of gliogenesis. The differentiation fates of endogenous precursors may be too limited to permit adequate differentiation fates of the endogenous precursors and too limited to allow their integration into varied portions of the brain. Third, the potential challenge is that it could be difficult to provide the precise combination and sequence of molecular signals necessary to induce endogenous precursors to proliferate efficiently and differentiate precisely into appropriate types of neurons deep in the brain. It is well documented that the timing of neurogenesis during development is highly correlated with neuronal laminar position and subsequent connectivity [71]. At this time, it is unknown if the same developmental sequence of events followed by neuroblasts in the developing brain are followed by NPCs and NSCs.
There is also another aspect to this challenge. Following CNS injury, body parts that were previously controlled by the injured part of the CNS are left affected or paralyzed due to the injury. If the limb or body part is mildly affected by the injury, and the patient is able to use it partially, the individual can be engaged in repetitive exercise treatments that eventually can help partially or completely restore the function of the affected limb or body part. This concept has been well demonstrated with the constraint-induced movement therapy (CIMT) [84].
However, when the neurological patient does not have any residual motor function, then one cannot deploy repetitive exercises/therapies, and the prospects of motor function recovery are reduced almost to zero. This can be explained by the loss or compromised control of the muscles by the responsible part of the CNS due to severe injury. Over time, the neuromuscular system associated with a particular limb drifts into two possible extreme “modes of operation,” flaccid paralysis or paralysis in which many of the limb muscles are contracted most of the time. Or the neuromuscular system assumes a “mode of operation” that is between these two possible extremes. No matter in which state the system “settles in” the patient is typically not able to voluntarily activate the muscles of interest and this with time results in “learned nonuse” of the affected limb or body part [84]. Once the patients reach the state of “learned nonuse” it is extremely difficult to help them relearn the affected motor task(s). Also, as the process of “learned nonuse” progresses, the remaining intact parts of the CNS that were before engaged in performing a desired motor task are with time “hijacked” by other motor, sensory, or cognitive tasks and become engaged in performing these new tasks. This process is known as neuroplasticity; more specifically, this is a form of neuroplasticity which is not necessarily desirable, especially in neurologic patients, as the “memory” of performing the tasks of interest slowly “fades” away with time.
The combination of cell, tissue, and circuit level challenges, as discussed above, is caused by the CNS injury and ultimately results in “learned nonuse” that frequently interferes with functional motor recovery. If any of these challenges could be addressed effectively, and, preferably, if few of them could be addressed simultaneously and successfully, one would be able to potentially help restore voluntary function in severe neurologic patients.
The Injured Neurological System and Functional Electrical Stimulation Therapy
How does FES therapy provide individuals with a neurological injury or neurodegenerative disease with the opportunity to recover sustained functional motor activity, while electrical stimulation does not? FES therapy is not simply the application of an electrical stimulus to the paralyzed muscle(s). We believe that FES therapy may be the ideal augmentative rehabilitation intervention. In addition to the physical rehabilitation benefits, recent studies suggest that FES therapy may facilitate or augment the repair of the injured nervous system. It has the potential to functionally reconstruct the damaged neural circuits through its potential ability to promote the self-regeneration capacity of the CNS by promoting:
-
1.
Robust regeneration and replenishment of neural cells.
-
2.
Robust regeneration and repair (myelination) of axons.
-
3.
Providing the necessary rhythmical and spatiotemporally organized efferent and afferent inputs to ensure that the synaptic connections are organized and operate according to the somatotopic maps and designated functions.
FES therapy uses electrical stimulation to guide the NPCs and NCSs to the site of the lesion. These electrical activity patterns influence a variety of developmental processes during corticogenesis, such as neurogenesis, apoptosis, neuronal migration, differentiation into a variety of different neurons, and network formation [85–88]. The information carried in these signals is critical not only during the initial organization of the nervous system but is perhaps even more critical during adulthood [89]. Later, experience-dependent, use-driven adaptations are encoded by the spatiotemporal pattern of sensory processing and intrinsic neural activity that lead to declarative learning and the acquisition of procedural skills [89]. Both forms of neural activity shape synaptic development [89].
Numerous studies have explored the role of electric fields in the CNS. Physiological direct current electric fields (dcEFs) play important roles during development and in tissue repair [86, 90–94] and have been shown to cathodally direct the turning of growth cones during axon elongation [91, 95]. Babona-Pilipos et al. [90, 94] have demonstrated that clonally derived pure populations of adult SE-derived NPCs exhibit rapid and directed galvanotaxis toward the cathode of a dcEF. This phenomenon is unique to undifferentiated NPCs. By inducing NPCs, maturation into differentiated phenotypes is associated with a loss of electrically induced migratory capacity. Thus the data indicate that externally applied dcEFs can stimulate and guide the migration of undifferentiated SE-derived NPCs, but not that of NPCs induced to differentiate into mature neural phenotypes. Similar studies have been performed on the NPCs that reside in the periventricular lining of the central canal. Therefore, FES therapy may assist in the self-repair and regeneration process by selectively increasing the volume of the neural cells at the site of the lesion and providing critical functional spatiotemporal information during their differentiation and maturation phases.
Our research has definitely demonstrated that FES therapy promotes increased neural activity below the level of injury in incomplete spinal cord injury rats [84, 96]. Experiments conducted by our team using animal models have shown that the FES therapy promotes rewiring of the neuronal circuitry below the level of spinal cord injury and that it also promotes propagation of the afferent signals over the site of injury to the somatosensory cortex [96]. These changes in the CNS activity following short-term FES therapy for walking (therapy was delivered to incomplete spinal cord injury rats—dose 15 min per session, three times per day, for 7 days) were not observed in the control group that was implanted with the FES system, but did not receive the FES therapy.
We have clearly established that it is critical that FES is administered while the patient is trying to perform the task. By doing that we are essentially generating proper muscle activation and proper sequence of muscle activities needed to carry out a desired task. This in turn produces popper muscle tension that is essential for producing needed afferent signals. Only muscles that are contracted with the proper level of intensity generate adequate afferent signals produced by muscle spindles, Golgi tendons, and other sensory receptors. If the muscles are not active, and they do not move along the desired “muscle contraction profile” they do not produce relevant afferent signals. This is why passive limb movements produced manually or using robotic systems are not providing sufficient afferent feedback needed for retraining motor tasks. It is this volley of afferent input combined with motor task planning and proper efferent input that are essential for the retraining of the injured CNS. More specifically, our clinical studies suggest that if a neurologic patient who attempts to execute a motor task is assisted with the FES therapy to carry out that task, he/she is effectively voluntarily generating the motor command. FES therapy is then providing both efferent input and afferent feedback (system’s output), indicating that the command was executed properly, successfully, and in physiologically correct manner. We hypothesize that by providing both the “command input” and “system’s output” to the CNS repetitively over enough time, this type of treatment facilitates functional reorganization within the sensorimotor network. We believe that the combination of performing diverse and meaningful tasks with high repetition and subject’s persistent active engagement (i.e., subject has to devote 100 % of his/her attention to the tasks performed) is playing a critical role in retraining voluntary motor functions. These strategies are fully in tune with the recent findings in the field of neuroplasticity [97] and suggest that the proposed FES therapy is potentially very effective method that can be used to retrain the neuromuscular system.
The CNS is a distributed system. This essentially means that even though some parts of the CNS are “more” responsible for performing a particular task, other parts of the system are also engaged. Therefore, following the injury to the CNS a part of the subsystem that is mainly responsible for carrying out a particular task may be damaged, but the other “less engaged” subsystems may remain intact and receptive to retraining. Currently, scientists have focused their research at exploring how the cortex can be “plastically” changed to accommodate these changes and retraining. It is our belief that phylogenetically older brain structures, such as the brain stem, may also have a capacity to relearn some motor tasks. The fact that severe stroke patients following FES therapy for reaching and grasping often relearn how to voluntarily grasp and release objects but are unable to relearn fine finger motor tasks may suggest that phylogenetically older brain structures have been engaged in the process of reaching and grasping task relearning [18]. In SCI patients, relearning fine finger motor tasks may not be such a challenge as it is in severe stroke patients. This suggests that in SCI patients the neuronal recovery occurs at the level of spinal cord and it allows more complex, cortical (supraspinal) commands to flow to the spinal cord below the level of lesion, allowing patient to relearn fine motor tasks.
We recently completed a clinical study with a chronic severe stroke patient [23]. We observed that even if the FES protocol is not a 100 % accurate representation of the actual upper limb movement and it is not engaging all relevant muscles, but only most prominent ones, the CNS will fast realize which muscles also need to be activated, in addition to the stimulated ones, to generate proper hand or arm movement. As a result, after 5–10 sessions the CNS itself will start engaging all relevant muscles in order to perform the task of interest. We were able to observe this finding by measuring voluntary EMG activities on both stimulated and nonstimulated muscles. Not only did the CNS demonstrate ability to regain voluntary control over the stimulated muscles following the FES therapy, but it was also able to regain voluntary control over the muscles that were not stimulated but are important for the correct performance of the desired task. We interpret this finding in the following manner. The volleys of the efferent input and afferent feedback indicate to the CNS that it is asked to perform a task which it until recently was performing all the time. The CNS then “recognizes” the tasks and voluntarily attempts to perform it. As its ability to recruit stimulated muscles increases the CNS automatically starts engaging both stimulated and other relevant muscles needed to carry out the task. Essentially, the “memory” of the neuromuscular systems is being refreshed and as this memory is becoming more and more engaged the system starts engaging all muscles of relevance in physiologically correct manner in order to carry out the desired task.
Finally, in addition to the clinically relevant and meaningful improvements in voluntary motor function we have achieved using FES therapy, we have also observed a myriad of other clinical benefits that FES therapy offers. Many of our patents experienced immediate reduction in spasticity and muscle tone, which later persisted following therapy completion. Others reported reduced pain, better posture, improved bladder and bowel function (especially patients who took part in the FES therapy for walking), and improved muscle and skin condition. All stroke patients reported that shoulder subluxation ceased to be a problem after 10+ therapy sessions. Also, one chronic stroke subject (>2 years poststroke), who received FES therapy for reaching and grasping, experienced dramatic improvement in speech following 20 treatments. As this is not the topic of this article, we will discuss these findings at another more opportune time.
Conclusion
We propose that the FES therapy restores functional motor activity by supporting the functional reconstruction and reorganization of the neural circuits in the CNS. The FES therapy does that by (1) enhancing neurogenesis (the recruitment, regeneration, and differentiation of neural progenitor stem cells) at lesion; (2) spatially and topographically organizes synaptogenesis (axonal regeneration and collateral sprouting) and remyelination; (3) reactivates the “memory” in the neuromuscular system; (4) helps create new neural networks within the preserved parts of the CNS that will substitute the function of the damaged part of the nervous system to allow it to control and execute desired motor functions; (5) by repetitively providing proper efferent and afferent input, it helps create and retrain the neural networks described in (4); and (6) maintains the integrity of the neuromuscular system.
The clinical results achieved to date in restoring voluntary reaching and grasping function in severe stroke and SCI individuals suggest that these improvements are dramatic and clinically relevant and that the FES therapy has to be taken into serious consideration as the potential new best practice for restoring upper limb function, at least in these two patient populations. As for the walking therapy, more rigorous randomized control trials are needed before we can say with confidence that the FES therapy is effective in restoring voluntary locomotion function in stroke and SCI individuals, although the initial findings are encouraging.
References
Groah SL, Lichy AM, Libin AV, Ljungberg I. Intensive electrical stimulation attenuates femoral bone loss in acute spinal cord injury. PM R. 2010;2:1080–7.
Biering-Sørensen F, Hansen B, Lee BSB. Non-pharmacological treatment and prevention of bone loss after spinal cord injury: a systematic review. Spinal Cord. 2009;47:508–18.
Lai C-H, Chang WH-S, Chan WP, Peng C-W, Shen L-K, Chen J-JJ, et al. Effects of functional electrical stimulation cycling exercise on bone mineral density loss in the early stages of spinal cord injury. J Rehabil Med. 2010;42:150–4.
Kennelly MJ, Bennett ME, Grill WM, Grill JH, Boggs JW. Electrical stimulation of the urethra evokes bladder contractions and emptying in spinal cord injury men: case studies. J Spinal Cord Med. 2011;34:315–21.
Kutzenberger J, Domurath B, Sauerwein D. Spastic bladder and spinal cord injury: seventeen years of experience with sacral deafferentation and implantation of an anterior root stimulator. Artif Organs. 2005;29:239–41.
Gyawali S, Solis L, Chong SL, Curtis C, Seres P, Kornelsen I, et al. Intermittent electrical stimulation redistributes pressure and promotes tissue oxygenation in loaded muscles of individuals with spinal cord injury. J Appl Physiol. 2011;110:246–55.
Minassian K, Hofstoetter U, Tansey K, Mayr W. Neuromodulation of lower limb motor control in restorative neurology. Clin Neurol Neurosurg. 2012;114:489–97.
Popovic MR, Thrasher A. Neuroprostheses. In: Wnek GE, Bowling GL, editors. Encyclopedia of biomaterials and biomedical engineering. 2nd edn. CRC Press USA; 2008. p. 3434.
Masani K, Popovic MR. Functional electrical stimulation: applications in rehabilitation and neurorehabilitation. In: Kramme R, Hoffmann K-P, Pozos RS, editors. Handbook of medical technology. New York: Springer; 2011. p. 877–96.
Kuhn A, Keller T, Micera S, Morari M. Array electrode design for transcutaneous electrical stimulation: a simulation study. Med Eng Phys. 2009;31:945–51.
Micera S, Keller T, Lawrence M, Morari M, Popovic DB. Wearable neural prostheses. Restoration of sensory-motor function by transcutaneous electrical stimulation. IEEE Eng Med Biol Mag. 2010;29:64–9.
Popovic DB, Popovic MB. Automatic determination of the optimal shape of a surface electrode: selective stimulation. J Neurosci Methods. 2009;178:174–81.
Graupe D, Kohn KH. Functional neuromuscular stimulator for short-distance ambulation by certain thoracic-level spinal-cord-injured paraplegics. Surg Neurol. 1998;50:202–7.
Graupe D, Davis R, Kordylewski H, Kohn K. Ambulation by traumatic T4-12 paraplegics using functional neuromuscular stimulation. Crit Rev Neurosurg. 1998;8:221–31.
Smith B, Tang Z, Johnson MW, Pourmehdi S, Gazdik MM, Buckett JR, et al. An externally powered, multichannel, implantable stimulator-telemeter for control of paralyzed muscle. IEEE Trans Biomed Eng. 1998;45:463–75.
Kapadia NM, Zivanovic V, Furlan JC, Craven BC, McGillivray C, Popovic MR. Functional electrical stimulation therapy for grasping in traumatic incomplete spinal cord injury: randomized control trial. Artif Organs. 2011;35:212–6.
Kapadia NM, Nagai MK, Zivanovic V, Bernstein J, Woodhouse J, Rumney P, et al. Functional electrical stimulation therapy for recovery of reaching and grasping in severe chronic pediatric stroke patients. J Child Neurol. 2014;29:493–9.
Thrasher TA, Zivanovic V, McIlroy W, Popovic MR. Rehabilitation of reaching and grasping function in severe hemiplegic patients using functional electrical stimulation therapy. Neurorehabil Neural Repair. 2008;22:706–14.
Thrasher TA, Flett HM, Popovic MR. Gait training regimen for incomplete spinal cord injury using functional electrical stimulation. Spinal Cord. 2006;44:357–61.
Popovic MR, Thrasher TA, Adams ME, Takes V, Zivanovic V, Tonack MI. Functional electrical therapy: retraining grasping in spinal cord injury. Spinal Cord. 2006;44:143–51.
Popovic MR, Kapadia N, Zivanovic V, Furlan JC, Craven BC, McGillivray C. Functional electrical stimulation therapy of voluntary grasping versus only conventional rehabilitation for patients with subacute incomplete tetraplegia: a randomized clinical trial. Neurorehabil Neural Repair. 2011;25:433–42.
Kapadia N, Popovic MR. Functional electrical stimulation therapy for grasping in spinal cord injury: an overview. Top Spinal Cord Inj Rehabil. 2011;17:70–6.
Kawashima N, Popovic MR, Zivanovic V. Effect of intensive functional electrical stimulation therapy on upper-limb motor recovery after stroke: case study of a patient with chronic stroke. Physiother Can. 2013;65:20–8.
Kapadia N, Zivanovic V, Popovic MR. Restoring voluntary grasping function in individuals with incomplete chronic spinal cord injury: pilot study. Top Spinal Cord Inj Rehabil. 2013;19:279–87.
Kapadia N, Masani K, Catharine Craven B, Giangregorio LM, Hitzig SL, Richards K, et al. A randomized trial of functional electrical stimulation for walking in incomplete spinal cord injury: effects on walking competency. J Spinal Cord Med. 2014;37:511–24.
Giangregorio L, Craven C, Richards K, Kapadia N, Hitzig SL, Masani K, et al. A randomized trial of functional electrical stimulation for walking in incomplete spinal cord injury: effects on body composition. J Spinal Cord Med. 2012;35:351–60.
Hitzig SL, Craven BC, Panjwani A, Kapadia N, Giangregorio LM, Richards K, et al. Randomized trial of functional electrical stimulation therapy for walking in incomplete spinal cord injury: effects on quality of life and community participation. Top Spinal Cord Inj Rehabil. 2013;19:245–58.
Stein RB, Everaert DG, Thompson AK, Chong SL, Whittaker M, Robertson J, et al. Long-term therapeutic and orthotic effects of a foot drop stimulator on walking performance in progressive and nonprogressive neurological disorders. Neurorehabil Neural Repair. 2010;24:152–67.
Taylor PN, Burridge JH, Dunkerley AL, Wood DE, Norton JA, Singleton C, et al. Clinical use of the Odstock dropped foot stimulator: its effect on the speed and effort of walking. Arch Phys Med Rehabil. 1999;80:1577–83.
Burridge JH, Taylor PN, Hagan SA, Wood DE, Swain ID. The effects of common peroneal stimulation on the effort and speed of walking: a randomized controlled trial with chronic hemiplegic patients. Clin Rehabil. 1997;11:201–10.
Granat MH, Maxwell DJ, Ferguson AC, Lees KR, Barbenel JC. Peroneal stimulator; evaluation for the correction of spastic drop foot in hemiplegia. Arch Phys Med Rehabil. 1996;77:19–24.
Kralj A, Bajd T, Turk R. Enhancement of gait restoration in spinal injured patients by functional electrical stimulation. Clin Orthop Relat Res. 1988;233:34–43.
Popovic D, Tomović R, Schwirtlich L. Hybrid assistive system—the motor neuroprosthesis. IEEE Trans Biomed Eng. 1989;36:729–37.
Solomonow M, Baratta R, Hirokawa S, Rightor N, Walker W, Beaudette P, et al. The RGO generation II: muscle stimulation powered orthosis as a practical walking system for thoracic paraplegics. Orthopedics. 1989;12:1309–15.
Bailey SN, Hardin EC, Kobetic R, Boggs LM, Pinault G, Triolo RJ. Neurotherapeutic and neuroprosthetic effects of implanted functional electrical stimulation for ambulation after incomplete spinal cord injury. J Rehabil Res Dev. 2010;47:7–16.
Davis JA, Triolo RJ, Uhlir J, Bieri C, Rohde L, Lissy D, et al. Preliminary performance of a surgically implanted neuroprosthesis for standing and transfers—where do we stand? J Rehabil Res Dev. 2001;38:609–17.
Davis JA, Triolo RJ, Uhlir JP, Bhadra N, Lissy DA, Nandurkar S, et al. Surgical technique for installing an eight-channel neuroprosthesis for standing. Clin Orthop Relat Res. 2001;385:237–52.
Hardin E, Kobetic R, Murray L, Corado-Ahmed M, Pinault G, Sakai J, et al. Walking after incomplete spinal cord injury using an implanted FES system: a case report. J Rehabil Res Dev. 2007;44:333–46.
Bajd T, Kralj A, Stefancic M, Lavrac N. Use of functional electrical stimulation in the lower extremities of incomplete spinal cord injured patients. Artif Organs. 1999;23:403–9.
Wieler M, Stein RB, Ladouceur M, Whittaker M, Smith AW, Naaman S, et al. Multicenter evaluation of electrical stimulation systems for walking. Arch Phys Med Rehabil. 1999;80:495–500.
Chae J, Harley MY, Hisel TZ, Corrigan CM, Demchak JA, Wong Y-T, et al. Intramuscular electrical stimulation for upper limb recovery in chronic hemiparesis: an exploratory randomized clinical trial. Neurorehabil Neural Repair. 2009;23:569–78.
Chae J, Bethoux F, Bohine T, Dobos L, Davis T, Friedl A. Neuromuscular stimulation for upper extremity motor and functional recovery in acute hemiplegia. Stroke. 1998;29:975–9.
Chae J, Hart R. Intramuscular hand neuroprosthesis for chronic stroke survivors. Neurorehabil Neural Repair. 2003;17:109–17.
Francisco G, Chae J, Chawla H, Kirshblum S, Zorowitz R, Lewis G, et al. Electromyogram-triggered neuromuscular stimulation for improving the arm function of acute stroke survivors: a randomized pilot study. Arch Phys Med Rehabil. 1998;79:570–5.
Hendricks HT, IJzerman MJ, de Kroon JR, in’t Groen FA, Zilvold G. Functional electrical stimulation by means of the “Ness Handmaster Orthosis” in chronic stroke patients: an exploratory study. Clin Rehabil. 2001;15:217–20.
Kowalczewski J, Gritsenko V, Ashworth N, Ellaway P, Prochazka A. Upper-extremity functional electric stimulation-assisted exercises on a workstation in the subacute phase of stroke recovery. Arch Phys Med Rehabil. 2007;88:833–9.
Popovic DB, Popovic MB, Sinkjaer T, Stefanovic A, Schwirtlich L. Therapy of paretic arm in hemiplegic subjects augmented with a neural prosthesis: a cross-over study. Can J Physiol Pharmacol. 2004;82:749–56.
Popovic D, Stojanović A, Pjanović A, Radosavljević S, Popovic M, Jović S, et al. Clinical evaluation of the bionic glove. Arch Phys Med Rehabil. 1999;80:299–304.
Prochazka A, Gauthier M, Wieler M, Kenwell Z. The bionic glove: an electrical stimulator garment that provides controlled grasp and hand opening in quadriplegia. Arch Phys Med Rehabil. 1997;78:608–14.
Rebersek S, Vodovnik L. Proportionally controlled functional electrical stimulation of hand. Arch Phys Med Rehabil. 1973;54:378–82.
Sullivan JE, Hedman LD. A home program of sensory and neuromuscular electrical stimulation with upper-limb task practice in a patient 5 years after a stroke. Phys Ther. 2004;84:1045–54.
Sullivan JE, Hedman LD. Effects of home-based sensory and motor amplitude electrical stimulation on arm dysfunction in chronic stroke. Clin Rehabil. 2007;21:142–50.
Popovic MB. Control of neural prostheses for grasping and reaching. Med Eng Phys. 2003;25:41–50.
Popovic MB, Popovic DB, Sinkjaer T, Stefanovic A, Schwirtlich L. Restitution of reaching and grasping promoted by functional electrical therapy. Artif Organs. 2002;26:271–5.
Popovic MR, Keller T. Modular transcutaneous functional electrical stimulation system. Med Eng Phys. 2005;27:81–92.
Popovic MR, Thrasher TA, Zivanovic V, Takaki J, Hajek V. Neuroprosthesis for retraining reaching and grasping functions in severe hemiplegic patients. Neuromodulation. 2005;8:58–72.
Glanz M, Klawansky S, Stason W, Berkey C, Chalmers TC. Functional electrostimulation in poststroke rehabilitation: a meta-analysis of the randomized controlled trials. Arch Phys Med Rehabil. 1996;77:549–53.
Cauraugh JH, Kim S. Two coupled motor recovery protocols are better than one: electromyogram-triggered neuromuscular stimulation and bilateral movements. Stroke. 2002;33:1589–94.
Gritsenko V, Prochazka A. A functional electric stimulation-assisted exercise therapy system for hemiplegic hand function. Arch Phys Med Rehabil. 2004;85:881–5.
Popovic MR, Keller T, Papas IPI, Dietz V, Morari M. Surface-stimulation technology for grasping and walking neuroprostheses. IEEE Eng Med Biol Mag. 2001;20:82–93.
Alon G, Sunnerhagen KS, Geurts ACH, Ohry A. A home-based, self-administered stimulation program to improve selected hand functions of chronic stroke. NeuroRehabilitation. 2003;18:215–25.
Ring H, Rosenthal N. Controlled study of neuroprosthetic functional electrical stimulation in sub-acute post-stroke rehabilitation. J Rehabil Med. 2005;37:32–6.
Mangold S, Keller T, Curt A, Dietz V. Transcutaneous functional electrical stimulation for grasping in subjects with cervical spinal cord injury. Spinal Cord. 2005;43:1–13.
Emsley JG, Mitchell BD, Magavi SSP, Arlotta P, Macklis JD. The repair of complex neuronal circuitry by transplanted and endogenous precursors. NeuroRx. 2004;1:452–71.
Palmer TD, Ray J, Gage FH. FGF-2-responsive neuronal progenitors reside in proliferative and quiescent regions of the adult rodent brain. Mol Cell Neurosci. 1995;6:474–86.
Palmer TD, Markakis EA, Willhoite AR, Safar F, Gage FH. Fibroblast growth factor-2 activates a latent neurogenic program in neural stem cells from diverse regions of the adult CNS. J Neurosci. 1999;19:8487–97.
Shihabuddin LS, Ray J, Gage FH. FGF-2 is sufficient to isolate progenitors found in the adult mammalian spinal cord. Exp Neurol. 1997;148:577–86.
Kazanis I. Can adult neural stem cells create new brains? Plasticity in the adult mammalian neurogenic niches: realities and expectations in the era of regenerative biology. Neuroscientist. 2012;18:15–27.
Wang Y, Sheen VL, Macklis JD. Cortical interneurons upregulate neurotrophins in vivo in response to targeted apoptotic degeneration of neighboring pyramidal neurons. Exp Neurol. 1998;154(2):389–402.
Kobilo T, Potter MC, van Praag H. Neurogenesis and exercise. In: Thompson GFKLMF, editor. Encyclopedia of behavioral neuroscience. Oxford: Academic; 2010. p. 404–9.
Aimone JB, Deng W, Gage FH. Adult neurogenesis: integrating theories and separating functions. Trends Cogn Sci (Regul Ed). 2010;14:325–37.
Appleby PA, Wiskott L. Additive neurogenesis as a strategy for avoiding interference in a sparsely-coding dentate gyrus. Network. 2009;20:131–61.
Weisz VI, Argibay PF. A putative role for neurogenesis in neuro-computational terms: inferences from a hippocampal model. Cognition. 2009;112:229–40.
Kobilo T, Liu Q-R, Gandhi K, Mughal M, Shaham Y, van Praag H. Running is the neurogenic and neurotrophic stimulus in environmental enrichment. Learn Mem. 2011;18:605–9.
Winocur G, Becker S, Luu P, Rosenzweig S, Wojtowicz JM. Adult hippocampal neurogenesis and memory interference. Behav Brain Res. 2012;227:464–9.
Asakura R, Matsuwaki T, Shim J-H, Yamanouchi K, Nishihara M. Involvement of progranulin in the enhancement of hippocampal neurogenesis by voluntary exercise. Neuroreport. 2011;22:881–6.
Ma X, Hamadeh MJ, Christie BR, Foster JA, Tarnopolsky MA. Impact of treadmill running and sex on hippocampal neurogenesis in the mouse model of amyotrophic lateral sclerosis. PLoS One. 2012;7, e36048.
van Praag H. Neurogenesis and exercise: past and future directions. Neuromolecular Med. 2008;10:128–40.
Becker S, Wojtowicz JM. A model of hippocampal neurogenesis in memory and mood disorders. Trends Cogn Sci (Regul Ed). 2007;11:70–6.
Becker S, Macqueen G, Wojtowicz JM. Computational modeling and empirical studies of hippocampal neurogenesis-dependent memory: effects of interference, stress and depression. Brain Res. 2009;1299:45–54.
Wojtowicz JM. Adult neurogenesis. From circuits to models. Behav Brain Res. 2012;227:490–6.
Bigi S, Fischer U, Wehrli E, Mattle HP, Boltshauser E, Bürki S, et al. Acute ischemic stroke in children versus young adults. Ann Neurol. 2011;70:245–54.
Blom I, De Schryver ELLM, Kappelle LJ, Rinkel GJE, Jennekens-Schinkel A, Peters ACB. Prognosis of haemorrhagic stroke in childhood: a long-term follow-up study. Dev Med Child Neurol. 2003;45:233–9.
Boake C, Noser EA, Ro T, Baraniuk S, Gaber M, Johnson R, et al. Constraint-induced movement therapy during early stroke rehabilitation. Neurorehabil Neural Repair. 2007;21:14–24.
Becker D, Gary DS, Rosenzweig ES, Grill WM, McDonald JW. Functional electrical stimulation helps replenish progenitor cells in the injured spinal cord of adult rats. Exp Neurol. 2010;222:211–8.
Geremia NM, Gordon T, Brushart TM, Al-Majed AA, Verge VMK. Electrical stimulation promotes sensory neuron regeneration and growth-associated gene expression. Exp Neurol. 2007;205:347–59.
Udina E, Furey M, Busch S, Silver J, Gordon T, Fouad K. Electrical stimulation of intact peripheral sensory axons in rats promotes outgrowth of their central projections. Exp Neurol. 2008;210:238–47.
Li Q, Brus-Ramer M, Martin JH, McDonald JW. Electrical stimulation of the medullary pyramid promotes proliferation and differentiation of oligodendrocyte progenitor cells in the corticospinal tract of the adult rat. Neurosci Lett. 2010;479:128–33.
Kilb W, Kirischuk S, Luhmann HJ. Electrical activity patterns and the functional maturation of the neocortex. Eur J Neurosci. 2011;34:1677–86.
Babona-Pilipos R, Droujinine IA, Popovic MR, Morshead CM. Adult subependymal neural precursors, but not differentiated cells, undergo rapid cathodal migration in the presence of direct current electric fields. PLoS One. 2011;6, e23808.
Hotary KB, Robinson KR. Evidence of a role for endogenous electrical fields in chick embryo development. Development. 1992;114:985–96.
Borgens RB, Shi R. Uncoupling histogenesis from morphogenesis in the vertebrate embryo by collapse of the transneural tube potential. Dev Dyn. 1995;203:456–67.
Song B, Zhao M, Forrester JV, McCaig CD. Electrical cues regulate the orientation and frequency of cell division and the rate of wound healing in vivo. Proc Natl Acad Sci U S A. 2002;99:13577–82.
Babona-Pilipos R, Popovic MR, Morshead CM. A galvanotaxis assay for analysis of neural precursor cell migration kinetics in an externally applied direct current electric field. J Vis Exp. 2012;68:4193.
Fields RD. Nerve impulses regulate myelination through purinergic signalling. Novartis Found Symp. 2006;276:148–58 (discussion 158–61–233–7–275–81).
Beaumont E, Guevara E, Dubeau S, Lesage F, Nagai M, Popovic M. Functional electrical stimulation post-spinal cord injury improves locomotion and increases afferent input into the central nervous system in rats. J Spinal Cord Med. 2014;37:93–100.
Jurkiewicz MT, Mikulis DJ, Fehlings MG, Verrier MC. Sensorimotor cortical activation in patients with cervical spinal cord injury with persisting paralysis. Neurorehabil Neural Repair. 2010;24:136–40.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media New York
About this chapter
Cite this chapter
Nagai, M.K., Marquez-Chin, C., Popovic, M.R. (2016). Why Is Functional Electrical Stimulation Therapy Capable of Restoring Motor Function Following Severe Injury to the Central Nervous System?. In: Tuszynski, M. (eds) Translational Neuroscience. Springer, Boston, MA. https://doi.org/10.1007/978-1-4899-7654-3_25
Download citation
DOI: https://doi.org/10.1007/978-1-4899-7654-3_25
Published:
Publisher Name: Springer, Boston, MA
Print ISBN: 978-1-4899-7652-9
Online ISBN: 978-1-4899-7654-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)