Abstract
Controlled transgene expression via a promoter is particularly triggered in response to pathogen infiltration. This is significant for eliciting disease-resistant features in crops through genetic engineering. The germins and germin-like proteins (GLPs) are known to be associated with plant and developmental stages. The 1107-bp Oryza sativa root GLP2 (OsRGLP2) gene promoter fused to a β-glucuronidase (GUS) reporter gene was transformed into potato plants through an Agrobacterium-mediated transformation. The OsRGLP2 promoter was activated in response to Fusarium solani (Mart.) Sacc. and Alternaria solani Sorauer. Quantitative real-time PCR results revealed 4–5-fold increase in promoter activity every 24 h following infection. There was a 15-fold increase in OsRGLP2 promoter activity after 72 h of F. solani (Mart.) Sacc. treatment and a 12-fold increase observed with A. solani Sorauer. Our results confirmed that the OsRGLP2 promoter activity was enhanced under fungal stress. Furthermore, a hyperaccumulation of H2O2 in transgenic plants is a clear signal for the involvement of OsRGLP2 promoter region in the activation of specific genes in the potato genome involved in H2O2-mediated defense response. The OsRGLP2 promoter evidently harbors copies of GT-I and Dof transcription factors (AAAG) that act in response to elicitors generated in the wake of pathogen infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Genetic engineering of economically significant crop plants with preferred characters has been successfully attained through improved plant molecular transformation practices. In the field of biotechnology, potato (Solanum tuberosum L.) is considered a significant crop plant, providing a beneficial exemplary system for studying signaling processes (Banerjee et al. 2006). Genetic transformation offers the tools to direct single specific elements in more complicated pathways and the control of their spatial and temporal expression (Rosellini 2011). In transgene expressions, the practice of plant defense gene promoters is beneficial as they are triggered only when plants are under pest or pathogen stress. Another advantage of indigenous plant promoters is that they assist in preventing transgene silencing, which is frequently linked with the existence of non-plant promoters in the plant genetic machinery (Matzke and Matzke 1995). It has been demonstrated that potato plant can be genetically transformed for resistance to a wide range of pathogens through the activity of defense-related peptides under the regulation of a CaMV 35S promoter (Osusky et al. 2000). Progress in the areas of tissue culture, genetic engineering, and genomics has promoted the establishment of resistant “transgenic” plants. One of these is the use of tissue-specific (for example tuber) promoters to control transgene expression and prevent consumer unease about probable negative influence of the foreign proteins (Kumar et al. 2010; Meiyalaghan et al. 2006).
Plants are consistently open to a wide range of biological stresses and have developed different ways to survive them, as well as acquiring a number of defense barriers against phytopathogens. Fungal pathogens are known as the major cause of crop loss. For instance, the fungus Exobasidium vexans has been reported to be the causative agent of blister blight disease resulting in huge loss of tea plant Camellia sinensis, affecting the tea industry both qualitatively and quantitatively. Through genetic engineering, the transformation of S. tuberosum class I chitinase gene via Agrobacterium-mediated transformation of tea plant genome has resulted in improved resistance against fungal pathogen E. vexans (Singh et al. 2015). Moreover, plant tissue wounding can initiate production of various defense-related proteins and further offers a useful approach to isolate and analyze the defense-related genes and their promoters in transformed plants (Clarke et al. 1994; Zhang et al. 2011). Germins and germin-like proteins (GLPs) have been identified to play a vital role in plant defensive mechanism. In cereals, germins exhibit an oxalate oxidase activity, which generates hydrogen peroxide (H2O2) from the oxidative breakdown of oxalate, thus playing a defensive role in plants. Manosalva et al. (2009) reported that a GLP gene member located on chromosome 8 in rice provides resistance against rice blast and sheath blight disease. GLPs were studied by comparing the gene clustering in cereal genomes with rice, the model plant among grass species (Breen and Bellgard 2010). It was shown that the overlapping of gene clusters with specific QTLs associated with disease resistance contributes against pathogens. The chemistry between local gene duplications, small RNAs, and regulatory and transposable elements in promoters may possibly work together to influence GLP expression profile and its ability to protect plant cells via an extensive defensive system.
In a recent report, Hernandez-Garcia and Finer (2013) reviewed quick, proficient, and accurate evaluation of promoter elements through the enhancement and step-up of gene expression strategies. This will be significant for functional validation of the regulatory factors recognized as a result of genomic and transcriptomic investigation. During a computational analysis of rice root germin-like protein 2 (OsRGLP2) promoter, Mahmood et al. (2007) depicted the presence of five common regions (CR1–CR5)which are found to be repeated at three to six other locations in the 30-kb region in which this gene (OsRGLP2) driven by its promoter is located. Moreover, it was found that the genes driven by promoter harboring these common regions are GLPS or germins. Further analysis of this 30-kb stretch of germin and GLP genes clustered on chromosome 8 indicated a putative 40-bp promoter which was found to be a part of CR2. It was also reported that various already recognized regulatory regions are available in OsRGLP2 promoter. These regulatory regions may have a role in response to water and salt stress, plant growth hormones, light sensitivity, storage proteins in seeds, and biotic stress. Further, recently Mahmood et al. (2013)reported that the wound-inducible property of this promoter in transgenic tobacco plants provides valuable insight for generating transgenic crop plants with enhanced defense-related features. Moreover, tissue specific activity of OsRGLP2 promoter was detected in the outer and inner phloem region of midrib, in the cortical area next to the phloem, in the epidermal cells, and in the veins of petals.
To investigate the OsRGLP2 gene promoter activity in potato, an efficient transformation and regeneration protocol was established. The promoter activity of the OsRGLP2 gene stimulated in response to the fungal strains Fusarium solani (Mart.) Sacc. and Alternaria solani Sorauer infection was studied in potato leaves via an Agrobacterium-mediated transient expression analysis of the promoter fused to a β-glucuronidase (GUS) reporter gene by quantitative real-time PCR (qRT-PCR) analysis.
Materials and methods
Plant material and promoter construct
Agrobacterium tumefaciens-mediated transformation of Solanum tuberosum cv. desiree was selected and was obtained from the National Agriculture Research Centre, Islamabad, Pakistan. Country of desiree origin is the Netherlands and it was reported in 1962 (data source is from Federal Research Centre of Agriculture, Germany and Plant Breeding and Acclimatization Institute, Poland). TheOsRGLP2 gene promoter from Oryza sativa fused with GUS as the reporter gene in GV3101 Agrobacterium strain was utilized to produce transgenic plants.
In vitro propagation
The conditions for in vitro culturing, callus generation, and plant regeneration from the callus tissue had been previously optimized (Munir et al. 2011). Plantlets were micropropagated using nodal segments as explants. MS basal media (Murashige and Skoog 1962) containing 30 g/l sucrose and 1.5 mg/l gibberellic acid (GA3) was used. The cultures were maintained in a growth chamber under a light intensity of 2000 lx with a photoperiod of 8 h light and 16 h dark.
Plant transformation
The transformation experiment was optimized by using different explants, i.e., leaf discs, nodal and internodal segments, roots, and tuber sections. For the co-infection stage of transformation, different dilution of GV3101 culture (at OD600 of 0.3, 0.4, 0.5, 0.6, and 0.7) were used. The co-infection time was adjusted using different batches of transformation trials, i.e., 2, 4, 6, 8, 10, 12, and 15 min. Co-culturing time was optimized by shifting explants on MS medium having indole acetic acid (IAA 0.2 mg/l) and 6-benzylaminopurine (BAP 1.0 mg/l) for 24, 48, and 72 h under 16:8 light/dark cycle at 25 °C and 50 ± 5 % humidity. For selection, the hygromycin concentration was optimized by repeating the transformation experiments with different concentrations of hygromycin (10, 15, 20, 25, 30, 35, and 40 mg/l). Regenerated potato shoots were rooted on MS medium supplemented with indole acetic acid (IAA 0.2 mg/l) and hygromycin (30.0 mg/l).
PCR analysis of transgenic plants
Genomic DNA was extracted from transgenic potato plant samples using the CTAB protocol (Richards 1997). PCR reactions were carried out with primers for OsRGLP2 promoter (RGLP2 F and RGLP2 R) and hygromycin resistance gene (Hygro F and Hygro R). The sequences of these primers are RGLP2 F: 5′ CCCGGGCTGGTCTACTTGGCATTGT 3′, RGLP2 R: 5′ CCCGGGTTCTCTGCTGAATTATTTGCT 3′ and Hygro F: 5′ GCTCCATACAAGCCAACCAC 3′, Hygro R: 5′ CGAAAAGTTCGACAGCGTCTC 3′.
The 50 μl PCR reaction mixture comprised 2.0 μl of 25 pmol each primer, 5.0 μl of 10× PCR buffer, 3.0 μl of 25 mM MgCl2, 3.0 μl of 2.0 mM dNTPs, 50 ng/μl of genomic DNA, and 3.0 U Taq polymerase (Fermentas). Reactions were carried out in a gradient Multi Gene Thermal Cycler (Labnet) set for 35 cycles of denaturation at 94 °C for 30 s, annealing at 57 °C for 30 s, and extension at 72 °C for 1 min, followed by a single-step final extension at 72 °C for 10 min.
Exposure to fungal pathogens
Transgenic plantlets 7–8 cm long with four to seven leaves were infected with a conidial suspension of F. solani (Mart.) Sacc. and A. solani Sorauer obtained from Queensland Plant Pathology Herbarium, Australia. The plants were placed at 27 °C under 16 h light conditions for 7 days. The experiments were repeated three times. The strength of fungal suspension was 1 × 106 conidia/ml. Mock controls were created in each treatment. The treated and mock-control leaf samples were collected after 24, 48, and 72 h following infection. These were preserved under liquid nitrogen at −80 °C for RNA isolation and cDNA synthesis.
Hydrogen peroxide detection
The pictorial finding of H2O2 in fungal infected leaves was performed according to Dong et al. (2008). The leaves from transgenic plants infected with F. solani and A. solani strains for 12 h and mock-treated controls were kept in the substrate 3, 3-diaminobenzidine (DAB). After dechlorophyllization and treating with boiling ethanol, the leaves were digitally visualized and transverse sections were examined under the microscope.
RNA extraction and cDNA synthesis
Total RNA was isolated from plant tissues using the NucleoSpin® RNA plant extraction kit (MACHEREY-NAGEL) and stored at −80 °C. The RNA quality and integrity was examined by 1.5 % agarose gel electrophoresis followed by SYBR Green staining. The primary strand cDNA synthesis was done by using 2 μl of purified RNA per 20 μl consisting of 4 μl of 25 mM MgCl2, 2 μl of 5× reaction buffer, 2 μl of 10 mM dNTP mix, 0.5 μl RiboLock RNase inhibitor (20 U), 0.6 μl RevertAid Reverse Transcriptase (15 U) (Fermentas), and 1 μl oligo (dT)15 primer 25 pM (e-oligos), made up to 20 μl with nuclease-free water. The reaction mixture was incubated at 42 °C for 1 h followed by 95 °C for 5 min to stop the reaction. Finally, the mixture was cooled down to 5 °C for 10 min before storage at −20 °C until further use.
Quantitative real-time PCR (qRT-PCR)
RT-PCR was performed using a quantitative method with double-strand DNA binding dye “SYBR” as reporter by using the Light Cycler® 96 System. The reaction volume was 25 μl containing 1 μl of cDNA (1:5 dilution), 12 μl of SYBR Green master mix (InvitrogenTM), 2 μl of 25 pM primer mix (forward and reverse), and 10 μl of nuclease-free water. The reaction was performed by using a specific set of primers for GUS as reporter gene and for action as a housekeeping gene. The sequence of primers is GUS F: 5′ CGGCAGAGAAGGTACTGGAA 3′, GUS R: 5′ ATATCCAGCCATGCACACTG 3′ and Actintac F: 5′ GGAATCCACGAGACTACA 3′, Actintac R: 5′ TGAGGGAAGCCAAGATAG 3′.
The following reaction conditions were used: pre-denaturation at 95 °C for 10 min followed by 40 cycles of denaturation at 95 °C for 30 s, annealing at 55 °C for 1 min, and extension at 72 °C for 10 s. These reactions were run and the florescence was detected. The data was evaluated in terms of promoter activity in reference to the expression of the reporter gene (GUS) in response to fungal infection. Relative quantification of RT data was performed through the 2−ΔΔCt method.
Results
An efficient potato transformation, regeneration, and microtuberization protocol was established. For the transformation of different types of explants (leaf discs, nodes, and internodes), various dilutions of Agrobacterium suspension at OD600 (0.1–0.7), co-infection time period (1–15 min), and antibiotic concentration at various steps of transformation were applied that resulted in an optimized potato transformation protocol with successful production of transgenic potatoes harboring OsRGLP2 promoter. Best transformation efficiency (95 %) was obtained when nodes were used as explant with bacterial suspension of OD600 = 0.5 and co-infection time of 5 min. Moreover, 750 mg/l cefotaxime concentration was found suitable for controlling excessive Agrobacterium. Hygromycin was optimized over a concentration range between 10 and 40 mg/l, and its optimal concentration was found to be 30 mg/l. Regeneration from nodes started after 2 weeks and regenerated shoots were shifted to the fresh selection medium after every 10 days. Rooting started after 15 days after transfer into MS rooting medium. Total time required from co-infection to rooting stage to obtain healthy transgenic potato plantlets (7–8 cm) was 5 weeks.
To obtain a good number of transformed microtubers, various parameters such as carbon supply, in vitro temperature, nitrogen supplements (N-supplements), plant growth regulators (PGRs), and H2O2 pre-treatment were adjusted. In response to various sucrose concentrations (30–70 g/l) applied, the best optimized sucrose concentration was 70 g/l that resulted in formation of microtubers in 87 % of the explants with 1600 mg fresh weight per explant. Different in vitro temperatures (20–31 °C) were also monitored and maximum microtuber production, i.e., a frequency of 76 % with 955 mg per explant, was observed at 22 °C. Furthermore, microtuberization production was studied under the treatment of N-supplements including NH4NO3 and KNO3 in a range of 50–500 mg/l where 300 mg/l was found to be the optimal concentration. The microtuberization yield in response to various concentrations and combinations of PGRs (GA3, BAP, and kinetin) was also investigated. The best optimized hormonal combination was GA3, BAP, and kinetin at 1.5, 1.0, and 2.0 mg/l, respectively, that resulted in a microtuber yield at a frequency of 96 % with 1450 mg per explant. Microtuber production in potato was also studied in response to H2O2 pre-treatment. It was found that H2O2 pre-treatment promoted the microtuber formation and resulted in early sprouting (the emergence period was reduced by 2 1/2 weeks). These optimized in vitro treatments resulted in a considerable positive effect on microtuber production. It may be important to mention that there is little available data on the microtuberization productivity in transgenic potato plants.
OsRGLP2 promoter-GUS gene expression in response to fungal infection
The transgenic potato plants harboring the OsRGLP2 promoter-GUS gene construct were evaluated for the response of promoter towards infection with two potato pathogens, i.e., F. solani and A. solani. The untransformed leaves were found to be severely damaged by the fungal infiltration. Intensity of the infection increased with duration of exposure (Fig. 1a, c) in control leaves. On the other hand, no noticeable lesion was observed in leaves obtained from transgenic plants in the first 48 h post-infection, while small lesions appeared after 72 h post-infection. Response to both F. solani and A. solani was the same as shown in Figs. 1 and 2.
Fusarium solani (Mart.) Sacc. infection in untransformed and transgenic potato leaves indicated by arrows. a Infection lesions over the untransformed potato leaf after 24 h of infection. b Transformed potato leaf with no obvious infection after 24 h of treatment. c Untransformed leaf with well-marked infection lesions after 48 h. d Transformed leaf with minor infection mark after 48 h of infection. e Distinct F. solani infection lesions in untransformed potato leaf following 72 h of infection. f Transformed potato leaf with small patches of infection. Bars indicate 1000 μm
Alternaria solani Sorauer infectivity in untransformed and transgenic potato leaves indicated by arrows. a Leaf lesion after 24 h of infection in untransformed leaf. b Transformed potato leaf with little infection mark after 24 h of treatment. c Untransformed leaf with increasing dark brown lesions after 48 h. d Transformed leaf with few infection initiation marks after 48 h of infection. e “Bull eye” pattern leaf lesions spreading in untransformed potato leaf following 72 h of infection. f Transformed potato leaf with tiny infection lesion after 72 h. Bars indicate 1000 μm
The histochemical GUS assay following infection with the pathogens showed low expression in control transgenic plant tissues, while in fungus-infiltrated tissues there was a noticeable increase in the intensity of blue staining which increased with the passage to time (Fig. 3). Another important observation was that the histochemical GUS expression was observed not only in infected leaves but was also evident in the distant leaves, suggesting the local as well as systemic response of the OsRGLP2 promoter-GUS gene towards fungal stress.
Transgenic potato plants were further tested for the H2O2 production in the leaves following fungal infection. The level of H2O2 was found to be intense, as revealed by dark brown blotches in OsRGLP2 gene promoter transformed leaves, while the mock-control plants had light brown spots after staining with DAB (Fig. 4). These findings are a clear signal for the presence of H2O2 responsive regulatory elements in the OsRGLP2 promoter that was triggered in response to fungal infection. Such infection responsive regulatory factors may well control the activation of specific defensive genes in potato genome.
Quantitative RT-PCR analysis
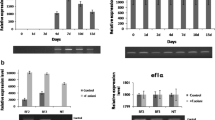
For expression analysis, the transgenic leaf samples under fungal stress at different time points (0, 24, 48, and 72 h) were evaluated by qRT-PCR. A “0 h” experimental time point indicates unstressed samples. The GUS gene expression level in mock-control samples was equal to the relative expression value at the 0 h experimental time point. There was a constant signal by the actin gene acting as an internal control at all time points. Under the pathogen stress by F. solani and A. solani infection, there was a considerable up-regulation of the OsRGLP2 promoter activity. In the case of F. solani-infected samples, there was a 5-fold increase in the expression level of GUS gene harboring the OsRGLP2 promoter at each time point, which reached a maximum level of 15-fold (p = 0.001) increase after 72 h following infection (Fig. 5a). There was an up-regulation pattern of GUS gene expression with A. solani infection as well, but the level of activity was slightly low as compared to F. solani treatment. There was a regular 4-fold increase in promoter activity at each time point that reached the highest level of 12-fold (p = 0.001) in A. solani-inoculated samples at 72 h (Fig. 5b).
Discussion
Numerous phytopathogens including fungi, bacteria, and viruses have a negative effect on the potato crop yield at both pre- and post-harvest stages. Moreover, considerable damage occurs in storage. Genetic engineering techniques can help in generating disease-resistant potato plants. Research investigation was carried out in two diploid Solanum tuberosum clones, 07506-01 and 12120-03, in response to a soil-borne fungus, Verticillium dahlia Kleb., infection; different levels of infection symptoms were observed in two potato studied clones, and for this the genetic variation in response to fungal pathogen was found to be the underlying reason. Furthermore, 07506-01 potato clone was found to be tolerant with two susceptible alleles of Ve2 gene, while 12120-03 clone having one Ve2 resistant and one susceptible allele was partially resistant (Tai et al. 2013).
Microtubers are vital for acquiring germ-free seeds, disease-free plantlets, and for healthy germplasm exchange to obtain beneficial disease-resistant crop plants. Transformation protocol was established by Chakravarty and Wang-Pruski (2010) for potato cv. Bintje, with an objective to evaluate the wide range functionality of potato genome. Compared to their results, our transformation protocol was found to be more efficient as the total time required from co-infection to rooting stage was 5 weeks (with 95 % transformation efficiency), while their data showed 6–7 weeks’ time period (with 93 % transformation efficiency). The transformation efficiency highly depends on genotype; in an earlier report, potato transformation efficiency (with npII gene) in three potato cultivars was found to be 90.9 % for Dragaevka, 76.9 % for Jelica, and 86.4 % for cultivar desiree (Cingel et al. 2010).
Few other similar studies such as the GLP13 promoter-GUS construct (Yang et al. 2013) in Arabidopsis thaliana and Nicotiana tabacum and GhRGP1 promoter-GUS construct from cotton (Wu et al. 2006) in transgenic tobacco were analyzed via GUS gene expression. The data indicated an intense expression in seeds, trichomes, and stem vascular tissues, demonstrating the spatial and temporal regulation. Moreover, these promoters were found to be strong wound-inducible promoters. In general, under stress conditions such as wounding, phytopathogens and herbivory plants mount some defense to guard themselves against damage especially at the site of stress. Certain wound healing proteins like ZmPAO from Zea mays (Angelini et al. 2008) and Atpep1 from Arabidopsis (Huffaker et al. 2006) are involved in wound healing process conferring plant. Such proteins can get triggered as a result of wounding so as to toughen and restore the damage.
Hence, in the present study with OsRGLP2, a strong wound-inducible promoter under fungal stress conditions has triggered GUS expression indicating that this promoter is fungal stress responsive. This, in turn, enhanced the H2O2 accumulation in plants due to the possible presence of H2O2 responsive elements. The production of H2O2 may confer plant defense and wound-repairing response. An important outcome of biotic and abiotic stresses is an intense formation of cellular reactive oxygen species which are consequently changed into H2O2. Besides being a toxicant, H2O2 has been considered as a vital stress signal in plants, having a significant role in the regulation of defensive gene expression (Hung et al. 2005). H2O2 in peroxisomes is found to be linked with biotic stress resistance (Chaouch et al. 2010). The role of H2O2 in response to biotic stress was reported by Dong et al. (2008) in Brassica napus, and it was found that varying intense H2O2 accumulation resulted after infection with Sclerotinia sclerotiorum, which is in accordance to the present study.
One of the most important reporter genes utilized in the qualitative and quantitative evaluation of gene expression in transgenic plant is GUS gene having an extensive application in biotechnological studies to follow the promoter activities in genetically engineered plants (Huttly 2009; Delporte et al. 2013). In the present study, the OsRGLP2 promoter activity was studied via qPCR analysis of GUS gene expression used as a reporter gene. In a recent study, Tanabe et al. (2015) analyzed the IbRbcS1 promoter (from sweet potato) fused to the GUS reporter gene in transgenic Arabidopsis plants, and their results depicted the promoter activity in a photo-inducible and tissue-specific way via the expression analysis of GUS reporter gene. In another earlier investigation by Delporte et al. (2013), the characterization of Nictaba promoter in Nicotiana tabacum was done using the promoter-GUS construct. Therefore, the GUS gene plays a vital and useful role in different expression analyses.
In a recent report, Yogendra et al. (2014) quantitatively analyzed the pathogen resistance-related genes of linked enzymes, tyrosine decarboxylase (TyDC), CoA ligase (4-CL), and tyraminehydroxycinnamoyltransferase (THT), in the pathogen-inoculated resistant and susceptible potato genotype, so as to validate the significance of these genes in the improvement of potato resistance in response to pathogen infection. Furthermore, in an earlier study, Yevtushenko et al. (2004) reported that the win3.12T wound-inducible promoter from poplar was systemically active in aerial regions of potato plants under fungal stress. In another study, Lee et al. (2007) gave an account of local and systemic induction of the pepper pathogen-induced gene promoter CAPIP2 in response to Xanthomonas campestris pv. vesicatoria infection. This promoter activity was found to be driven by CARAV1 and CAZFP1 pepper transcription factors. The systemic stimulation of the CAPIP2 promoter indicated that there was translocation of promoter activating signals to the far-off tissues after local infectivity as a result of bacterial pathogenicity. Our results also indicated that OsRGLP2 promoter is biotic stress responsive.
In another study, the expression analysis of two promoters, including one from plant origin, the apple CaM and another from a virus, under biotic and abiotic stress was conducted. Both promoters were found to be activated at different levels under various stress conditions. The apple CaM promoter was found to play a vital part in vascular tissues, as revealed by GUS fluorometric, histochemical, and real-time PCR analysis. That suggested that the plant defensive role against various phytopathogens was confined in the phloem tissue (Maghuly et al. 2008). The computational analysis of the OsRGLP2 promoter suggested that this strong wound-inducible promoter has various regulatory factors contributing to the defensive system against biotic stress (Mahmood et al. 2007).
The wound inducibility of OsRGLP2 promoter is already known (Mahmood et al. 2013); the novelty of the present study is that this promoter is functional in different host systems, in this case against fungal infection. The present study has illustrated that the OsRGLP2 promoter has strong local and systemic activity to F. solani and A. solani infection, and this response was greater than simple mechanical wounding. This study brings to light the biotechnological relevance of the OsRGLP2 promoter to be utilized as a strong biotic stress responsive promoter. On the basis of the present study, the OsRGLP2 promoter is found to be stimulated locally and systemically by fungal infection and it is a strong pathogen-inducible promoter.
References
Angelini R, Tisi A, Rea G, Chen MM, Botta M, Federico R, Cona A (2008) Involvement of polyamine oxidase in wound healing. Plant Physiol 146:162–177
Banerjee AK, Prat S, Hannapel DJ (2006) Efficient production of transgenic potato (S. tuberosum L. andigena) plants via Agrobacterium tumefaciens mediated transformation. Plant Sci 170:732–738
Breen J, Bellgard M (2010) Germin-like proteins (GLPs) in cereal genomes: gene clustering and dynamic roles in plant defense. Funct Integr Genomics 10:463–476
Chakravarty B, Wang-Pruski G (2010) Rapid regeneration of stable transformants in cultures of potato by improving factors influencing Agrobacterium mediated transformation. Adv Biosci Biotechnol 1:409–416
Chaouch S, Queval G, Vanderauwera S, Mhamdi A, Vandorpe M, LangloisMeurinne M, Van Breusegem F, Saindrenan P, Noctor G (2010) Genetic reversion of cell death in the Arabidopsis cat2 knockout mutant shows that peroxisomal H2O2 is coupled to biotic responses by isochorismate synthase 1 in a day length-related manner. Plant Physiol 153:1692–1705
Cingel A, Vinterhalter B, Vinterhalter D, Ali-Dragosavac D, Smigocki A, Ninkovic S (2010) Agrobacterium-mediated transformation of two Serbian potato cultivars (Solanum tuberosum L. cv. Dragaevka and cv. Jelica). Afr J Biotechnol 9:4644–4650
Clarke HR, Davis JM, Wilbert SM, Bradshaw HD Jr, Gordon MP (1994) Wound induced and developmental activation of a poplar tree chitinase gene promoter in transgenic tobacco. Plant Mol Biol 25:799–815
Delporte A, Sofie VH, Els JM, Damme V (2013) Qualitative and quantitative analysis of the Nictaba promoter activity during development in Nicotiana tabacum. Plant Physiol Biochem 67:162–168
Dong X, Ji R, Guo X, Foster SJ, Chen H, Dong C, Liu Y, Hu Q, Liu S (2008) Expressing a gene encoding wheat oxalate oxidase enhances resistance to Sclerotinia sclerotiorum in oilseed rape (Brassica napus). Planta 228:331–340
Hernandez-Garcia CM, Finer JJ (2013) Identification and validation of promoters and cis acting regulatory elements. Plant Sci 217–218:109–119
Huffaker A, Pearce G, Ryan C (2006) An endogenous peptide signal in Arabidopsis activates components of the innate immune response. Proc Natl Acad Sci U S A 103:10098–10103
Hung SH, Yu CW, Lin CH (2005) Hydrogen peroxide functions as a stress signal in plants. Bot Bull Acad Sin 46:1–10
Huttly A (2009) Reporter genes. Methods Mol Biol 478:39–69
Kumar M, Chimote V, Singh R, Mishra GP, Nail PS, Pandey SK, Chakrabarti SK (2010) Development of Bt transgenic potatoes for effective control of potato tuber moth by using cry1Ab gene regulated by GBSS promoter. Crop Prot 29:121–127
Lee SC, Kim DS, Kim NH, Hwang BK (2007) Functional analysis of the promoter of the pepper pathogen-induced gene, CAPIP2, during bacterial infection and abiotic stresses. Plant Sci 172:236–245
Maghuly F, Khan MA, Fernandez EB, Druart P, Watillon B, Laimer M (2008) Stress regulated expression of the GUS-marker gene (uidA) under the control of plant calmodulin and viral 35S promoters in a model fruit tree rootstock: Prunus incisa × serrula. J Biotechnol 135:105–116
Mahmood T, Hyder MZ, Naqvi SMS (2007) Cloning and sequence analysis of germin-like protein gene 2 promoter from Oryza sativa L. ssp indica. DNA Seq 18:26–32
Mahmood T, Yasmin T, Haque MI, Naqvi SMS (2013) Characterization of a rice germin-like protein gene promoter. Genet Mol Res 12:360–369
Manosalva PM, Davidson RM, Liu B, Zhu X, Hulbert SH, Leung H, Leach JE (2009) A germin-like protein gene family functions as a complex quantitative trait locus conferring broad-spectrum disease resistance in rice. Plant Physiol 149:286–296
Matzke MA, Matzke AJM (1995) How and why do plants inactivate homologous (trans) genes? Plant Physiol 107:679–685
Meiyalaghan S, Jacobs JME, Butler RC, Wratten SD, Conner AJ (2006) Expression of cry1Ac9 and cry9Aa2 genes under a potato light-inducible Lhca3 promoter in transgenic potatoes for tuber moth resistance. Euphytica 147:297–309
Munir F, Naqvi SMS, Mahmood T (2011) In vitro culturing and assessment of somaclonal variation of Solanum tuberosum var. desiree. Turk J Biochem 36:296–302
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Osusky M, Zhou G, Osuska L, Hancock RE, Kay WW, Misra S (2000) Transgenic plants expressing cationic peptide chimeras exhibit broad-spectrum resistance to phytopathogens. Nat Biotechnol 18:1162–1166
Richards EJ (1997) Preparation of plant DNA using CTAB. In: Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K (eds) Short protocols in molecular biology, 3rd edn. John Wiley, New York, pp 10–11, 2
Rosellini D (2011) Selectable marker genes from plants: reliability and potential. In Vitro Cell Dev Biol Plant 47:222–223
Singh HR, Deka M, Das S (2015) Enhanced resistance to blister blight in transgenic tea (Camellia sinensis [L.] O. Kuntze) by overexpression of class I chitinase gene from potato (Solanum tuberosum). Funct Integr Genomics 15:461–480
Tai HH, Goyer C, Platt HW, De Koeyer D, Murphy A, Uribe P, Halterman D (2013) Decreased gene expression in tolerance versus resistance to Verticillium dahliae in potato. Funct Integr Genomics 13:367–378
Tanabe N, Tamoi M, Shigeoka S (2015) The sweet potato RbcS gene (IbRbcS1) promoter confers high level and green tissue-specific expression of the GUS reporter gene in transgenic Arabidopsis. Gene 567:244–250
Wu AM, Ling C, Liu JY (2006) Isolation of a cotton reversibly glycosylated polypeptide (GhRGP1) promoter and its expression activity in transgenic tobacco. J Plant Physiol 163:426–435
Yang L, Li T, Zhang SC, Gao GL, Yang CW (2013) Characterization of the GLP13 gene promoter in Arabidopsis thaliana. Biol Plant 57:231–237
Yevtushenko DP, Sidorov VA, Romero R, Kay WW, Misra S (2004) Wound-inducible promoter from poplar is responsive to fungal infection in transgenic potato. Plant Sci 167:715–724
Yogendra KN, Pushpa D, Mosa KA, Kushalappa AC, Murphy A, Mosquera T (2014) Quantitative resistance in potato leaves to late blight associated with induced hydroxycinnamic acid amides. Funct Integr Genomics 14:285–298
Zhang H, Jin J, Tang L, Zhao Y, Gu X, Gao G, Luo J (2011) Plant TFDB 2.0: update and improvement of the comprehensive plant transcription factor database. Nucleic Acids Res 48:1114–1117
Acknowledgments
We are thankful to the Higher Education Commission, Islamabad, Pakistan for providing financial assistance. We are grateful to the Centre for Integrative Legume Research—CILR lab, the University of Queensland, Australia for the qRT-PCR facility and to the Queensland Plant Pathology Herbarium, Australia for providing the potato-specific fungal strains.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Munir, F., Hayashi, S., Batley, J. et al. Germin-like protein 2 gene promoter from rice is responsive to fungal pathogens in transgenic potato plants. Funct Integr Genomics 16, 19–27 (2016). https://doi.org/10.1007/s10142-015-0463-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10142-015-0463-y