Abstract
Germin like proteins (GLPs) are a large group of related and ubiquitous plant proteins which are considered to be involved in different processes important for plant development and defense. Multiple functional copies of this gene family have been reported in a number of species (wheat, barley, rice, soybean mosses and liverwort), and their role is being evaluated by gene regulation studies and transgenic approaches. To analyze the role of a rice (Oryza sativa) root expressed germin like protein1 OsRGLP1, for its antifungal activity, transgenic potato plants were developed. These transgenic potato plants were molecularly characterized and biologically assessed after inoculation with Fusarium oxysporum f. sp. tuberosi. Functional analysis showed high accumulation of H2O2, increased Superoxide Dismutase (SOD) activity and no oxalate oxidase activity (OxO) in transgenics in comparison to nontransformed control. This increased SOD activity, resistance to heat and sensitivity to H2O2 suggest it is a Fe-like SOD. OsRGLP1 expression in potato plants exhibited enhanced resistance in comparison to nontransformed wild type plants suggesting its role in providing protection against Fusarium oxysporum f. sp. tuberosi through elevated SOD level. Overall, results suggest that OsRGLP1 is a candidate for the engineering of potato plants with increased fungal tolerance however, the greater height and tuber number was observed. This phenotype associated with the resistance needs to be evaluated to determine if this is a positive or negative feature.
Resumen
Las proteínas tipo germins (GLP) son un grupo grande de proteínas relacionadas de plantas y ubicuas, que se les considera que estan involucradas en diferentes procesos importantes para el desarrollo y defensa de la planta. Se han reportado múltiples copias funcionales de esta familia de genes en muchas especies (trigo, cebada, arroz, soya, musgos, plantas hepáticas), y su papel esta siendo evaluado mediante estudios de regulación génica y enfoques transgénicos. Para analizar la función de una proteína 1 tipo germins, OsRGLP1, expresada en la raíz de arroz (Oryza sativa), por su actividad antifúngica, se desarrollaron plantas de papa transgénicas. Éstas fueron caracterizadas molecularmente y evaluadas biológicamente después de la inoculación con Fusarium oxysporum f. sp. tuberosi. El análisis funcional mostró alta acumulación de H2O2, aumento de la actividad de la superóxido-dismutasa (SOD) e inactividad de la oxalato-oxidasa (OxO) en las transgénicas en comparación con las testigos no transformadas. Este aumento en la actividad de la SOD, resistencia al calor y sensibilidad a H2O2 sugiere que es una Fe-SOD. La expresión de OsRGLP1 en plantas de papa exhibió incremento en la resistencia en comparación con las plantas tipo silvestre no transformadas, lo que sugiere que su función es proporcionar protección contra Fusarium oxysporum f. sp. tuberosi mediante un nivel elevado de SOD. En general, los resultados sugieren que OsRGLP1 es una candidata para la ingeniería de plantas de papa con aumento de tolerancia a los hongos, no obstante, se observó la mayor altura y número de tubérculos. Este fenotipo asociado con la resistencia necesita evaluarse para determinar si esta es una característica positiva o negativa.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Germins and germin-like proteins (GLPs) are a large, ubiquitous and extremely diverse family of plant glycoproteins belonging to the cupin superfamily. Despite the considerable sequence similarity between germins and GLPs, they possess different enzymatic activities. Germins exhibit oxalate oxidase activity (Lane 2002) while Superoxide dismutase (SOD) activity is associated with most of the GLPs (Woo et al. 2000). Germin-like proteins (GLPs)/SODs catalyze the conversion of superoxide to H2O2 which is of major importance in protecting living cells from toxicity produced by superoxide anion under oxidative stress conditions. Hydrogen peroxide (H2O2) functions as a signaling molecule, inducing a range of defense responses either directly or indirectly and is also involved in strengthening of cell wall by promoting lignification and papillae formation (Thordal-Christensen et al. 2000). Over the last 15 years, GLPs have been broadly characterized because of their divergent roles in various plant processes, for instance germination, developmental stages and stress-related signaling (Davidson et al. 2009). Additionally, germins and GLPs contribute in providing defense against a wide range of fungal pathogens, including Sclerotinia sclerotiorum, Blumeria graminis, Verticillium longisporum, Rhizoctonia solani, Magnaporthe oryzae, and Aspergillus flavus (Godfrey et al. 2007; Davidson et al. 2009; Knecht et al. 2010; Wang et al. 2013).
Potato (Solanum tuberosum L.) is the world’s fourth most agronomically significant crop in terms of demand and production (Chakravarty et al. 2007) and ranked third among food crops after rice and wheat. It is an important vegetable crop because of its natural prospects for high production, cost-effective income and nutritious values (Bhutta 2008). In Pakistan, potato is ranked fifth in total production and people mostly consume at least one tuber a day while in Europe and South America it is considered as an essential food (Haq et al. 2008). Potato crop faces loss in production due to biotic stresses that include fungal, viral and bacterial pathogen (Malik 1995 and Bhutta et al. 2004) and unavailability of disease free seed and lack of information about integrated disease management (Bhutta and Hussain 2002). Therefore strategies that allow betterment of commercial potato varieties are of distinctive relevance for developed and developing countries.
The introduction and expression of defense related proteins into plant genomes have shown that the progress of phytopathogenic fungi can be reduced significantly. Introduction of more than one defense gene has shown more potential in providing disease resistance than a single transgene introgression (Punja 2001). Overexpression of GLPs in transgenic plants and silencing has been shown to play role in pathogen attack in wheat, rice and barley (Banerjee and Maiti 2010; Banerjee et al. 2010; Himmelbach et al. 2010). In rice, a cluster of GLP genes was found on chromosome 8, overlapping with QTLs associated with disease resistance and contributes against pathogens (Manosalva et al. 2009). Oryza sativa root GLP2 gene promoter (OsRGLP2) activity was enhanced against fungal attack on transgenic potato plants due to the presence of several GT-1 and DOF binding sites (Munir et al. 2015). Several regulatory elements putatively involved in biotic stress response were found in the promoter region of OsRGLP1 by in silico analysis (Yasmin et al. 2008).
The present study was aimed at generating transgenic potato lines using a rice root-expressed GLP1 (OsRGLP1) gene and challenging them with Fusarium oxysporum f. sp. tuberosi to assess the potential role of this gene as inhibitor of fungal pathogenesis and to evaluate its effectiveness in transgenics plants.
Materials and Methods
Plant Material and Fungal Strain
Solanum tuberosum cv. Desiree was used to generate transgenic plants. Pure culture of Fusarium oxysporum f. sp. tuberosi was maintained on PDA (potato dextrose agar) at 27 ± 5 °C for fungal assays.
Expression Vector and Plant Transformation
The Gateway expression vector 35S:OsRGLP1, containing the coding sequence of OsRGLP1 cDNA, under the transcriptional control of 35S promoter and terminator, was used to generate transgenic plants. The vector contained the hygromycin phosphotransferase resistance gene as selectable marker for transformed plants selection (Supplementary Fig. 1). The recombinant vector was electroporated into Agrobacterium tumefaciens strain GV3101 (pMP90). Transgenic plants were generated using a protocol developed at the potato breeding and genetics lab Michigan State University, USA (Douches et al. 1998). The internode cuttings of stems were taken as explants from tissue culture plantlets. 1 to 1.5 cm long shoots were carefully excised and transferred to rooting medium (MS medium+15 mg/L hygromycin) in test tubes and were placed at 25 ± 3 °C in an incubator with a light intensity of approximately 2000 lx with a photoperiod of 16 h. To guarantee that regenerated plants were derived from independent transformation events, a single shoot was removed from each callus. Hygromycin selected rooted transformants were maintained by micropropagation. Five independent transgenic lines were obtained by this method.
Genomic DNA was isolated by using DNeasy plant mini Kit (QIAGEN Valencia, CA). Presence of OsRGLP1 gene in transgenic plants was confirmed through PCR with a specific pair of primers: forward primer for 35S (CaMV) promoter and reverse primer specific for gene (Supplementary Table 1). The thermal profile adopted for PCR was 94 °C for 3 min followed by 35 cycles each of 94 °C for 30 s, 55 °C for 30 s and 72 °C for 1 min, and final extension at 72 °C for 10 min.
Confirmation of OsRGLP1 Gene at Transcriptional Level
Total RNA was extracted from transgenic as well as nontransformed control plants by using RNeasy Plant Mini Kit (QAIGEN Valencia, CA). DNA contamination was removed from RNA preparation by using TURBO DNA-free™ Kit. For detection of expression of OsRGLP1 one step RT-PCR was done using Platinum taq system (Invitrogen, Life Technologies). Thermal cycler was programmed so that cDNA synthesis was followed automatically with PCR amplification. Twenty μL reactions were conducted containing 10 μL 2X Reaction Mix, 2 μL template RNA, 1 μL forward primer (10 μM), 1 μL reverse primer (10 μM), 1 μL RT/Platinum Taq Mix and 5 μL autoclaved distilled water. The first step of PCR included 1 cycle of 45 °C for 30 min followed by pre-denaturation for 2 min on 94 °C. The second step included 35 cycles of: denaturation at 94 °C for 15 s, annealing at 55 °C for 30 s and extension at 72 °C for 40 s. The third step included 1 cycle of 72 °C for 10 min for final extension. Reaction was cooled down and stored at 4 °C until used. Primer sets specific for the coding region of OsGLP1 and housekeeping gene elongation factor 1 subunit α (EF-1α) were used. (Supplementary Table 1). Amplified products were analyzed by electrophoresis using 1% agarose gel according to Sambrook and Russel (2001).
Quantitation of Transgene Transcript
RNA from nontransformed wild type as well as PCR positive lines was isolated using Thermo Scientific GeneJET Plant RNA Purification Mini Kit according to manufacturer’s instructions. The extracted total RNA was used directly for cDNA synthesis which was synthesized using RevetAid First Strand cDNA Synthesis Kit (Thermo Scientific cat #K1621). Before quantifying the expression, the cDNA from all PCR positive lines and wild type control were subjected to routine PCR using primers for EF-1α and OsRGLP1 that were designed to quantify the expression (Supplementary Table 1). The thermal profile used for PCR was initial denaturation at 94 °C for 3 min followed by 35 cycles each of 94 °C for 30 s, 56 °C for 30 s and 72 °C for 40 s, and final extension at 72 °C for 10 min and the result was seen on 1% agarose gel. Relative quantification of expression of OsRGLP1 in the independent transgenic lines was performed by real-time PCR. Maxima SYBR Green qPCR Master Mix (2X) kit (Fermentas Life Sciences Cat# K0221) was used for the purpose. Thermal profile for real-time PCR included one cycle of pre-amplification denaturation at 95 °C for ten minutes, followed by 40 cycles of denaturation at 95 °C for 15 s, annealing at 56 °C for 30 s and extension at 72 °C for 30 s. The signal was detected after extension of each cycle. Line-Gene K Fluorescence Quantitative PCR Detection System (BIOER) was used for relative quantification with EF-1α as a reference gene. The method used by software for the measurement of relative quantification was the 2-[delta] [delta] Ct method.
Morphology and Growth Analysis
Morphology of 3-month-old nontransformed wild type plant and high expression lines was observed. Nine plants each of four transformed events and the nontransformed plant were grown in the greenhouse. Data were collected for plant height, number of shoots, number of leaves and tubers harvested per plant.
Functional Evaluation of Transgene in Solution Superoxide Dismutase Activity
Protein concentration of wild type and transgenic plants was determined by Lowry’s method (Lowry et al. 1951) before the SOD assay. The assay was based on Beauchamp and Fridovich method (1971) with some modifications. To the reaction mixture (13 mM methionine, 0.1 mM EDTA, 75 μM Nitroblue Tetrazolium (NBT), 0.05 M potassium phosphate buffer (pH 7.8), 2 μM riboflavin, enzyme extract) distilled water was added to make the volume up to 1.5 mL in a cuvette. The reactions (carried out in triplicate) were initiated by inserting the cuvettes in an illumination chamber. The reaction was stopped after 15 min by taking out the cuvettes from the illumination chamber. Similarly, the replicates were placed in dark to determine the % inhibition. The blanks of both light and dark reactions, lacking protein gave the maximum reduction of NBT by highest absorbance at OD595. The SOD activity was determined by taking the difference between the absorbance of the samples in light and dark. The % inhibition was calculated by taking the difference between OD of blank and OD of sample and divided that by OD of blank. The amount of SOD required to inhibit the photochemical reduction of NBT by 50% was defined as 1 unit of SOD.
Added SOD activity in transgenic potato lines was evaluated for its heat tolerance, by preheating leaf extracts before measuring SOD activity at 90 °C for 15 min. To foresee the metal ion cofactor attached to OsGLP1/SOD, its sensitivity to KCN and H2O2 was studied by treating the leaf extracts with 100 mM KCN and H2O2 separately for 30 min before SOD measurement.
Oxalate Oxidase (OXO) Activity Assay
Tissue localization of oxalate oxidase activity was determined by histochemical assay according to the method established by Liang et al. (2001). Leaf discs and stem sections from control and transgenic plants were incubated with oxalic acid (2.5 mM) in a succinate buffer (25 mM succinic acid, 3.5 m M EDTA, pH 4.0) containing 4-chloro-1-naphthol (0.6 mg/ml) as staining reagent. The incubation was carried out at room temperature in dark for 24 h. Germinating wheat seeds were used as a positive control. Plant tissues were photographed with a digital camera. The experiment was repeated three times with at least 6 explants in each trial.
Detection of Localized H2O2 Level by ‘DAB-Uptake Method’
Hydrogen peroxide level was detected through 3, 3′-diaminobenzidine (DAB) staining in wild type control and transgenic leaf samples (Thordal-Christensen et al. 1997). Leaf disks, ten of each type, were placed in DAB solution (1 mg/ml) and incubated overnight. Chlorophyll was removed by heating the leaf discs with 96% ethanol for 10 min, then the leaf tissues were observed for H2O2 level.
Disease Incidence Assay of Selected Highly Expressed Potato Lines with F. oxysporum f. Sp. tuberosi
Pathogen resistance of transformed and nontransformed potato plants was determined against F. oxysporum f. sp. tuberosi. Isolates were purchased from First Fungal Culture Bank of Pakistan (FCBP), Institute of Agricultural Sciences (IAGS), Punjab University Lahore. Cultures were multiplied on PDA media supplemented with 250 mg/ml streptomycin. Microscopic characteristics were observed under light microscope. Fungal inoculum was prepared using sorghum seeds according to the method of (Akhtar et al. 2005). The sterilized clay sand mixture was inoculated with weighed inoculum of F. oxysporum f.sp. tuberosi, mixed and incubated for one week at 25 °C. The pots were filled with the infected clay sand mixture. Six weeks old potato plants from greenhouse wild type control (D-wt) as well as high expression transgenic lines (DG-1, DG-2, DG-4) were shifted to the pots and watered as required. Plants in pots without inoculum served as a negative control. Pots were kept in greenhouse at 25 °C under natural light. Fusarium oxysporum generally produces symptoms such as wilting, chlorosis, necrosis, premature leaf drop, browning of the vascular system, stunting, and damping-off. The most important of these is vascular wilt. Symptoms of leaf yellowing and wilting were scored at 12, 15, 18 and 21 days after planting based on modified scale of Silva and Bettiol (2005).
Statistical Analysis
Data was scored for statistical analysis. Analysis of variance (ANOVA) and Duncan’s multiple range test (DMRT) was calculated using software MSTATC.
Results
Plant Transformation and Selection of Transgenic Potato Plants Expressing OsRGLP1
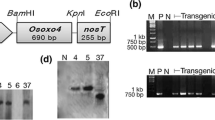
Transformation efficiency was calculated for the OsRGLP1 gene introduced into potato via Agrobacterium-mediated transformation. A total of 200 internodes were co-cultured with GV3101 (pMP90), out of which, 150 explants were regenerated on selection medium containing 15 mg/L hygromycin (Table 1; Fig. 1). Plants selected on hygromycin were confirmed by PCR amplification of the 910 bp fragment of the OsRGLP1 gene (Supplementary Fig. 2). No amplification was observed in case of wild type control plants. Among regenerated rooted shoots, 15 plantlets were selected for DNA isolation and detected for presence of gene of interest by PCR. When calculated according to Jo et al. (2014) marker-assisted transformation efficiency was 80% depicted as the percentage of PCR-positive rooted shoots while our transformation frequency was 60% (Table 1). Five independent transgenic lines were obtained. All five PCR positive transgenic lines selected from 35S:OsRGLP1 transformation were transcript positive showing a 675 bp band which represented the coding sequence of OsRGLP1 gene (Supplementary Fig. 2). The transgenic plants grown in the greenhouse from tissue culture plants were characterized by greater height, number of leaves and tuber number (Fig. 3) in comparison to the wild-type.
Different stages of potato transformation from tissue culturing to greenhouse condition. A: two-week-old explants on selection medium B: four-week-old explants on selection medium C: nontransformed wild type control plant on MS medium D: Desiree plants transformed with 35S:OsRGLP1 E: Soil acclimatized transgenic plants transformed with plasmid 35S:OsRGLP1 F: Soil acclimatized nontransformed wild type plant G: Minitubers from transgenic lines H: Minitubers from nontransformed wild type plants
Relative Quantitation of OsRGLP1 Transcript
The relative quantification of OsRGLP1 gene expression was studied by employing real-time PCR. EF-1α was selected as reference gene to normalize the expression level of the OsRGLP1 gene. Before quantifying the expression, the cDNA from all PCR positive lines and wild type plants were subjected to routine PCR using primers for EF-1α and OsRGLP1 that were designed to quantify the expression (Supplementary Table 1) and the amplicons were resolved on 1% agarose gel in the form of 281 bp and 265 bp bands (Fig. 2A). Real-time PCR results indicated that all five transgenic lines were actively expressing the transgene (Fig. 2B).
Quantification of OsRGLP1 transcript levels in transgenic lines. A: Standard PCR with wild type control (D-wt) and five independent transgenic lines (DG-1…DG-5) using primers for OsRGLP1and EF1α cDNA alternatingly, amplifying product of 265 bp and 281 bp respectively. B: Real-time PCR. Relative expression levels for OsRGLP1 were calculated using EF-1α cDNA as a reference. Statistical grouping was determined with the DMRT (p < 0.05) is indicated by letters
Comparative Analysis of Morphology and Growth
Experiments were designed to study different morphological characteristic of high expression transgenic lines. The parameters included plant height, number of leaves, shoots and tubers harvested per plant. Observations were made on 3-month-old plants of wild type Desiree and the three high expressing lines. All high expressing lines revealed greater plant height, number of shoots, number of leaves and number of tubers as compared to wild type plants (Fig. 3).
Morphology and growth analysis of OsRGLP1 transgenic lines and nontransformed control Transgenic lines (DG-1, DG-2, DG-4) and nontransformed control (D-wt) were grown for 3 months, phenotypes were assessed and averages of 6 plants are provided. Error bars represent standard error. Statistical grouping was determined with DMRT (P < 0.05) (A) plant height. (B) number of shoots. (C) number of leaves. (D) number of tubers harvested per plant for each line as well as nontransformed wild type control
Functional Evaluation of Transgene
SOD, OxO and H2O2 increase determination and fungal assays were performed to evaluate OsRGLP1 function in highly expressed transgenic potato lines.
Superoxide Dismutase Activity Determination
The high-expressing OsRGLP1 potato lines were subjected to the SOD assay to assess the contribution of OsRGLP1 gene. A significantly enhanced specific SOD activity was observed in transgenic potato lines in contrast to wild type plants (Fig. 4A, B).
SOD activity measurement in transgenic potato lines and effect of heat and inhibitors on SOD activity A): SOD activity in total protein extract from leaf samples of nontransformed control (D-wt) and selected high OsRGLP1 expression lines (DG-1, DG-2, DG-4). B) Effect of high temperature treatment on SOD activity. C) KCN treatment effect on SOD activity. D) H2O2 treatment effect on SOD activity in nontransformed wild type control and transgenic samples. Bars represent standard error (SE) based on three independent experiments. The letters designate significance in each group according to the DMRT (P < 0.05)
It was observed that the SOD activity of transgenic lines remained significantly higher than the control after heat treatment (Fig. 4C). SOD activity native to the plant decreased 60% in wild type plants. In case of transgenic potato lines SOD activity after subtraction of the native activity, was same in treated and untreated samples (Fig. 4D). The results suggested that the added SOD activity in transgenic potato plants may be attributed to some heat stable SOD and thus may be because of expression of OsRGLP1.
Since SOD is present in all aerobic organisms and most subcellular compartments that generate activated oxygen, it has been assumed that SOD has a central role in the defense against oxidative stress. There are three distinct types of SODs classified on the basis of the metal factor: Mn-SOD is resistant to KCN and H2O2, Cu/Zn-SOD is sensitive to both while Fe-SOD is resistant to KCN and sensitive to H2O2.
The transgenic potato lines and wild type samples were subjected to 3 mM KCN and 10 mM H2O2 treatment before the SOD assay. SOD activity of KCN treated and untreated samples from transgenic plants showed no significant difference (Fig. 4E), while it was reduced to 50% in wild type samples treated with KCN demonstrating the existence of endogenous Cu/ZnSOD which is known to be KCN sensitive. Added SOD activity detected in samples from transgenic plants was insensitive to KCN, proposing that it was not Cu/ZnSOD (Fig. 4F). SOD activity in H2O2 treated transgenic samples was significantly decreased in comparison to untreated samples, while the activity remained unaffected in control wild type samples (Fig. 4 G&H). These results suggested that added SOD activity conferred by transgene OsRGLP1 in transgenic plants sample is sensitive to H2O2 suggesting its Fe-SOD nature.
Oxalate Oxidase (OXO) Activity Assay
Leaf discs and stem sections from wild type and high expression lines were used for this assay to characterize OsRGLP1 protein for the presence of any OXO activity associated. Germinating wheat seeds served as positive control while plant tissues incubated in solution without oxalic acid served as negative control. The production of OXO was achieved by incubating plant tissues in a buffer comprising oxalic acid and dye 4-chloro-1-naphthol as substrate. The degradation of oxalic acid by OXO produces H2O2, which is used by endogenous peroxidases, causing the development of a dark blue precipitate. No detectable OXO activity was observed in plant tissues after incubation with the activity creator solution in the presence or absence of oxalic acid, while the control wheat seeds exhibited a dark blue color in embryonic region (Supplementary Fig. 3).
In Situ Detection of H2O2 in Potato Leaves
After DAB staining leaf cuttings from wild type and transformed plants developed brown color on leaf surface that represents the polymerization product of DAB which ultimately displays the localization of H2O2. High H2O2 accumulation was detected in transgenic leaf samples as compared to wild type control. (Supplementary Fig. 4).
Anti-Fungal Assays with Selected Highly Expressed Lines with F. oxysporum f. sp. tuberosi
To assess the role of OsRGLP1 in pathogenic fungi interaction the high expression selected transformed lines and nontransformed potato plants were infected with F. oxysporum f. sp. tuberosi. The infected plants were observed daily and were scored after every three days and categorized into the six classes from 1 to 6, established on the expansion of the disease symptoms (Table 2). Strong differences were detected in the progression of the disease symptoms between transgenic and wild type plants (Table 2; Fig. 5). Twelve days after infection, disease symptoms were seen on wild-type plants (D-wt), in the form of yellowing of leaves, mainly on older leaves. Transgenic leaves showed no symptoms after 12 days, slight yellowing of leaves was seen 21 days after infection (Table 2; Fig. 5). After 21 days wild type plants suffered seriously from fungal infection, and more than 70% of the leaves displayed severe infection symptoms (score 5 to 6), while for transgenic plants very minor infection symptoms were noticeable on the oldest leaves represented by scoring 1 to 2 (Table 2). Survival rate percentage was calculated 12, 15, 18 and 21 days post inoculation. Significant differences were observed between transgenic lines and wild type plants when data was analyzed using DMRT (Fig. 6). These findings advocate that the over-expression of OsRGLP1 gene in potato lessens the susceptibility of the potato plants to Fusarium oxysporum f. sp. tuberosi.
Visual comparison between infected transgenic potato plants expressing OsRGLP1 and nontransformed wild-type plants. Nontransformed wild-type control (D-wt) and high OsRGLP1 expression lines (DG-1, DG-2, DG-4) infected with Fusarium oxysporum f.sp. tuberosi in soil 21 days after post-inoculation. Uninfected plants served as control
Percentage survival rate of potato plants infected with Fusarium oxysporum f.sp. tuberosi. Plants of three independent OsRGLP1 transgenic potato lines (DG-1, DG-2, DG-4 and nontransformed D-wt) were infected with F. oxysporum f.sp. tuberosi. Six week old potato plantlets were shifted to infection mixture (sand + clay + inoculum) and the survival rate in % was calculated 12, 15, 18, and 21 days post-inoculation. Bars denote standard errors, based on three independent experiments. The data represents significant differences in plant survival rates for transgenic lines and the wild-type control plants based on DMRT (p < 0.05), as indicated by letters
Discussion
Over the years sufficient evidence has accumulated that suggests that germin and germin-like proteins play a role in defense against a broad range of pathogenic fungi (Zimmermann et al. 2006). Wei et al. (1998) isolated from barley, an OXO-like protein, which expresses resistance to powdery mildew fungus. Christensen et al. (2004) described HvGLP4 as a functional SOD, and studied the transient expression of TaGLP4 and HvGLP4, and observed their contribution in resistance to fungal diseases in wheat and barley. A BvGLP1 gene from sugar beet when transformed into Arabidopsis thaliana showed enhanced resistance to soil born phytopathogen Rhizoctonia solani (Knecht et al. 2010). Banerjee et al. 2010 documented OsGLP1 as a defense related protein that possesses SOD activity and accumulates H2O2 when transgenically expressed in tobacco.
The study of QTLs on chromosome 8 of rice (Manosalva et al. 2009) demonstrated the difficulty of assessing the role of GLPs and their participation in high disease resistance against pathogenic fungus, the Magnaporthe oryzae and Rhizoctania solani. Rietz et al. (2012) showed the GLP family in rape (Brassica napus) for the first time, and strongly suggested that members of BnGLP with SOD activity in B. napus may add to decrease the exposure of fungal pathogen Sclerotinia sclerotiorum. The introduction and expression of defense-related proteins into plant genomes have shown that the progress of phytopathogenic fungi can be reduced significantly. Our results further support the use of defense-related proteins for pathogen resistance.
The Agrobacterium-mediated transformation was performed using strain GV3101 (pMP90) harboring OsRGLP1 gene using internode/stem pieces as explants. A number of transgenic potato lines were obtained and verified by PCR amplification of the OsRGLP1 gene. A Gateway expression vector containing OsRGLP1 gene, 35S:OsRGLP1, was transformed in potato and five independent transgenic lines were selected for further analysis. All the plants were OsRGLP1 transcript positive and transformation efficiency calculated was 80% when depicted as PCR positive rooted shoots (Table 1), suggesting that Gateway recombinant vectors are good system to integrate T-DNA into the plant genome and for their efficient transcription.
Real-time PCR is considered as a very sensitive method for the detection and relative quantification of low abundance mRNAs (Bustin 2000), and can be used for the analysis of expression of gene at specific tissue (Bustin et al. 2000), and for plant studies (Gachon et al. 2004). Real-time PCR revealed one high expression line and four lines with similar but lower expression. GLPs may play regulatory role in different development stages of plants such as leaf, root, flower, seed and fruit development (Dunwell et al. 2008). Constitutive expression of OsRGLP1 in potato could affect its morphology. Significant differences were observed in plant height, number of leaves, shoots and tubers in transformed lines in comparison to wild type plants. If vector backbone integration did not play any role, this increase in plant height in transgenic line could be due to OsRGLP1 expression, as GLPs are identified as proteins which are involved in developmental regulation of plants. It has already been proposed that production of OH− from H2O2 may take part in loosening of cell wall through polysaccharide cleavage (Fryer et al. 2002; Liszkay et al. 2004). OsRGLP1 seems to possess SOD activity (Yasmin et al. 2015) and generate high H2O2, and therefore may be involved in expansion of the cell wall. Banerjee and Maiti (2010) exposed the efficient role of rice OsRGLP1 in plant height regulation by establishing transgenic rice plants through gene silencing. The increase in leaf number in transgenic plants can be attributed to the involvement and over-expression of OsRGLP1 gene. An increase in leaf number per plant was observed in comparison to wild type control. Increase in leaf number can be correlated to the role of GLPs in plant height as one report has shown that OsGLP1 regulates plant height development (Banerjee and Maiti 2010). Over-expression of GLP gene in transgenic plant may play a role in the better development of morphological characteristics. Oxalate oxidase activity (OxO), H2O2 level determination, SOD activity and fungal assays were performed to evaluate the OsRGLP1 function in highly expressed transgenic potato lines. OxO is an oxidoreductase enzyme which in the presence of O2 converts oxalic acid into CO2 and H2O2 (Lane 1994). No detectable OxO activity was observed in plant tissues in the presence or absence of oxalic acid, while the control wheat seeds exhibited a dark blue color in embryonic region. Similar results were observed when OsRGLP1 was overexpressed in a model plant Nicotiana tabacum (Yasmin 2009). Germins are generally described to have OxO while GLPs are mostly SODs (Zimmermann et al. 2006; Godfrey et al. 2007). Reactive oxygen species such as superoxide (•O2−), hydroxyl radical (OH−), hydrogen peroxide (H2O2), singlet oxygen, and lipid hydro peroxides are produced when partial reduction of oxygen takes place. These very unstable reactive oxygen species (ROS) are only able to be detected by end product measurement. These end products are the result of their reaction with a particular substance and can be measured by changes in their color fluorescence, or luminescence. Reactive oxygen species such as hydrogen peroxide and superoxide have been usually detected by staining methods (Jambunathan 2010). High H2O2 accumulation was detected in transgenic leaf samples as compared to wild type, these results are in accordance with the previous study of OsRGLP1 in tobacco by Yasmin et al. (2015). How a plant protects itself when it experiences oxidative burst is a complex mechanism and involves together enzymatic and non-enzymatic antioxidant components. One of the main enzymes is superoxide dismutase that plays an important role in plant defense mechanism, through detoxifying superoxide radicals by converting them into H2O2 (Bowler et al. 1992). In the present study the three highest expressing OsRGLP1 potato lines were subjected to SOD assay to assess the contribution of OsRGLP1. Increased SOD level was observed in transgenic potato lines contrast to wild type plants, earlier reported by (Yasmin 2009) in transgenic tobacco. GLPs exhibiting SOD activity are known to be heat stable (Carter and Thornburg 2000; Yasmin et al. 2008; Yasmin 2009). It was observed that the SOD activity of transgenic samples remained significantly higher than the control after heat treatment. SOD are ubiquitous metalloenzymes and plants generally possess three types of SODs. Based on their prosthetic metal ion they are named as: manganese SOD, iron SOD, and copper/zinc SOD. These SOD isoforms are located in different subcellular compartments; FeSODs are generally present in chloroplasts, MnSODs in peroxisomes and mitochondria, and Cu/ZnSODs are found in the cytosol, peroxisomes, chloroplasts and apoplasts. It is reported that FeSOD is sensitive to H2O2 and is resistant to KCN treatment. (Alscher et al. 2002). To predict the metal ion cofactor attached to OsGLP1/SOD, its sensitivity to KCN and H2O2 was studied. These results suggested that added SOD activity conferred by transgene OsRGLP1 in transgenic plants sample is sensitive to H2O2 and resistant to KCN.
To assess the role of OsRGLP1 in pathogenic fungi interaction the high expression selected transformed lines and nontransformed potato plants were infected with F. oxysporum f. sp. tuberosi. Minor infection symptoms were noticeable on the oldest leaves in comparison to wild type plants suffered seriously from fungal infection. A promising description for the antifungal function of OsRGLP1 in a potato plant could be the production of H2O2 due to its enzymatic activity (SOD). H2O2 helps in papillae formation by crosslinking cell wall proteins at the infection sites. This cell wall strengthening helps the cell to protect itself against fungal penetration (Wei et al. 1998; and Christensen et al. 2004). H2O2 produced by OsRGLP1 may also function as a signaling molecule by triggering other defense responses in plants so OsRGLP1 may be employed for general defense against fungal infections.
Conclusively, the results from the present study showed that the protein product of the OsRGLP1 gene is perhaps a heat stable iron like SOD that may play a role in stress conditions that generate ROS. The high expression transgenic potato plants were not completely protected against infection by F. oxysporum f.sp. tuberosi, however, the delay in disease progression was observed, over-expression of this gene may provide a general defense system against fungi at least in potato. It may be concluded that the OsRGLP1 could be a favorable candidate gene for crop improvement through genetic engineering strategy.
Abbreviations
- GLPs:
-
Germins and germin-like proteins
- SOD:
-
Superoxide dismutase
- H2O2 :
-
Hydrogen peroxide
- PDA:
-
Potato dextrose agar
- NBT:
-
Nitroblue Tetrazolium
- OXO:
-
Oxalate Oxidase
- DAB:
-
3, 3′-diaminobenzidine
- ANOVA:
-
Analysis of variance
- DMRT:
-
Duncan’s multiple range test
- ROS:
-
Reactive oxygen species.
References
Akhtar, H., S. Anita, and S.P. Kumar. 2005. Studies on the management of root-knot nematode, Meloidogyne incognita-wilt fungus, Fusarium oxysporum disease complex of green gram, Vigna radiata cv. ML-1108. Journal of Zhejiang University Science 6( (8): 736–742.
Alscher, R., G.N. Erturk, and L.S. Heath. 2002. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. Journal of Experimental Botany 53 (372): 1331–1341.
Banerjee, J., and M.K. Maiti. 2010. Functional role of rice germin-like protein1 in regulation of plant height and disease resistance. Biochemical and Biophysical Research Communications 394 (1): 178–183.
Banerjee, J., N. Das, P. Dey, and M.K. Maiti. 2010. Transgenically expressed rice germin-like protein1 in tobacco causes hyper-accumulation of H2O2 and reinforcement of the cell wall components. Biochemical and Biophysical Research Communications 402 (4): 637–643.
Beauchamp, C., and I. Fridovich. 1971. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gel. Analytical Biochemistry 44 (1): 276–287.
Bhutta, A.R. 2008. Survey of tuber born diseases of potato in northern areas, Pakistan. Pak. Journal of Phytopathology 20 (1): 20–33.
Bhutta, A.R., and J.I. Hussain. 2002. Production of healthy seed potato in Pakistan. Science, Technology and Development 21 (3): 1–9.
Bhutta, A.R., M.Q. Muhammad, and J.I. Hussain. 2004. Pathological survey of potato crop in northern areas, 2003, 109 pp. DOA, Islamabad: FSC & RD / AKRSP.
Bowler, C., M. Van Montague, and D. Inzé. 1992. Superoxide dismutase and stress tolerance. Annual Review of Plant Physiology and Plant Molecular Biology 43 (1): 83–116.
Bustin, S.A. 2000. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. Journal of Molecular Endocrinology 25 (2): 169–193.
Bustin, S.A., V.G. Gyselman, S. Siddiqi, and S. Dorudi. 2000. Cytokeratin 20 is not a tissue specific marker for the detection of malignant epithelial cells in the blood of colorectal cancer patients. International Journal of Surgical Investigation 2 (1): 49–57.
Carter, C., and R.W. Thornburg. 2000. Tobacco Nectarin I: Purification and characterization as a germin-like, manganese superoxide dismutase implicated in the defense of floral reproductive tissues. The Journal of Biological Chemistry 275 (47): 36726–36733.
Chakravarty, B., G. Wang-Pruski, B. Flinn, V. Gustafson, and S. Regan. 2007. Genetic transformation in potato: Approaches and strategies. American Journal of Potato Research 84 (4): 301–311.
Christensen, A.B., H. Thordal-Christensen, G. Zimmermann, T. Gjetting, M.F. Lyngkjaer, R. Dudler, and P. Schweizer. 2004. The germin-like protein GLP4 exhibits superoxide dismutase activity and is an important component of quantitative resistance in wheat and barley. Molecular Plant-Microbe Interactions 17 (1): 109–117.
Davidson, R.M., P. Reeves, P.M. Manosalva, and J.E. Leach. 2009. Germins: A diverse protein family important for crop improvement. Plant Science 177: 499–510.
Douches, D.S., A.L. Westedt, K. Zarka, and E.J. Grafius. 1998. Transformation of CryV-Bt transgene combined with natural resistance mechanisms for resistance to tuber moth in potato (Solanum tuberosum L.). Hortscience 33 (6): 1053–1056.
Dunwell, J., J.G. Gibbings, T. Mahmood, and S.M. Saqlan Naqvi. 2008. Germin and Germin-like proteins: Evolution, structure, and function. Critical Reviews in Plant Sciences 27 (5): 342–375.
Fryer, M.J., K. Oxobough, P.M. Mullineaux, and N.R. Baker. 2002. Imaging of photo-oxidative stress responses in leaves. Journal of Experimental Botany 53 (372): 1249–1254.
Gachon, C., A. Mingam, and B. Charrier. 2004. Real-time PCR: What relevance to plant studies. Journal of Experimental Botany 55 (402): 1445–1454.
Godfrey, D., A.J. Able, and I.B. Dry. 2007. Induction of a grapevine germin-like protein (VvGLP3) gene is closely linked to the site of Erysiphe necator infection: A possible role in defense. Molecular Plant-Microbe Interactions 20 (9): 1112–1125.
Haq, I., A. Rashid, and S.A. Khan. 2008. Relative efficacy of various fungicides, chemicals and biochemicals against late blight of potato. Pak. Journal of Phytopathology 21 (1): 129–133.
Himmelbach, A., L. Liu, U. Zierold, L. Altschmied, H. Maucher, F. Beier, D. Müllera, G. Hensela, A. Heiseb, A. Schützendübelc, J. Kumlehna, and P. Schweizera. 2010. Promoters of the barley germin-like GER4 gene cluster enable strong transgene expression in response to pathogen attack. The Plant Cell 22 (3): 937–952.
Jambunathan, N. 2010. Determination and detection of reactive oxygen species (ROS), lipid peroxidation, and electrolyte leakage in plants. Methods in Molecular Biology 639: 292–298.
Jo, K., C. Kim, S. Kim, T. Kim, M. Bergervoet, M.A. Jongsma, R.G.F. Visser, E. Jacobsen, and J.H. Vossen. 2014. Development of late blight resistant potatoes by cisgene stacking. BMC Biotechnology 14 (1): 1–50.
Knecht, K., M. Seyffarth, C. Desel, T. Thurau, I. Sherameti, B. Lou, R. Oelmüller, and D. Cai. 2010. Expression of BvGLP1 encoding a Germin-like protein from sugar beet in Arabidopsis Thaliana leads to resistance against Phytopathogenic fungi. Molecular Plant-Microbe Interactions 23 (4): 446–475.
Lane, B.G. 1994. Oxalate, germin and the extracellular matrix of higher plants. The FASEB Journal 8 (3): 294–301.
Lane, B.G. 2002. Oxalate, Germins, and higher-plant pathogens. IUBMB Life 53 (2): 67–75.
Liang, H., C.A. Maynard, R.D. Allen, and W.A. Powell. 2001. Increased Septoria musiva resistance in transgenic hybrid poplar leaves expressing a wheat oxalate oxidase gene. Plant Molecular Biology 45 (6): 619–629.
Liszkay, A., E. Van der Zalm, and P. Schopfer. 2004. Production of reactive oxygen intermediates (O2, H2O2, and OH) by maize roots and their role in wall loosening and elongation growth. Plant Physiology 136 (2): 3114–3123.
Lowry, O.H., N.J. Rosbrough, A.L. Farr, and R.J. Randall. 1951. Protein measurement with the Folin phenol reagent. The Journal of Biological Chemistry 193 (1): 265–275.
Malik, N.I. 1995. Potatoes in Pakistan. PARC, Islamabad: A Hand Book Pak-Swiss Project.
Manosalva, P.M., R.M. Davidson, B. Liu, X. Zhu, S.H. Hulbert, and H. Leung. 2009. A Germin-like protein gene family functions as a complex quantitative trait locus conferring broad-Spectrum disease resistance in Rice. Plant Physiology 149 (1): 286–296.
Munir, F.S. Hayashi, J. Batley, S.M.S. Naqvi, and T. Mahmood. 2015. Germin-like protein 2 gene promoter from rice is responsive to fungal pathogens in transgenic potato plants. Functional & Integrative Genomics 16 (1): 19–27.
Punja, Z.K. 2001. Genetic engineering of plants to enhance resistance to fungal pathogens, a review of progress and future prospects. Canadian Journal of Plant Pathology 23 (3): 216–235.
Rietz, S., F.E. Bernsdorff, and D. Cai. 2012. Members of the Germin-like protein family in Brassica Napus are candidates for the initiation of an oxidative burst that impedes pathogenesis of Sclerotinia Sclerotiorum. Journal of Experimental Botany 63 (15): 5507–5519.
Sambrook, J., and D.W. Russel. 2001. Preparation of plasmid DNA by alkaline lysis with SDS: Minipreparation. In Molecular cloning: A laboratory manual, ed. J. Sambrook and D.W. Russel, 3rd ed., 1.32–1.34. New York: Cold Spring Harbor.
Silva, J. C. and W. Bettiol. (2005) Potential of non-pathogenic Fusarium oxysporum isolates for control of Fusarium wilt of tomato. Fitopatologia Brasileira 30 (4): 409–412.
Thordal-Christensen, H., Z. Zhang, Y. Wei, and D.B. Collinge. 1997. Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during powdery-mildew interaction. The Plant Journal 11 (6): 1187–1194.
Thordal-Christensen, H., P.L. Gregersen, and D.B. Collinge. 2000. The barley/Blumeria (syn. Erysiphe) graminis interaction: a case study. In Mechanism of resistance to plant diseases, ed. A.J. Slusarenko, R.S.S. Fraser, and L.C. Van Loon, 77–100. Dordrecht: Kluwer Academic Publishers.
Wang, T., X. Chen, F. Zhu, H. Li, L. Li, Q. Yang, X. Chi, S. Yu, and X. Liang. 2013. Characterization of peanut Germin-like proteins, AhGLPs in plant development and defense. PLoS One 8 (4): 61722.
Wei, Y.D., Z. Zhang, C.H. Andersen, E. Schmelzer, P.L. Gregersen, D.B. Collinge, V. Smedegaard-Petersen, and H. Thordal-Christensen. 1998. An epidermis/papilla-specific oxalate oxidase-like protein in the defense response of barley attacked by the powdery mildew fungus. Plant Molecular Biology 36 (1): 101–112.
Woo, E.J., J.M. Dunwell, P.W. Goodenough, A.C. Marvier, and R.W. Pickersgill. 2000. Germin is a manganese containing Homohexamer with Oxalateoxidase and superoxide dismutase activities. Nature Structural Molecular Biology 7 (11): 1036–1040.
Yasmin, T. 2009. Cloning and Over-Expression of a Germin-Like Protein Gene for its Functional Analysis. Ph.D. Thesis, Univ. Arid Agric. Rwp., p. 24–27.
Yasmin, T., T. Mahmood, M.Z. Hyder, S. Akbar, and S.M.S. Naqvi. 2008. Cloning, sequencing and in silico analysis of Germin-like protein gene 1 promoter from Oryza sativa L. Ssp. Indica. Pakistan Journal of Botany 40 (4): 1627–1634.
Yasmin T., A. Mumtaz, T. Mahmood, M.Z. Hyder, and S.M.S. Naqvi. 2015. A germin-like protein gene of rice increased superoxide dismutase activity in transformed tobacco. Biologia Plantarum 59 (3): 456–462.
Zimmermann, G., H. Baumlein, H.P. Mock, A. Himmelbach, and P. Schweitzer. 2006. The multigene family encoding germin-like proteins of barley; regulation and function in basal host resistance. Plant Physiology 142 (1): 181–192.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 3272 kb)
Rights and permissions
About this article
Cite this article
Majeed, N., Javaid, B., Deeba, F. et al. Enhanced Fusarium oxysporum f. sp. tuberosi Resistance in Transgenic Potato Expressing a Rice GLP Superoxide Dismutase Gene. Am. J. Potato Res. 95, 383–394 (2018). https://doi.org/10.1007/s12230-018-9639-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12230-018-9639-z