Abstract
Healthy tendons play an important role in joint movements and subjected to a group of pathologies called tendinopathy due to multiple factors. Tendons have a slowly repairing process due to the low vascularity and cellularity. Treatment options aimed at potentiating the healing response and relieving symptoms. Phototherapy and platelet-rich plasma were novel treatment modalities in tendons based on photobiomodulation and growth factors during healing, and the results were encouraging suggesting calibrating treatment parameters. This study utilizes cell culture to explore the potential effect of light-emitting diode and/or growth factors in the form of platelet-rich plasma (PRP) on the activity of tenocytes isolated from sheep Achilles tendons by measuring the cell metabolism and cell mobility using cell viability and migration assays to proof safety and confirm activity. Results showed that sheep tenocyte-cultured groups treated with 5% platelet-rich plasma alone or combined with 4 J/cm2 light-emitting diode have increased viability significantly when compared to control group after a 48 h, while light-emitting diode treatment has not decreased cell migration significantly when compared with control. Result suggests that using platelet-rich plasma alone or combined with light-emitting diode might have potential to enhance healing response at the conditions applied. PRP could enhance proliferation while LED could enhance migration and proliferation. Further research is needed at longer durations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tendinopathy is a pathology affecting tendons with a multifactorial etiology characterized histologically as a disruption in matrix collagen fibers and cells. It is manifested clinically with pain and dysfunction of the musculoskeletal system, and it has a significant impact on national health systems and at the indivudal level especially workers and atheltes [1]. In tendinopathy, healing phases are largely dependent on tenocyte activity; therefore, treatment options aimed at potentiating tenocyte activities, such as cell division and metabolism, recruitment of tendon cells at the injured site, to restore tendon matrix normal function with no symptoms [2].
Current treatment options demonstrate safety at the parameters used with different outcomes and with different start times of application. Eccentric exercise is the treatment choice used by many physiotherapists; however, it cannot be applied directly after injury as it may worsen the condition [3]. Other treatments used by physiotherapist include phototherapy and growth factors which can be applied immediately after injury, although it has showed safety but with different outcomes [1].
Phototherapy treatments such as light-emitting diode (LED) and laser are based on photobiomodulation, which involves the absorption of photon energy by tissue molecules which results in photochemical or photothermal reactions that lead ultimately to cellular effects [4]. LED optical devices produce a monochromatic, non-coherent light with favorite properties such as large spot size, high performance, and relatively safer device [5]. LED devices have showed positive effects on connective tissue disorders such as wound healing, acne treatment, and skin rejuvenation [6, 7], by modulating: inflammatory mediators, collagen synthesis, gene expression [8, 9], angiogenesis [10], uniformity of collagen fibers [11], ATP synthesis, reactive oxygen species (ROS) and nitrous oxide production, and blood flow leading to enhancement of cell enzyme activity, mitochondrial respiration which affects cell proliferation and tissue regeneration [12, 13].
LED with different energies and wavelengths has been explored for tendon disorders at cellular, preclinical animal studies and clinical human studies, and results have shown increased cellularity of chicken embryo fibroblast cultures [14], increased collagen synthesis in a low-serum fibroblast cultures [15], enhanced blood supply and reduced inflammation in a tendinitis-induced in sheep [16], modulation of gene expression and inflammatory mediators in a rat Achilles tendinitis model [8, 9], improved tissue remodeling in a rat tenotomized Achilles tendon [17], and improved pain and function in patients suffering painful knee conditions [18]. However, in other trials, no significant effect was reported, and this was attributed to different light parameters (energy delivered, duration, spot size, and frequency) [19, 20], and factors related to the patients include age, body mass index, type of tendon, and nature of lesion [6] which need further research both at clinical and preclinical level to refine parameters efficiently [21].
Growth factors derived from platelet such as vascular endothelial growth factor (VEGF), insulin-like growth factor (IGF)-1, and platelet-derived growth factor (PDGF) have been used for the treatment of tendinopathy in single formulation or as a platelet-rich plasma (PRP). PRP is a source of growth factors used to potentiate the healing of tendon diseases by increased collagen expression, increased cell viability, increased fiber organization, and angiogenesis [22]. Application of PRP in in vitro studies had increased fibroblast proliferation, VEGF, and procollagen type I C-peptide expression [23], decreased inflammatory response, elevated vascularity, and increased fiber arrangement [24]. Adding plasma to ovine tenocyte 3D model enhances mitosis and attachment while adding platelet-enhanced metabolism and mitosis [25]. A recent systematic review has suggested a role of PRP in the treatment of tendinopathy; however, it suggested further research to refine the treatment regimens [26].
Recently, combined treatments were explored for tendinopathy rather than single treatment [2, 27]. Moreover, in vitro models are important research tool in investigating different treatment options to confrm safety and to provide an evidence-based treatment for clinical trials [28]. This motivates us to investigate the effect of LED and/or growth factors at the cellular level using different outcome measures with the aim to potentiate the healing response and supports the use of combined treatments. Our hypthesis is that LED and/or PRP have positive effects on the metabolism and mobility of cultured tenocyte monolayer extracted from sheep Achilles tendons.
Methodology
In vitro animal model: development and optimization
Sheep adult hind limbs (n = 10, age 6–10 months) were obtained from local slaughterhouse, and tissue biopsies (1 mm3) were harvested from the Achilles tendons and cultured in 75-cm2 flasks to allow cells to be released and sub-cultured until it reached third passage. Ten cell lines were cryopreserved ready for the experiments. Culture conditions of serum concentration, seeding density, LED parameters (energy and frequency), and PRP (dose) treatment parameters were determined based on literature and refined empirically. Viable cell number was counted using the Trypan blue method. RPMI 1640 media supplemented with 10% FBS, 5% penicillin and streptomycin, 5% HEPES buffer, 5% Amphotericin B, and 5% glutamine was used in all experiments under standard culture conditions of 5% CO2 and 95% humidity.
Initially, a pilot study was run to optimize serum concentration in culture medium using 0, 5, and 10% v/v of fetal bovine serum (FBS). For seeding density determination, a starting concentration of 250,000 tenocytes/ml of culture media was prepared and used to prepare serial dilutions up to a concentration of 244 tenocytes/ml. Triplicates of 200 μl of each cell solution were cultured in a black 96-well plate. Cell viability was measured using an Alamar blue assay every 24 h and three readings were made at 24, 48, and 72 h of seeding.
Alamar blue assay
Tenocyte viability was determined using the Alamar blue assay (Invitrogen, USA) based on the metabolism of formazan into red fluorescent solution. Simply, 10% (v/v) Alamar blue solution was added to wells of a black 96-well clear bottom plate and incubated for 3 h under normal culture conditions in the dark, and subsequently, the fluorescence intensity was measured using a fluorescence reader (Bio-tek FLx 800, USA), at 560 nm/590 nm excitation/emission filters according to manufacturer’s instructions.
Platelet-rich plasma (PRP) preparation and counting and treatment protocol
Platelet-rich plasma harvested from whole sheep blood purchased from local manufacturer and a two-spin method was used. Briefly, a fine centrifugation of 2500 rpm for 6 min was made for the whole blood sample to separate plasma with platelet followed by a hard centrifugation (4200 rpm, 6 min) to separate platelet from plasma. Platelet pellet was mixed with 25% of the supernatant plasma to yield a platelet-rich plasma which was kept at 25 °C ready to use within 5 days. The concentrated platelet (PRP) ratio relative to the whole blood was determined. A 5% (v/v) PRP was added to the RPMi 1640 culture media and used as a treatment in all PRP protocols based on literature (Kanno et al. 2005; Choi et al. 2005; Markopoulou et al. 2009).
LED device and treatment parameters
LED treatments were applied using a Photizo® device (Photon Therapy Systems LTD, 2012, South Africa)) (Table 1). LED energy parameters (i.e., energy fluence (J/cm2) and frequency of treatment (per day)) used in this study were determined empirically by conducting a pilot study using different LED energy densities and applying LED either daily or every other day.
Experimental design
Based on the optimized culture conditions and treatment parameters, a third passage cryopreserved cell lines were used to set up all experiments in triplicate using 96-well black plates with a clear bottom for the viability assays (Alamar blue) and 24-well plates for the scratch assays. Four treatment groups were used: group 1: control group received no LED or PRP treatments; group 2: LED group received 4 J/cm2 LED; group 3: PRP group received 5% PRP; and group 4: LED and PRP group received 4 J/cm2 LED and 5% PRP. Culture period includes an initial 24 h to allow cells attached to the plate followed by 48-h treatment period. A seeding density of 10,000 cells/well was used for 96-well black plates and 50,000 cells/well in 24-well plates. In experiments designed for histocytochemistry, a 24-well plate was used, and coverslips were placed at the well bottoms before culturing. LED treatment (i.e., a single energy dose of 4 J/cm2 (18 min)) and 5% PRP or their combination were applied immediately following the first 24 h of seeding. LED large probe was used and applied through the top surface of a black 96-well plate.
Scratch assay
A simple assay-simulating wound healing in vitro, based on producing a scratch in confluent cultured monolayer cells and quantifying the cell migration during the closure of the scratch. Sheep tenocytes were cultured in a 24-well plate (Corning, USA) until it reaches confluency; at this point, a scratch line was made by 100-μl micropipette tip followed by applying optimized treatment parameters of a single dose of 4 J/cm2 LED and/or 5%PRP. Images were taken using a phase-contrast inverted microscope at different time intervals (including zero time) until gap closed completely.
Statistical analysis
Statistical analysis was performed using IBM Statistical Package for the Social Sciences software (SPSS, version 20). Distribution of data was tested using Shapiro–Wilk test. Parametric tests (i.e., ANOVA and t test) were employed for normally distributed data while non-parametric tests (i.e., Kruskal–Wallis tests) were applied on non-normally distributed data. Multiple comparisons were made for the different treatment groups accordingly. Statistical significance was determined at p value less than 0.05.
Results
Optimization of the model and culture conditions
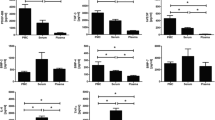
Serum concentration in culture medium results showed that in the absence of FBS, no increase in cell number or viability was seen while adding 10% FBS increased cell numbers as well as viability significantly in the presence and absence of 5% PRP (Online Resource 1). Seeding density results are presented in Fig. 1, which shows a minimal increase in cell viability after the initial 24 h as cells are attaching to culture vessels not proliferating (i.e., lag phase). After 48 and 72 h of seeding, a gradual increase was evident with a cell density reaching the maximum growth (measured as viability) at 31,250 and 3900 cells/well, respectively. This peak represents the end of the log phase after which a gradual decline in cell viability starts which represent the stationary phase. Platelet counts in PRP were 533,000/μl of serum, and this was approximately 2.4 times that of whole blood in sheep.
LED energy and frequency optimization
LED energy and frequency optimization pilot study results are presented in Figs. 2 and 3. LED energy results showed that 4 J/cm2 has better results than 8 and 20 J/cm2 after 48 h of treatment (Fig. 2). Results of analysis of variances (ANOVA) and post hoc (Fisher’s least significant difference (LSD) test) tests compared to control (0 energy) revealed insignificant increase in viability at 4 J/cm2 and insignificant decrease at 8 J/cm2 while a significant decrease in viability at 20 J/cm2. Frequency of LED treatment (i.e., treatment period) results showed a decrease in viability when cells were treated daily for 48 h while a slight increase in cell viability is seen after 48-h treatment period (Fig. 3). Statistical analysis showed no significant change in viability between both treatments when compared with control (independent sample t test, p value = 0.110); however, a significance difference was seen in the viability of tenocytes between the daily (24 hourly) and every other day (48 hourly) LED treatments (independent sample t test, p value = 0.017).
Viability—Alamar blues assay
Change in the viability of cultured tenocytes measured during the LED and/or PRP treatment periods (i.e., in 48 h) was calculated as means and stander error of the mean (means ± SEM). LED treatment alone showed no change in cell viability compared with control group (Mann-Whitney test, p value = 0.304) while adding PRP to the culture media alone or combined with LED has increased cell viability significantly (Mann-Whitney test, p value = 0.013 and 0.002, respectively) (Fig. 4).
Scratch assay
Data of scratch assay extracted from images taken at different times (Online Resource 2). revealed that scratch treated with PRP tends to slow down cell migration (i.e., slow closure) while LED-treated scratch showed a tendency to accelerate cell migration (i.e., fast closure). Statistically, LED treatments increase cell migration but not significantly while treating cells with 5% PRP alone would decrease cell migration significantly but not when combined with LED (post hoc test, Tukey’s HSD test; p = 0.01 and 0.073, respectively) (Fig. 5).
Discussion
Model development and optimization
In this study, an in vitro sheep superficial Achilles tendon model was developed, optimized, and used to investigate the potential effect of different parameters of phototherapy (LED) and/or PRP on the cell viability and migration. Initially, tenocytes were mobilized from tendon explants slowly (5 days) into the culture medium without using enzymatic dissociation which prevents any injury to cells although the risk of contamination increases. A culture medium (RPMI 1640) with phenol red was used in our in vitro culture system to mimic normal conditions as tendons are vascular connective tissues and the presence of phenol red will absorb certain wavelengths similar to blood hemoglobin [29].
Bovine serum is essential for cell culture survival, and a concentration of 10% was selected to maintain cell viability in the log phase and allow to detect potential effects based on our pilot study results (data not shown). A cell density of 10,000 cells/well was used to determine serum concentration based on our previous study using bovine primary cell cultures [30] and in line with other in vitro studies [31]. Blank wells filled with media were added at the periphery of the tested wells to minimize the effect of evaporation due to the 48 h of culturing. Alamar blue assay was chosen over MTT assay as it measures viability of cells by fluorescence intensity units (not affected by background and depends on cell metabolism) in cell number range between 500 and 50,000 [32] which represent cells at exponential phase, while MTT assay measures viability of cells using absorbance intensity units (affected by background and depends on cell number) and readings close to 0.7 are used to determine seeding density [33]. Moreover, the change in cell viability during the treatment period was calculated which compensates for background effect, pipetting errors, and seeding number variations.
In this study, a third passage was used in all experiments which gave consistent results and represent an active cell line before a senescence started to take place [28]. Moreover, our seeding density results suggest a 10,000 cells per well of 96-well plate, and a 100,000 cells per well of a 24-well plate are appropriate seeding density to start our experiments and to allow the measurement of the treatment effect before confluence was reached and this was in accordance with other in vitro studies using seeding density ranges between 5000 and 10,000 for 96-well plates with different cell lines [32, 34]. Therefore, choosing a seeding densities which represent the midpoint of the log phase (exponential growth) of the growth curve allowed the detection of any changes in cell growth as a result of treatment [28]. PRP used was prepared from sheep whole blood and used within 2 days which ensures a full efficiency and eliminates the potential adverse immune response [35]. A 5% (V/V) of PRP supplied with media was used as treatment for tenocytes, and this concentration was recommended in previous studies [31, 36, 37].
LED energy and frequency optimization results revealed that a fluence of 4 J/cm2 applied every other day (48-h treatment period) is expected to result in best parameters and maintain safety at the same time. A 48-h LED treatment period was in accordance with the World Association of Laser Therapy (WALT) (World Confederation for Physical Therapy, 2017) and were comparable with previous literature [38, 39]. For example, Xavier et al. (2010) use rats as a model of partial injury; LED treatment was applied every 48 h; in the other hand, Casalechi et al. (2009) used a 24-h LED treatment period; results from both in vivo animal studies improved tendon healing [9, 17]. LED treatment application was applied from the top of the plate which is comparable to tendons that are enclosed with skin and tendon sheaths. LED energy density-optimized results (4 J/cm2) were in accordance with several previous studies investigating photobiomodulation upon healing of animal models for cartilage, tendon, and muscle disorders utilizing low energies [8, 9, 40,41,42,43,44,45,46,47,48,49,50,51].
LED and/or PRP effects on cell viability and migration
Cell proliferation, metabolism, and migration are key phases in tendon repair process [7]. Results showed that PRP alone or combined with LED increased significantly tenocyte viability while LED alone could enhance cell migration. PRP seems to enhance proliferation and metabolism and impede cell migration. These results suggested that factors released from platelets such as growth factors [22, 23] are more potent than LED-induced effect on cell proliferation and metabolism at the parameters used and under the same conditions. Previous in vitro studies applying PRP have showed similar outcomes of increased proliferation rate of tenocytes [23, 37] and fibroblast [52, 53]. In our study, cells responded to PRP with/without LED with slow migration, and this could be explained as cells were very active in proliferation and metabolism rather than migration as cells were in the initial phase of healing response actively involved in metabolism to synthesize factors such as chemotactic factors to recruit other cells simultaneously [22]. The previous study results showed that platelet factors such as histamine have different effect on cell migration and proliferation when applied at different cell types; for example, in the endothelial cell, only cell division increased while in smooth muscles, both migration and proliferation increased [57]. A recent study supports the proliferative effect of PRP but contradicts our results of slow migration effect which could be due to the human skin fibroblast used instead of sheep tenocytes [58].
Interestingly, LED treatment of skin fibroblasts and epithelial cells has increased proliferation and migration at wavelengths of 638 nm and 518 nm which supports our results [54]. However, other studies did not find significance potential effects for light therapy which could be attributed to the different light devices or different light parameters used [27, 55, 56]. Photobiomodulation-induced effects such as anti-inflammatory have been demonstrated in different animal models addressing musculoskeletal disorders involving tendons, cartilage, and muscles [8, 9, 40,41,42,43,44,45,46,47,48,49,50,51]. For example, a tendinitis model showed that 3 j of 810-nm light therapy was effective in lowering inflammatory mediators such as prostaglandins and MMPs [43, 48] and a photobiomodulation anti-inflammatory effect at the level of gene expression using a 4 J of 808 nm light upon an in vivo arthritis model; however, both treatments apply a monochromatic laser light therapy [49, 51]. Moreover, anti-inflammatory and antioxidant effects because of photobiomodulation treatment were shown in experimental models of skeletal muscle myopathy [41, 42, 44, 46] and cartilage-induced inflammation [45, 47]. Dystrophin gene and protein parameters have improved in a myopathy rat model following a 3 and 10 J of 870-nm and 640-nm LED probes combined with 905-nm laser probe [40]. Similar photobiomodulation protective effect upon a trauma-induced myopathy model at low energies of 1 and 3 J of 660-nm and 905-nm lasers was seen [50]. These experimental studies support the effect of phototherapy on disorders involving tendons, cartilage, and muscles at different energies, different wavelengths, and different optical devices and more precisely at low energies less than 10 J and within a therapeutic wavelength window of 600 to 900 nm, and these conclusions were in harmony with our in vitro results as 4 J/cm2 of 625 nm and 850 nm LED was delivered.
In conclusion, our model safety was confirmed, and results indicate that adding PRP would significantly decrease migration (scratch assay) and increase viability (viability assay-Alamar blue), while LED could enhance migration and proliferation. Results suggested that using PRP alone or combined with LED might have the potential to enhance healing response at the conditions applied; however, this is an in vitro study which could be confirmed with future in vivo studies. In future studies, it is recommended to investigate longer culturing periods, higher passage number, and different serum levels. Further research is needed to tackle effects on metabolism especially collagen and proteoglycans.
References
Riley G (2008) Tendinopathy—from basic science to treatment. Nat Rev Rheumatol 4(2):82
Allahverdi A, Sharifi D, Takhtfooladi MA, Hesaraki S, Khansari M, Dorbeh SS (2015) Evaluation of low-level laser therapy, platelet-rich plasma, and their combination on the healing of Achilles tendon in rabbits. Lasers Med Sci 30(4):1305–1313
Maisels MJ, McDonagh AF (2008) Phototherapy for neonatal jaundice. N Engl J Med 358(9):920–928
Steiner R (2011) Basic laser physics. Laser and IPL Technology in Dermatology and Aesthetic Medicine. Springer, In, pp 3–22
Chang M-H, Das D, Varde P, Pecht M (2012) Light emitting diodes reliability review. Microelectron Reliab 52(5):762–782
Opel DR, Hagstrom E, Pace AK, Sisto K, Hirano-ALi SA, Desai S, Swan J (2015) Light-emitting diodes: a brief review and clinical experience. J Clin Aesthet Dermatol 8(6):36
Seo Y-K, Park J-K, Song C, Kwon S-Y (2014) Comparison of light-emitting diode wavelength on activity and migration of rabbit ACL cells. Lasers Med Sci 29(1):245–255
Xavier M, de Souza RA, Pires VA, Santos AP, Aimbire F, Silva JA, Albertini R, Villaverde AB (2014) Low-level light-emitting diode therapy increases mRNA expressions of IL-10 and type I and III collagens on Achilles tendinitis in rats. Lasers Med Sci 29(1):85–90
Xavier M, David DR, de Souza RA, Arrieiro AN, Miranda H, Santana ET, Silva JA, Salgado MAC, Aimbire F, Albertini R (2010) Anti-inflammatory effects of low-level light emitting diode therapy on achilles tendinitis in rats. Lasers Surg Med 42 (6):553–558
Salate AC, Barbosa G, Gaspar P, Koeke PU, Parizotto NA, Benze BG, Foschiani D (2005) Effect of in-Ga-Al-P diode laser irradiation on angiogenesis in partial ruptures of Achilles tendon in rats. Photomed Laser Ther 23(5):470–475
Fillipin LI, Mauriz JL, Vedovelli K, Moreira AJ, Zettler CG, Lech O, Marroni NP, González-Gallego J (2005) Low-level laser therapy (LLLT) prevents oxidative stress and reduces fibrosis in rat traumatized Achilles tendon. Lasers Surg Med 37(4):293–300
Wong-Riley MT, Bai X, Buchmann E, Whelan HT (2001) Light-emitting diode treatment reverses the effect of TTX on cytochrome oxidase in neurons. Neuroreport 12(14):3033–3037
Hamblin MR, Huang Y (2013) Handbook of photomedicine. Taylor & Francis
Vinck EM, Cagnie BJ, Cornelissen MJ, Declercq HA, Cambier DC (2003) Increased fibroblast proliferation induced by light emitting diode and low power laser irradiation. Lasers Med Sci 18(2):95–99
Huang P-J, Huang Y-C, Su M-F, Yang T-Y, Huang J-R, Jiang C-P (2007) In vitro observations on the influence of copper peptide aids for the LED photoirradiation of fibroblast collagen synthesis. Photomed Laser Surg 25(3):183–190
de Mattos LHL, Álvarez LEC, Yamada ALM, Hussni CA, Rodrigues CA, Watanabe MJ, Alves ALG (2015) Effect of phototherapy with light-emitting diodes (890 nm) on tendon repair: an experimental model in sheep. Lasers Med Sci 30 (1):193–201
Casalechi HL, Nicolau RA, Casalechi VL, Silveira L, De Paula AM, Pacheco MT (2009) The effects of low-level light emitting diode on the repair process of Achilles tendon therapy in rats. Lasers Med Sci 24 (4):659–665
Leal-Junior ECP, Johnson DS, Saltmarche A, Demchak T (2014) Adjunctive use of combination of super-pulsed laser and light-emitting diodes phototherapy on nonspecific knee pain: double-blinded randomized placebo-controlled trial. Lasers Med Sci 29(6):1839–1847
Bjordal JM, Lopes-Martins RA (2013) Lack of adherence to the laser dosage recommendations from the world Association for Laser Therapy in Achilles study. Arch Phys Med Rehabil 94(2):408
Chen M-H, Huang Y-C, Sun J-S, Chao Y-H, Chen M-H (2015) Second messengers mediating the proliferation and collagen synthesis of tenocytes induced by low-level laser irradiation. Lasers Med Sci 30(1):263–272
Enwemeka CS, Reddy GK (2000) The biological effects of laser therapy and other physical modalities on connective tissue repair processes. Laser Ther 12:22–30
James R, Kesturu G, Balian G, Chhabra AB (2008) Tendon: biology, biomechanics, repair, growth factors, and evolving treatment options. J Hand Surg 33(1):102–112
Anitua E, Andia I, Sanchez M, Azofra J, del Mar Zalduendo M, de la Fuente M, Nurden P, Nurden AT (2005) Autologous preparations rich in growth factors promote proliferation and induce VEGF and HGF production by human tendon cells in culture. J Orthop Res 23(2):281–286. https://doi.org/10.1016/j.orthres.2004.08.015
Dragoo JL, Braun HJ, Durham JL, Ridley BA, Odegaard JI, Luong R, Arnoczky SP (2012) Comparison of the acute inflammatory response of two commercial platelet-rich plasma systems in healthy rabbit tendons. Am J Sports Med 40(6):1274–1281
Kelly BA, Proffen BL, Haslauer CM, Murray MM (2016) Platelets and plasma stimulate sheep rotator cuff tendon tenocytes when cultured in an extracellular matrix scaffold. J Orthop Res 34(4):623–629
Nourissat G, Ornetti P, Berenbaum F, Sellam J, Richette P, Chevalier X (2015) Does platelet-rich plasma deserve a role in the treatment of tendinopathy? Joint Bone Spine 82(4):230–234
Tumilty S, McDonough S, Hurley DA, Baxter GD (2012) Clinical effectiveness of low-level laser therapy as an adjunct to eccentric exercise for the treatment of Achilles' tendinopathy: a randomized controlled trial. Arch Phys Med Rehabil 93(5):733–739
Freshney RI (2015) Culture of animal cells: a manual of basic technique and specialized applications. John Wiley & Sons
Faber DJ, Mik EG, Aalders MC, van Leeuwen TG (2003) Light absorption of (oxy-) hemoglobin assessed by spectroscopic optical coherence tomography. Opt Lett 28 (16):1436–1438
Alzyoud JA, Khan IM, Rees SG (2017) In vitro studies to evaluate the effect of varying culture conditions and IPL fluencies on tenocyte activities. Lasers Med Sci 32(7):1561–1570
Kanno T, Takahashi T, Tsujisawa T, Ariyoshi W, Nishihara T (2005) Platelet-rich plasma enhances human osteoblast-like cell proliferation and differentiation. J Oral Maxillofac Surg 63(3):362–369
O'brien J, Wilson I, Orton T, Pognan F (2000) Investigation of the Alamar blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. FEBS J 267(17):5421–5426
Hamid R, Rotshteyn Y, Rabadi L, Parikh R, Bullock P (2004) Comparison of alamar blue and MTT assays for high through-put screening. Toxicol in Vitro 18(5):703–710
Borenfreund E, Babich H, Martin-Alguacil N (1988) Comparisons of two in vitro cytotoxicity assays—the neutral red (NR) and tetrazolium MTT tests. Toxicol in Vitro 2(1):1–6
McCarrel TM, Minas T, Fortier LA (2012) Optimization of leukocyte concentration in platelet-rich plasma for the treatment of tendinopathy. JBJS 94(19):e143
Markopoulou C, Markopoulos P, Dereka X, Pepelassi E, Vrotsos I (2009) Effect of homologous PRP on proliferation of human periodontally affected osteoblasts. In vitro preliminary study. Report of a case. J Musculoskelet Neuronal Interact 9(3):167–172
de Mos M, van der Windt AE, Jahr H, van Schie HT, Weinans H, Verhaar JA, van Osch GJ (2008) Can platelet-rich plasma enhance tendon repair? A cell culture study. Am J Sports Med 36 (6):1171–1178
da Costa Santos VB, de Paula Ramos S, Milanez VF, Corrêa JCM, de Andrade Alves RI, Dias IFL, Nakamura FY (2014) LED therapy or cryotherapy between exercise intervals in Wistar rats: anti-inflammatory and ergogenic effects. Lasers Med Sci 29(2):599–605
Whelan HT, Smits RL Jr, Buchman EV, Whelan NT, Turner SG, Margolis DA, Cevenini V, Stinson H, Ignatius R, Martin T (2001) Effect of NASA light-emitting diode irradiation on wound healing. J Clin Laser Med Surg 19(6):305–314
Albuquerque-Pontes GM, Casalechi HL, Tomazoni SS, Serra AJ, Ferreira CSB, Brito RBO, de Melo BL, Vanin AA, Monteiro K, Delle H, Frigo L, Marcos RL, de Carvalho PTC, Leal-Junior ECP (2018) Photobiomodulation therapy protects skeletal muscle and improves muscular function of mdx mice in a dose-dependent manner through modulation of dystrophin. Lasers Med Sci 33(4):755–764. https://doi.org/10.1007/s10103-017-2405-5
de Almeida P, Tomazoni SS, Frigo L, de Carvalho Pde T, Vanin AA, Santos LA, Albuquerque-Pontes GM, De Marchi T, Tairova O, Marcos RL, Lopes-Martins RA, Leal-Junior EC (2014) What is the best treatment to decrease pro-inflammatory cytokine release in acute skeletal muscle injury induced by trauma in rats: low-level laser therapy, diclofenac, or cryotherapy? Lasers Med Sci 29(2):653–658. https://doi.org/10.1007/s10103-013-1377-3
de Almeida P, Lopes-Martins RA, Tomazoni SS, Albuquerque-Pontes GM, Santos LA, Vanin AA, Frigo L, Vieira RP, Albertini R, de Carvalho Pde T, Leal-Junior EC (2013) Low-level laser therapy and sodium diclofenac in acute inflammatory response induced by skeletal muscle trauma: effects in muscle morphology and mRNA gene expression of inflammatory markers. Photochem Photobiol 89(2):501–507. https://doi.org/10.1111/j.1751-1097.2012.01232.x
Marcos RL, Leal-Junior EC, Arnold G, Magnenet V, Rahouadj R, Wang X, Demeurie F, Magdalou J, de Carvalho MH, Lopes-Martins RA (2012) Low-level laser therapy in collagenase-induced Achilles tendinitis in rats: analyses of biochemical and biomechanical aspects. J Orthop Res 30(12):1945–1951. https://doi.org/10.1002/jor.22156
Tomazoni SS, Frigo L, Dos Reis Ferreira TC, Casalechi HL, Teixeira S, de Almeida P, Muscara MN, Marcos RL, Serra AJ, de Carvalho PTC, Leal-Junior ECP (2017) Effects of photobiomodulation therapy and topical non-steroidal anti-inflammatory drug on skeletal muscle injury induced by contusion in rats-part 1: morphological and functional aspects. Lasers Med Sci 32(9):2111–2120. https://doi.org/10.1007/s10103-017-2346-z
Tomazoni SS, Leal-Junior EC, Pallotta RC, Teixeira S, de Almeida P, Lopes-Martins RA (2017) Effects of photobiomodulation therapy, pharmacological therapy, and physical exercise as single and/or combined treatment on the inflammatory response induced by experimental osteoarthritis. Lasers Med Sci 32(1):101–108. https://doi.org/10.1007/s10103-016-2091-8
Tomazoni SS, Frigo L, Dos Reis Ferreira TC, Casalechi HL, Teixeira S, de Almeida P, Muscara MN, Marcos RL, Serra AJ, de Carvalho PTC, Leal-Junior ECP (2017) Effects of photobiomodulation therapy and topical non-steroidal anti-inflammatory drug on skeletal muscle injury induced by contusion in rats-part 2: biochemical aspects. Lasers Med Sci 32(8):1879–1887. https://doi.org/10.1007/s10103-017-2299-2
Tomazoni SS, Leal-Junior EC, Frigo L, Pallotta RC, Teixeira S, de Almeida P, Bjordal JM, Lopes-Martins RA (2016) Isolated and combined effects of photobiomodulation therapy, topical nonsteroidal anti-inflammatory drugs, and physical activity in the treatment of osteoarthritis induced by papain. J Biomed Opt 21(10):108001. https://doi.org/10.1117/1.Jbo.21.10.108001
Marcos RL, Leal Junior EC, Messias Fde M, de Carvalho MH, Pallotta RC, Frigo L, dos Santos RA, Ramos L, Teixeira S, Bjordal JM, Lopes-Martins RA (2011) Infrared (810 nm) low-level laser therapy in rat achilles tendinitis: a consistent alternative to drugs. Photochem Photobiol 87(6):1447–1452. https://doi.org/10.1111/j.1751-1097.2011.00999.x
dos Santos SA, Alves AC, Leal-Junior EC, Albertini R, Vieira RP, Ligeiro AP, Junior JA, de Carvalho Pde T (2014) Comparative analysis of two low-level laser doses on the expression of inflammatory mediators and on neutrophils and macrophages in acute joint inflammation. Lasers Med Sci 29 (3):1051–1058. doi:https://doi.org/10.1007/s10103-013-1467-2
Santos LA, Marcos RL, Tomazoni SS, Vanin AA, Antonialli FC, Grandinetti Vdos S, Albuquerque-Pontes GM, de Paiva PR, Lopes-Martins RA, de Carvalho Pde T, Bjordal JM, Leal-Junior EC (2014) Effects of pre-irradiation of low-level laser therapy with different doses and wavelengths in skeletal muscle performance, fatigue, and skeletal muscle damage induced by tetanic contractions in rats. Lasers Med Sci 29(5):1617–1626. https://doi.org/10.1007/s10103-014-1560-1
da Rosa AS, dos Santos AF, da Silva MM, Facco GG, Perreira DM, Alves AC, Leal Junior EC, de Carvalho Pde T (2012) Effects of low-level laser therapy at wavelengths of 660 and 808 nm in experimental model of osteoarthritis. Photochem Photobiol 88(1):161–166. https://doi.org/10.1111/j.1751-1097.2011.01032.x
Liu Y, Kalén A, Risto O, Wahlström O (2002) Fibroblast proliferation due to exposure to a platelet concentrate in vitro is pH dependent. Wound Repair Regen 10(5):336–340
Graziani F, Ivanovski S, Cei S, Ducci F, Tonetti M, Gabriele M (2006) The in vitro effect of different PRP concentrations on osteoblasts and fibroblasts. Clin Oral Implants Res 17(2):212–219
Fushimi T, Inui S, Nakajima T, Ogasawara M, Hosokawa K, Itami S (2012) Green light emitting diodes accelerate wound healing: characterization of the effect and its molecular basis in vitro and in vivo. Wound Repair Regen 20(2):226–235
Haker EH, Lundeberg TC (1991) Lateral epicondylalgia: report of noneffective midlaser treatment. Arch Phys Med Rehabil 72(12):984–988
Saunders L (1995) The efficacy of low-level laser therapy in supraspinatus tendinitis. Clin Rehabil 9(2):126–134
Bell L, Madri JA (1989) Effect of platelet factors on migration of cultured bovine aortic endothelial and smooth muscle cells. Circ Res 65(4):1057–1065
Anitua E, Pino A, Orive G (2016) Plasma rich in growth factors promotes dermal fibroblast proliferation, migration and biosynthetic activity. J Wound Care 25(11):680–687. https://doi.org/10.12968/jowc.2016.25.11.680
Acknowledgments
J. A. M. Alzyoud is grateful to Hamdi Mango Centre for Scientific Research, The University of Jordan, Amman, Jordan, for their support.
Funding
This research is partially supported by Hamdi Mango Centre for Scientific Research, The University of Jordan, Amman, Jordan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
No informed consent is needed (in vitro study).
Electronic supplementary material
ESM 1
(DOCX 287 kb)
Rights and permissions
About this article
Cite this article
Alzyoud, J.A.M., Al Najjar, S.A., Talat, S. et al. Effect of light-emitting diodes, platelet-rich plasma, and their combination on the activity of sheep tenocytes. Lasers Med Sci 34, 759–766 (2019). https://doi.org/10.1007/s10103-018-2657-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-018-2657-8