Abstract
Tendons are dense, fibrous connective tissues which carry out the essential physiological role of transmitting mechanical forces from skeletal muscle to bone. From a clinical perspective, tendinopathy is very common, both within the sporting arena and amongst the sedentary population. Studies have shown that light therapy may stimulate tendon healing, and more recently, intense pulsed light (IPL) has attracted attention as a potential treatment modality for tendinopathy; however, its mechanism of action and effect on the tendon cells (tenocytes) is poorly understood. The present study therefore investigates the influence of IPL on an in vitro bovine tendon model. Tenocytes were irradiated with IPL at different devise settings and under variable culture conditions (e.g. utilising cell culture media with or without the pH indicator dye phenol red), and changes in tenocyte viability and migration were subsequently investigated using Alamar blue and scratch assays, respectively. Our data demonstrated that IPL fluencies of up to 15.9 J/cm2 proved harmless to the tenocyte cultures (this was the case using culture media with or without phenol red) and resulted in a significant increase in cell viability under certain culture conditions. Furthermore, IPL treatment of tenocytes did not affect the rate of cell migration. This study demonstrates that irradiation with IPL is not detrimental to the tenocytes and may increase their viability under certain conditions, thus validating our in vitro model. Further studies are required to elucidate the effects of IPL application in the clinical situation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tendon is a highly organised, dynamic connective tissue which performs the essential physiological role of transmitting tensile forces from muscle to bone [1]. From a clinical perspective, tendinopathy (defined as a syndrome of tendon pain, tenderness and swelling that affects function) is very common, both within the sporting arena and amongst the sedentary population [2, 3]. The maintenance of the normal physiological function and healing processes within tendon is dependent on tendon cell (tenocyte) activities (i.e. viability, mobility and proliferation [4]). Moreover, changes in the tendon extracellular environment are sensed by the tenocytes, which, in turn, results in a cascade of responses (e.g. cell activation, alterations in the expression of matrix proteins and inflammatory mediators) [4,5,6]. Thus, the nature of the tenocyte response is key to normal tendon functioning, as well as during the initiation of pathological processes [7].
Optical devices have been demonstrated to have a photobiomodulatory effect on different connective tissues, such as tendons and skin [8, 9]. In tendon, the outcomes of phototherapy (i.e. using laser and, to lesser extent, light emitting diode [LED] light) in the treatment of tendinopathy have been described as varying, and this has been attributed to the diversity in targets and light parameters [9,10,11]. In addition, most of the positive outcomes reported used monochromatic light-producing optical devices (laser and LED) and were based on clinical trials, with a limited number of in vitro laboratory research studies evaluating the effect of light-culture system interactions and their impact on safety; however, the information gleaned from such studies is important in informing the design of clinical trials [12]. Moreover, in vitro cell culture models are critical tools pre-clinically in investigating treatment modalities, as they provide a better understanding of pathophysiology, as well as in optimising parameters for successful treatment [6, 13]. For example, laser treatment of in vitro tenocyte cultures has been shown to stimulate cell proliferation and to increase cell viability and collagen synthesis [14, 15], whilst LED light has been demonstrated to have a positive effect on growth and migration of tendon cell cultures [16]. However, different cell types have different thresholds for cell thermal injury, according to their internal chromophore absorption, and in cell cultures, this threshold is also dependent on the light parameters and the overall rise in temperature of the culture medium [12, 17]. Importantly, an understanding of these factors is key to the validity and design of in vitro model systems.
Intense pulsed light (IPL) devices produce a polychromatic light, and their use is now popular in aesthetic medicine [8]; this is supported by in vitro studies demonstrating that irradiation of skin fibroblasts with IPL has a stimulatory effect, e.g. improved cell viability and up-regulation of collagen expression [18]. IPL devices have recently attracted attention as a potential treatment modality for tendinopathy [19]; however, to our knowledge, no studies to date have examined the effect of IPL on tenocyte viability and migration (which forms part of the tendon healing response). Since light treatments may have various effects at the cellular level which could enhance or compromise cell activity, the safety and efficiency of light treatment are dependent upon the light parameters and cell culture conditions [12]. For example, the type and nature of the tissue culture media used will have a bearing on the amount of light energy reaching the cells (media containing the pH indicator dye, phenol red, have a distinctive absorption peak not seen in phenol red-free media), and such factors need to be taken into account when developing in vitro models [12]. Therefore, the aim of our study was to investigate the effect of various IPL fluencies on these parameters, in order to establish the safety and efficacy of our bovine tenocyte model system. We specifically examined the influence on light on cell viability and migration using specialised assays (namely Alamar blue and scratch assays, respectively), with tenocytes cultured in the absence or presence of phenol red-containing media, with varying concentration of foetal bovine serum (FBS).
Materials and methods
Tendon cell isolation
Tendon cells (tenocytes) were isolated from adult bovine deep digital flexor tendon (DDFT) [20]. Briefly, tendon explants were chopped into small pieces (approximately 1 mm3) using a sterile scalpel and washed with phosphate-buffered saline (PBS), supplemented with penicillin and streptomycin (100 U/ml) (Gibco Life Technologies, UK). Explants were subsequently cultured at 37 °C in a humidified air/CO2 atmosphere (19:1) in Dulbecco’s Modified Eagle’s Medium (DMEM; Gibco Life Technologies, UK) containing 10% (v/v) FBS (Gibco Life Technologies, UK) for 1 week using 24-well tissue culture plates; this allowed cells to be released from the tissue into the culture media and adhere to the plate.
Optimisation of cell culture conditions
Primary cell lines (third passage) harvested from bovine DDFT (as described above) were used in setting up subsequent experiments. Briefly, bulk volumes of DMEM with or without phenol red (all Gibco Life Technologies, UK), and supplemented with 10% (v/v) FBS, were prepared to a cellular density of 10,000 cells per ml and allocated to 24-well plates. Blank wells containing bulk media (without cells) were included on plates as controls, and coverslips were placed inside the wells assigned for subsequent live/dead staining prior to cell seeding. All experiments were conducted in quadruplicate and repeated four times.
Plates were incubated for 24 h under normal culture conditions to allow cell adherence. Thereafter, the culture media was replaced with fresh phenol red-containing or phenol red-free media supplemented in the absence or presence of FBS (5 or 10% [v/v]). Plates were cultured for a further 48 h in the absence of IPL treatment and were thus designated as control cultures. The viability of cultured tenocytes was measured as fluorescence intensity using Alamar blue assay (Life Technologies, UK), performed at the termination of culture in accordance with the manufacturer’s protocol; measured data was expressed as a fold change relative to the control group (i.e. in the absence of FBS), in the absence or presence of phenol red-containing DMEM culture media. Live and dead tenocytes were then labelled with Cell Tracker Green 5-Chloromethylfluorescein Diacetate (CMFDA) and ethidium homodimer-II dye, respectively, following the manufacturer’s guidelines (Life Technologies, UK), with 4′,6-Diamidino-2-Phenylindole (DAPI) as a nuclear counterstain. Sections were viewed under epifluoresence using a Carl Zeiss microscope (Axio Imager 2 model), equipped with AxioVision software for digital image acquisition.
Optimisation of IPL fluence
Tenocyte cell lines were irradiated with an IPL system (iPulse i300, CyDen Ltd., Wales, UK; features as described previously [21] (Table 1)) at three distinct fluencies over a 96-h treatment period (based on the World Association for Laser Therapy (WALT) recommended dosage) [11, 22]; using the IPL devise settings of 10, 15 and 20 J/cm2, the actual energy delivered was determined to be 7.3, 10.8 and 15.9 J/cm2, respectively (J. Alzyoud—unpublished observations). In these experiments, tenocyte cultures were prepared and cultured as described, except that media supplemented with 10% (v/v) FBS was used, as this was found to be the optimum concentration based on the results of the previous experiments. The first round of IPL treatment was applied directly to the primary cell monolayer through the under surface of the culture plates, and the viability of cultured tenocytes determined using the Alamar blue assay after 48 h in culture. The culture media was then changed and a second round of IPL treatment applied immediately to the cells, and the plates cultured for a further 48 h, before Alamar blue assay and live/dead staining, were performed. Experimental controls included (i) non-irradiated cell cultures and (ii) irradiated media samples (in the absence of cells). All experiments were conducted in quadruplicate and repeated four times.
Scratch assay (wound healing assay)
A scratch assay was performed as previously described [23] to determine the effect of IPL treatment on tenocyte migration under the optimum culture conditions of phenol red-containing DMEM culture media supplemented with 10% (v/v) FBS and using a 15.9 J/cm2 IPL fluence. Tenocytes harvested from four confluent cell line cultures (1 million cells) were plated evenly onto 60 mm culture dishes coated with 5 μg/ml fibronectin in duplicate, designated as control and IPL-treated. Cells were cultured under normal culture conditions until confluent, before a scratch gap was created. A single IPL treatment (15.9 J/cm2) was applied to the culture dishes through the under surface, and a phase-contrast inverted microscope (Axiovert 40C, ZEISS) used to inspect the scratch gap and to acquire the first image at zero time; subsequent images were acquired every 2 h until the scratch gap closed. The scratch assay images were subsequently analysed for gap area at all time points using ImageJ software, and results were expressed graphically as the gap area percentage relative to the first image for each time point. A time point representing a 50% gap closure was selected for testing of cell migration.
Statistical analysis
Statistical data were analysed using SPSS software. Data were presented as means plus or minus the standard error of the means. Parametric data were tested with one-way ANOVA to compare means, whilst the Kruskal-Wallis H-test was used as for non-parametric data. Significance was determined at a P value of 0.05.
Results
Effect of FBS on tenocyte viability
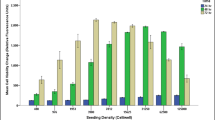
Analyses revealed that the viability of cultured tenocytes was very low in the absence of FBS; however, this showed a concentration-dependent change in the presence of 5 and 10% (v/v) FBS, with an approximate 1700- and 2600-fold increase in Alamar blue assay fluorescent intensity, respectively (Fig. 1I). This trend was similar in tenocytes cultured in the absence or presence of phenol red-containing culture media, where cell viability was found to be unaffected at the corresponding concentrations of FBS. Statistically, there was a significant difference when comparing the mean fold percentage change in primary tenocyte viability utilising culture media with and without FBS (i.e. 0, 5 and 10%), and this trend was similar using clear or phenol red-containing culture media (p = 0.001, one way ANOVA). Post hoc tests also revealed a significant difference in cell viability between all FBS concentrations, for both types of culture media (p = 0.001, Dunnett T3 and Games-Howell). However, there was no significant difference in the viability of tenocytes cultured in clear or phenol red-containing media, when comparing the corresponding FBS concentrations (p > 0.05, one-way ANOVA).
Analyses of the viability of tenocytes cultured with or without phenol red-containing culture media, supplemented in the absence or presence of FBS. I Alamar blue viability assay of tendon cultures. II Images representing the results of tenocytes subjected to live/dead staining in the absence (control) or presence (5 or 10% [v/v]) of FBS in the culture media (panels A and D, control; B and E, 5% FBS; and C and F, 10% FBS). The upper row (A, B and C) represents cells cultured in clear DMEM culture media, whilst the lower row (D, E and F) represents those cultured in phenol red-containing media. Green, live cells; red, dead cells; and blue, nuclear counterstain
The viability of tenocytes cultured in the absence or presence of FBS was further assessed using immunolabelling (live/dead staining), revealing cell death and a complete lack of cell proliferation in plates cultured without FBS (Fig. 1IIA, D); in contrast, there was a marked increase in the number of viable cells when the FBS concentration in the media was increased from 5% (Fig. 1IIB, E) to 10% (Fig. 1IIC, F). These findings were similar in plates cultured using clear (Fig. 1IIA–C) or phenol red-containing DMEM (Fig. 1IID–F).
Effect of IPL on tenocyte viability
Analyses revealed that tenocytes cultured in clear DMEM and irradiated with increasing IPL fluencies (7.3, 10.8 and 15.9 J/cm2) showed no significant difference in viability (p > 0.05, one-way ANOVA) compared with the control group (i.e. no treatment) following the first and second rounds of IPL treatment (Fig. 2IA, B). Similarly, there was no significant effect of IPL treatment on the viability of tenocytes cultured in phenol red containing-DMEM following one round of IPL treatment (Fig. 2IC). In contrast, tenocytes cultured in phenol red-containing DMEM and subjected to two IPL treatments over the 96-h culture period (Fig. 2ID) showed a significant difference in viability between treatment groups (p = 0.01, one-way ANOVA); furthermore, post hoc analyses revealed that tenocytes treated with a fluence of 15.9 J/cm2 showed a significant increase in viability when compared with the control group and 7.3 and 10.8 J/cm2 IPL treatment groups (p = 0.037, 0.002, 0.037 respectively, Tukey HSD).
Analyses of the viability of tenocytes cultured with or without phenol red-containing culture media in control (0) and IPL-treated (7.3, 10.8 and 15.9 J/cm2) samples. I Cell viability was measured using the Alamar blue viability assay after 48 h in culture following one round of IPL treatment (A, C) and again after 96 h in culture following a second round of IPL treatment (B, D); FI fluorescent intensity. II Images representing the results of tenocytes treated with or without IPL and subjected to live/dead staining at the end of the 96-h culture period. Panels A and E, no IPL treatment; B and F, 7.3 J/cm2 fluence; C and G, 10.8 J/cm2 fluence; D and H: 15.9 J/cm2 fluence. Upper row (A–D), cells cultured in clear DMEM. Lower row (E–H), cells cultured in phenol red-containing DMEM. Green, live cells; red, dead cells; and blue, nuclear counterstain
The viability of tenocytes treated with or without IPL was further assessed at the end of the 96-h culture period using live/dead staining (Fig. 2II), and these data were consistent with the results of the Alamar blue assay (Fig. 2I). Analyses revealed the presence of confluent, healthy cells in control cultures, with no evidence of cell death in the absence (Fig. 2IA) or presence (Fig. 2IE) of phenol red-containing culture media. Furthermore, data demonstrated that cell viability was not affected by IPL treatment at any energy level, in the absence (Fig. 2IB–D) or presence (Fig. 2IF–H) of phenol red.
Effect of IPL on tenocyte migration
Tenocyte migration, in the absence or presence of IPL treatment (using a maximum IPL fluence of 15.9 J/cm2), was assessed using the scratch assay. In these experiments, it was first established that the cell migration rate was dependent on cell passage number, using data analysed for cultured finite cell lines. For example, the results of the assay utilising third passage cell lines revealed that it took 18 h for the cells to achieve a 50% scratch gap closure (Fig. 3I and II, C1–C3 and D1–D3). This was in contrast to seventh passage cell lines, which took 30 h to achieve 50% closure of the scratch gap (Fig. 4I and II, C1–C3 and D1–D3). Furthermore, complete closure of the gap had occurred after 48 h with cells at third passage (Fig. 3I and II, E1–E3 and F1–F3), compared with only a 40% closure with seventh passage cells (Fig. 4I). Secondly, analyses of control and IPL-treated cultures revealed no statistically significant difference in the rate of cell migration at lower (third; Fig. 3I) or higher passages at any time point measured (p > 0.05, Kruskal-Wallis test).
Analyses of tenocyte migration in the absence or presence of IPL treatment, using third passage primary tenocytes. I Graph showing percentage of scratch gap relative to time zero in control and IPL-treated cultures. Black arrow denotes the 50% closure of the scratch area (18 h). II Images representing the scratch assay for tenocytes treated with or without IPL (n = 3). Panels A1–A3, C1–C3, and E1–E3, control cultures at 0, 18 and 48 h, respectively; B1–B3, D1–D3 and F1–F3, IPL-treated cultures at 0, 18 and 48 h, respectively. Scale bar = 250 μm
Analyses of tenocyte migration in the absence or presence of IPL treatment, using seventh passage primary tenocytes. I Graph showing percentage of scratch gap relative to time zero in control and IPL-treated cultures. Black arrow denotes the 50% closure of the scratch area (30 h). II Images representing the scratch assay for tenocytes treated with or without IPL (n = 3). Panels A1–A3 and C1–C3, control cultures at 0 and 30 h, respectively; B1–B3 and D1–D3, IPL-treated cultures at 0 and 30 h, respectively. Scale bar = 250 μm
Discussion
Phenol red is added to culture media for the purpose of monitoring pH changes during culturing [24]. In our experiments, the viability of finite cell lines harvested from bovine tendons was unaffected by the absence or presence of phenol red within the DMEM, and these results are in agreement with studies utilising cultured HeLa cells, which found no evidence of cytotoxicity in association with high concentrations of this pH indicator [25]. Other studies have also shown that the presence of phenol red-containing culture media did not interfere with Alamar blue viability assay fluorescence intensity readings [26], which is consistent with our findings (Fig. 1). Interestingly, our data showed that cell viability increased in the presence of phenol red when treated at the highest IPL energy (Fig. 2I). This may be due to the fact that phenol red increases energy absorption and heating of the culture media, which results in a photobiostimulation effect through thermal reaction [27], as the phenol red molecules are in close proximity to the cell monolayer. Another possible explanation may be that there is increased reflection of IPL light back to cells, which further enhances viability. Interestingly, in studies examining the impact of cell culture equipment on energy loss, the use of phenol red-free media was recommended, as media containing phenol red was found to have a distinctive absorption peak at 560 nm [12]. However, our results show that this absorbance peak may have a positive impact on cell viability and that the optical window for photobiomodulation is still valid.
Serum provides various essential components, such as growth factors and other proteins necessary for the survival of cultured cells [28, 29]. Indeed, our studies demonstrate that cultures of primary tenocytes required supplementation with FBS in order to survive and proliferate, with increased FBS levels associated with high viability (Fig. 1). Our results are in agreement with previous studies which report a dose-dependent effect of FBS (0–20% v/v) on fibroblast proliferation rate [30]. Furthermore, ex vivo tendon tissue cultured using serum-rich medium was found to enhance cell proliferation in the epitenon layer [31]. Whilst adding FBS to culture media was shown to be important for tendon proteoglycan (PG) synthesis [32], conversely a low rate of PG synthesis was reported when tendon explants were cultured in low-serum media [33, 34]. Therefore, serum-rich media (5–10%) is required for the culturing of bovine tendon [3, 28, 35].
In our experiments, tenocyte cultures were irradiated every 48 h, consistent with WALT (2012) recommended dosages, as well as other published literature incorporating laser or LED photobiomodulation treatment regimens. Consequently, the actual maximum IPL dose received by the tenocytes was in line with WALT recommendations (i.e. 12 J/cm2) [22, 36, 37]. Importantly, our results show that IPL irradiation did not have a detrimental effect on cell viability (i.e. there was no decrease compared with untreated cultures); moreover, an increased cell proliferation was seen when higher levels of energy were delivered to the cells, with a significant increase in cell viability at the highest fluency, thus confirming the safety/validity of the devise in our in vitro system (Fig. 2). This may be explained by the fact that higher energies enable deeper penetration and absorption of light into tissue targets, especially at higher wavelengths (600–900 nm) [38]. In addition, high fluency IPL treatments have been shown to be safer than higher energy laser treatments, which supports the use of the former in the clinical situation. Our findings are also consistent with a number of laboratory and clinical studies investigating the effects of IPL treatment on skin using high fluencies (up to 75 J/cm2), which report increased cell proliferation and viability with no evidence of cytotoxicity, as well as improved clinical assessment scores with few side effects [18, 39, 40]. Proposed mechanisms for IPL enhancement of cell viability include the reversal of cell senescence via reduced reactive oxygen species production and enhanced telomerase activity, as well as activation of cytochrome-c oxidase and release of nitrous oxide, leading to activation of cell signalling pathways [8, 38, 41, 42].
In our studies, the cell migratory rate, as well as the population doubling time, slowed as the cell passage number increased (third versus seventh passage) with or without IPL treatment (Figs. 3 and 4). This may be explained by the progressive ageing of the tenocytes in culture (senescence), as their proliferative ability, motility and metabolic rate decreases, leading to a decline in their regenerative ability [43,44,45], and is in keeping with previous studies. However, in contrast to our data, low-level laser irradiation has been found to stimulate tenocyte migration using an in vitro model [14, 15]. The lack of detection of any effect of IPL on cell migration in our system might be due to a rapid proliferation of cells over a short period, or the lack of cumulative effect of multiple treatments over a longer period [14]. Future studies could therefore be aimed at investigating a longer dosing regimen.
Conclusion
This study investigates for the first time the effect of IPL on a bovine tendon model system; IPL fluencies of up to 15.9 J/cm2 were proven harmless to the tenocyte cultures, and this was the case using culture media with or without phenol red. Furthermore, there was a significant increase in cell viability following irradiation with the highest energy under certain culture conditions. Future translational studies should be carried out to elucidate the effects of IPL application in the clinical situation.
References
Kannus P (2000) Structure of the tendon connective tissue. Scand J Med Sci Sports 10(6):312–320
Riley G (2004) The pathogenesis of tendinopathy. A molecular perspective. Rheumatology 43(2):131–142
Rees S, Dent C, Caterson B (2009) Metabolism of proteoglycans in tendon. Scand J Med Sci Sports 19(4):470–478
Sharma P, Maffulli N (2005) Tendon injury and tendinopathy: healing and repair. J Bone Joint Surg 87(1):187–202
McNeilly C, Banes A, Benjamin M, Ralphs J (1996) Tendon cells in vivo form a three dimensional network of cell processes linked by gap junctions. J Anat 189(Pt 3):593
O'brien M (1997) Structure and metabolism of tendons. Scand J Med Sci Sports 7(2):55–61
Cook J, Purdam CR (2009) Is tendon pathology a continuum? A pathology model to explain the clinical presentation of load-induced tendinopathy. Br J Sports Med 43(6):409–416
Babilas P, Schreml S, Szeimies RM, Landthaler M (2010) Intense pulsed light (IPL): a review. Lasers Surg Med 42(2):93–104
Allemann IB, Kaufman J (2011) Laser principles. In: Allemann IB, Goldberg DJ (eds) Basics in Dermatological Laser Applications, vol 42. Curr Probl Dermatol, Karger, Basel, pp7-23
Calderhead RG (2007) The photobiological basics behind light-emitting diode (LED) phototherapy. Laser Ther 16(2):97–108
Tumilty S, Munn J, McDonough S, Hurley DA, Basford JR, Baxter GD (2010) Low level laser treatment of tendinopathy: a systematic review with meta-analysis. Photomed Laser Surg 28(1):3–16
Davies LB, Kiernan MN, Bishop JC, Thornton CA, Morgan G (2014) The impact of cell culture equipment on energy loss. Lasers Med Sci 29(1):195–202
Franchi M, Alessandra T, Marilisa Q, Ester O, Victoria O (2007) Collagen structure of tendon relates to function. Sci World J 7:404–420
Tsai W-C, Cheng J-W, Chen J-L, Chen C-Y, Chang H-N, Liao Y-H, Lin M-S, Pang J-HS (2014) Low-level laser irradiation stimulates tenocyte proliferation in association with increased NO synthesis and upregulation of PCNA and cyclins. Lasers Med Sci 29(4):1377–1384
Tsai W-C, Hsu C-C, Pang J-HS, Lin M-S, Chen Y-H, Liang F-C (2012) Low-level laser irradiation stimulates tenocyte migration with up-regulation of dynamin II expression. PLoS One 7(5):e38235
Seo Y-K, Park J-K, Song C, Kwon S-Y (2014) Comparison of light-emitting diode wavelength on activity and migration of rabbit ACL cells. Lasers Med Sci 29(1):245–255
Lapotko DO, Zharov VP (2005) Spectral evaluation of laser-induced cell damage with photothermal microscopy. Lasers Surg Med 36(1):22–30
Wong W, Shyu W, Tsai J, Hsu K, Pang J (2009) Intense pulsed light effects on the expression of extracellular matrix proteins and transforming growth factor beta-1 in skin dermal fibroblasts cultured within contracted collagen lattices. Dermatol Surg: Off Publ Am Soc Dermatol Surg [et al] 35(5):816–825
Hutchison A, Pallister I, Evans R, Bodger O, Topliss C, Williams P, Beard D (2013) Intense pulsed light treatment of chronic mid-body Achilles tendinopathy. Bone Joint J 95(4):504–509
Crockett RJ, Centrella M, McCarthy TL, Thomson JG (2010) Effects of cyclic strain on rat tail tenocytes. Mol Biol Rep 37(6):2629–2634
Hutchison A, Beard D, Bishop J, Pallister I, Davies W (2012) An investigation of the transmission and attenuation of intense pulsed light on samples of human Achilles tendon and surrounding tissue. Lasers Surg Med 44(5):397–405
World Association of Laser Therapy (2006) Consensus agreement on the design and conduct of clinical studies with low-level laser therapy and light therapy for musculoskeletal pain and disorders. Photomed Laser Surg 24(6):761
Liang C-C, Park AY, Guan J-L (2007) In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc 2(2):329–333
Berthois Y, Katzenellenbogen JA, Katzenellenbogen BS (1986) Phenol red in tissue culture media is a weak estrogen: implications concerning the study of estrogen-responsive cells in culture. Proc Natl Acad Sci 83(8):2496–2500
Zhu Y, Zhang X, Zhu J, Zhao Q, Li Y, Li W, Fan C, Huang Q (2012) Cytotoxicity of phenol red in toxicity assays for carbon nanoparticles. Int J Mol Sci 13(10):12336–12348
Al-Nasiry S, Geusens N, Hanssens M, Luyten C, Pijnenborg R (2007) The use of Alamar blue assay for quantitative analysis of viability, migration and invasion of choriocarcinoma cells. Hum Reprod 22(5):1304–1309
Karu T (1999) Primary and secondary mechanisms of action of visible to near-IR radiation on cells. J Photochem Photobiol B Biol 49(1):1–17
Brunner D, Frank J, Appl H, Schöffl H, Pfaller W, Gstraunthaler G (2010) Serum-free cell culture: the serum-free media interactive online database. ALTEX 27(1):53
Van der Valk J, Brunner D, De Smet K, Svenningsen ÅF, Honegger P, Knudsen LE, Lindl T, Noraberg J, Price A, Scarino M (2010) Optimization of chemically defined cell culture media–replacing fetal bovine serum in mammalian in vitro methods. Toxicol in Vitro 24(4):1053–1063
Voytik-Harbin SL, Brightman AO, Waisner B, Lamar CH, Badylak SF (1998) Application and evaluation of the alamarBlue assay for cell growth and survival of fibroblasts. In Vitro Cell Dev Biol Anim 34(3):239–246
Abrahamsson SO, Lundborg G, Lohmander LS (1991) Long-term explant culture of rabbit flexor tendon: effects of recombinant human insulin-like growth factor-I and serum on matrix metabolism. J Orthop Res 9(4):503–515
Koob T, Vogel KG (1987) Proteoglycan synthesis in organ cultures from regions of bovine tendon subjected to different mechanical forces. Biochem J 246(3):589–598
Koob TJ, Clark PE, Hernandez DJ, Thurmond FA, Vogel KG (1992) Compression loading in vitro regulates proteoglycan synthesis by tendon fibrocartilage. Arch Biochem Biophys 298(1):303–312
Vogel K, Hernandez D (1992) The effects of transforming growth factor-beta and serum on proteoglycan synthesis by tendon fibrocartilage. Eur J Cell Biol 59(2):304–313
Rees S, Flannery C, Little C, Hughes C, Caterson B, Dent C (2000) Catabolism of aggrecan, decorin and biglycan in tendon. Biochem J 350:181–188
Bastos J, Lizarelli R, Parizotto N (2009) Comparative study of laser and LED systems of low intensity applied to tendon healing. Laser Phys 19(9):1925–1931
Bjordal JM (2012) Low level laser therapy (LLLT) and world Association for Laser Therapy (WALT) dosage recommendations. Photomed Laser Surg 30(2):61–62
Hamblin MR, Demidova TN (2006) Mechanisms of low level light therapy. Proc Spie 6140(61001):1-12.
Bahmer F, Drosner M, Hohenleutner U, Kaufmann R, Kautz G, Kimmig W, Landthaler M, Neumann R, Raulin C, Seeber N (2007) Recommendation for laser and intense pulsed light (IPL) therapy in dermatology. JDDG: J Dtsch Dermatol Ges 5(11):1036–1042
Bedewi AE, Khalafawy GE (2013) The use of synchrotron infrared microspectroscopy to demonstrate the effect of intense pulsed light on dermal fibroblasts. J Cosmet Laser Ther 15(6):305–309
Raulin C, Greve B, Grema H (2003) IPL technology: a review. Lasers Surg Med 32(2):78–87
Karu T (2013) Is it time to consider photobiomodulation as a drug equivalent? Photomed Laser Surg 31(5):189–191
Zhou Z, Akinbiyi T, Xu L, Ramcharan M, Leong DJ, Ros SJ, Colvin AC, Schaffler MB, Majeska RJ, Flatow EL (2010) Tendon-derived stem/progenitor cell aging: defective self-renewal and altered fate. Aging Cell 9(5):911–915
López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G (2013) The hallmarks of aging. Cell 153(6):1194–1217
Kohler J, Popov C, Klotz B, Alberton P, Prall WC, Haasters F, Müller-Deubert S, Ebert R, Klein-Hitpass L, Jakob F (2013) Uncovering the cellular and molecular changes in tendon stem/progenitor cells attributed to tendon aging and degeneration. Aging Cell 12(6):988–999
Acknowledgments
J. A. M. Alzyoud is grateful to the Hashemite University, Jordan for their financial sponsorship and support. The authors wish to thank Dr. Owen Bodger (Swansea University) for expert statistical advice and CyDen Ltd., in Wales for the IPL light device.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
Hashemite University of Jordan is the sponsorship for the corresponding author during his PhD degree and this study was part of his PhD thesis.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
No informed consent is needed (in vitro study).
Rights and permissions
About this article
Cite this article
Alzyoud, J.A.M., Khan, I.M. & Rees, S.G. In vitro studies to evaluate the effect of varying culture conditions and IPL fluencies on tenocyte activities. Lasers Med Sci 32, 1561–1570 (2017). https://doi.org/10.1007/s10103-017-2279-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-017-2279-6