Abstract
One of the objectives of rotator cuff repairs is to achieve biological healing and recovery in the tendon-bone zone. Some clinical evaluations reported the feasibility of tendon healing based on the stimulations of electric field and platelet-rich plasma (PRP). However, because of lack of appropriate tool for in vitro primary culture under complicated conditions, the efficacy and standard protocol of these healing approaches are still controversial among clinical experts. In this study, a novel co-culture device was developed for the study of tenocytes proliferation under single and combined stimulations of electric field and PRP. The device was a culture well divided into three sub-chambers separated by a barrier and embedded with a pair of parallel plate electrodes. Tenocytes and PRP gel could be respectively loaded into the sub-chambers and cultured with interlinked medium. Hence, tenocytes could concurrently receive a uniform electric field and platelet-derived growth factors by diffusion. Results revealed that the proliferation of tenocytes could be significantly enhanced by these stimulations. The device provides a precise and practical approach for the in vitro study of tendon healing, especially for PRP study. Moreover, optimization of the conditions of electric field and PRP could be determined by in vitro screening procedure before surgery to provide a personalized therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The function of rotator cuff is to stabilize humeral head in the center of the glenoid cavity by generating force coupling in the coronal and transverse planes. The prevalence of rotator cuff tears is approximately 45% of patients in their 70s and many of these patients report difficulty with routine activities of daily living (El-Azab et al., 2010; Tashjian, 2012). The rotator cuff repairs aims to achieve high initial fixation strength, maintain mechanical stability, minimize gap formation, and optimize the biology of the tendon-bone healing zone (Cole et al., 2007). More than 300,000 repairs were reported to be conducted each year. Although there were some newly developed techniques to increase the strength of repair construct, observed re-tear rates still ranged from 25% to 90% (Nho et al., 2007). Studies have continuously revealed that high incidence of recurrent tendon defect was occurred after the rotator cuff repairs (Tashjian et al., 2010; Toussaint et al., 2011). One important reason for such a high re-tear rate is because of minimal blood supply to the repair construct (Sanchez et al., 2007). Tenocytes are the basic cellular component of tendon tissue and contribute to synthesize all components of the extracellular matrix for the intrinsic repair process (O'Brien, 1997; Sharma & Maffulli, 2005). Low cell density in tendon tissue and minimal blood supply lead to the limited extracellular matrix restoration for tendon healing.
Alternatively, non-surgical approaches that are without anatomical reduction are used to achieve fracture and tendon healing based on external mechanical and electromagnetic stimulations (Tang et al., 2006; Carter et al., 1988; Brook et al., 2012; de Girolamo et al., 2013). Studies reported that micromovement at fracture sites can improve periosteal callus formation and bridging the bony fragments (Yamaji et al., 2001; Kanno et al., 2005). Low frequency pulsed electromagnetic field has been proven to enhance the healing of bone, cartilage, and tendon tissue (de Girolamo et al., 2013; Fini et al., 2005; Vavken et al., 2009). The above demonstrations showed external physical stimulations could enhance cells to synthesize the components of extracellular matrix and cytokines for cell proliferation. Moreover, direct-current (DC) electrical stimulation was reported for the acceleration of fracture healing (Goldstein et al., 2010; Jorgensen, 1977). For alternating current (AC) electric stimulation, electric field at low frequency (under 1 kHz) is used for excite tissues including nerve, muscle, and heart (Polk, 1995). At high frequency (above 1 MHz), tissue heating becomes dominant due to dielectric losses (Elson, 1995). Medical treatments such as diathermy and tumor ablation can be accomplished. Although electric field at intermediate frequency (kHz - MHz) has not yet been reported to have biological effect, but DC electric field was reported to stimulate vascular endothelial cells for inducing angiogenic responses (Bai et al., 2011; Zhao et al., 2004). We believe electric fields at the intermediate frequency could promote cell proliferation by stimulating cells to synthesize the growth factors. However, few works have been investigated for tendon healing using AC electric field at intermediate frequency.
Recently, the use of autologous platelet-rich plasma (PRP) gels was proposed to be an alternative biological healing approach to stimulate and accelerate the soft tissue and bone healing process (Everts et al., 2007; Gosens et al., 2011; Wrotniak et al., 2007). The effectiveness of the PRP lies in the generation of high concentration of platelet-derived growth factors that mimic the physiologic wound healing and reparative tissue processes. The clinical evaluations provided the foundation of the feasibility of using PRP. However, the efficacy of PRP application during arthroscopic rotator cuff repair has not been clearly demonstrated and remains controversial among medical experts (Jo et al., 2011). The optimal concentration of PRP and standardization in the preparation of PRP are yet to be determined and required a significant amount of basic science studies (Foster et al., 2009). In the past decades, because directly applying PRP to culture medium in a small culture well causes gelling of cells (Hoppe et al., 2013), studying PRP stimulation was generally to use the culture wells in large diameter, e.g., 12-well and 6-well plates (Boswell et al., 2014; Mazzocca et al., 2012a). However, it is known that multiple passaging of tenocytes leads to phenotypic drift (Almarza et al., 2008; de Wreede & Ralphs, 2009; Yao et al., 2006; Mazzocca et al., 2012b) and this poses a problem in tenocytes research because freshly cultured tenocytes are not manifold available in a sufficient amount. Normally, limited number of tenocytes could be isolated from human rotator cuff tendons. Hence, a culture well in small size is generally preferred to reduce the usage of tenocytes. Alternatively, indirect co-culture system with the use of Transwell filters with 0.4 μm pores was used for preventing gelling of cells (Zhai et al., 2012; Jo et al., 2012). Tenocytes and PRP were respectively added into the outer and inner chambers. Although this approach could prevent gelling of cells, but the diffusion across the filters was limited and that may influence the accuracy of the results.
In order to reduce the usage of tenocytes and provide a combined stimulation of electric field and PRP, a novel co-culture device was developed to respectively seed tenocytes and apply PRP gel in different sub-chambers under the interlinked medium. Hence, tenocytes cultured in the sub-chamber could receive platelet-derived growth factors by diffusion. In the current study, the co-culture device was also embedded with a pair of parallel plate electrodes when tenocytes were stimulated by electric field. By using the co-culture device, the combined stimulation of electric field and PRP could be realized for the investigation of the response of human tenocytes. Results indicated that tenocytes stimulated by AC electric field of 0.57 V/cm and 70 kHz were found to have the highest proliferation ratio. Increasing platelet ratio in PRP was positively proportional to cell proliferation. Also, results confirmed that PRP could compensate the cytotoxic effect of drug. Finally, combined stimulation of electric field and PRP was studied and superposition effect was found in cell proliferation and transforming growth factor-β1 (TGF-β1) in culture medium. In conclusion, the fabrication of the co-culture device is simple and it allows for large volume production for clinical study. Also, the processing of cell culture is compatible to general bio-technological skillset. Unlike other microfluidics-based device, tubing and external instrumentation are required to be operated by the trained engineering personnel. The current approach of co-culture experiment is expected to have a higher acceptance by the researchers in biological laboratories.
2 Materials and methods
2.1 Isolation of tenocytes obtained from human rotator cuff tendons
The approval of the study protocols used to collect tendon tissues and blood samples was provided by Institutional Review Board (IRB), Linkou Chang Gung Memorial Hospital, Taiwan (IRB No. 105-0162C). Inclusion criteria were those who had failure of 6 months of non-operative treatment, no episodes of shoulder instability, no radiographic signs of fracture of the glenoid or the greater or lesser tuberosity, MRI evidence of cuff tear and a repairable full-thickness tear of the rotator cuff found at the time of surgery. Exclusion criteria of this study included patients with a history of previous rotator cuff surgery, significant degenerative glenohumeral osteoarthritis, rheumatalogical, neuromuscular, or autoimmune disease. After obtaining written informed consent before surgery, tendon tissues were isolated from the edge of torn human rotator cuff tendons undergoing arthroscopic rotator cuff repair, as shown in Fig. 1(a). We used a previously published protocol to isolate tenocytes (Wang et al., 2012; Pauly et al., 2015). Briefly, tendon samples were digested in an enzymatic solution containing 4 mg/mL dispase (Roche, Burgess Hill, UK) and 300 U/mL collagenase Type II (Gibco, Invitrogen, Paisley, UK) at 37 °C for 16 h. After digestion, the mixture was filtered and centrifuged at 1000 rpm (400 × g) for 5 min at 37 °C. The cell pellet was then suspended and maintained in culture medium (minimum essential medium; α-MEM) supplemented with 10% FBS and 1% antibiotics in standard tissue culture flasks. After the first passage, the adherent cells was trypsinized and cells were seeded at 2 × 104 cells/cm2 in the co-culture device for the current study. Normal tenocyte morphological characteristics were confirmed by microscopy, as shown in Fig. 1(b). The cell number was counted using an automated cell counter (Model: Countess II FL; Invitrogen, USA). Tenocytes above three passages were discarded because phenotypic drift was reported (Mazzocca et al., 2012b). The tenocyte genotype was confirmed by quantitative real-time polymerase chain reaction (qPCR) for the tenocyte markers including type I collagen, type III collagen, decorin, tenascin-C, and scleraxis (data not shown).
2.2 Preparation of PRP products
The 20 mL whole blood sample collected from individual during surgery or volunteer was mixed with 1.25 mL anticoagulant solution (citrate phosphate dextrose adenine-1; CPDA-1) in a plain tube. PRP was prepared by a 2-step centrifugation process using a bench-top centrifuge machine (Model: 5430R; Eppendorf, Germany). The first step was a separating centrifugation of 1500 rpm for 5 min. After centrifugation, erythrocytes were sedimented and platelets remained in suspension. Then, the upper plasma layer was transferred to another clean plain tube for the second centrifugation. It was a condensation centrifugation of 6300 rpm for 15 min to condense the platelets. The lower 5 mL PRP was preserved and the supernatant layer (platelet-poor plasma) was discarded. A 1 mL sample of each native blood specimen and each preparation was analyzed by the blood laboratory at Linkou Chang Gung Memorial Hospital. Platelet number, red blood cells number, and white blood cells differentiation were determined by an automatic hematology analyzer (Model: Sysmex XT-1800i; Kobe, Japan). The platelet ratio in PRP was defined as the platelet number of the prepared PRP divided by the platelet number of the whole blood sample (DeLong et al., 2012).
2.3 Design and fabrication of the co-culture device
A co-culture device was designed to respectively seed tenocytes and apply PRP gel in different sub-chambers under the interlinked medium. Embedded with a pair of parallel plate electrodes, proliferation of human tenocytes was investigated under the stimulation of electric field and PRP. The culture well of the co-culture device was 15 mm in diameter and 7 mm in height, as shown in Fig. 2(a). It was composed of three sub-chambers separated by a barrier of 3 mm in height and 1 mm in width. Therefore, the growth area of each sub-chamber was calculated to be approximate 0.59 cm2, while 0.38 cm2 in 96-well plates, 1.9 cm2 in 24-well plates, and 3.8 cm2 in 12-well plates.
Design and experimental setup of the investigation of the proliferation of tenocytes under the stimulation of electric field and PRP. (a) Photograph of the co-culture device. (b) Experimental setup of tenocytes under the stimulation of electric field. (c) Experimental setup of tenocytes under the stimulation of PRP
The co-culture device consisted of a glass substrate and a polydimethylsiloxane (PDMS) (Model: Sylgard® 184; Dow Corning, USA) layer. The PDMS layer was fabricated by molding from a poly(methacrylate) (PMMA) mold with negative pattern of the culture wells machined by a CNC engraving machine (Model: EGX-400; Roland, Japan). The molding process is described briefly. PDMS mixture prepared by mixing with PDMS pre-polymer and curing agent in (w/w) 10:1 was poured to the PMMA mold. After solidification at 70°C for 1 h, the PDMS layer was peeled off from the mold. To apply an electric field to the culture well, the PDMS layer was bonded to an indium tin oxide (ITO)-glass substrates with surface resistance of 7–10 Ω (Uni-Onward Corp., Taiwan). For the study of cells stimulated by PRP, the PDMS layer was simply bonded to a glass substrate. Finally, the co-culture device was washed by phosphate-buffered saline (PBS; 50 mM phosphate, 150 mM NaCl, and 10 mM EDTA; pH 7.6) and kept under ultraviolet light for further experiments.
2.4 Tenocytes stimulated by the electric field
Cells at a density of 2×104 cells/cm2 were seeded on the sub-chambers of the co-culture device. Hence, three independent experiments were simultaneously conducted in three sub-chambers, as shown in Fig. 2(b). The culture medium was applied to top up the culture well. Then, an ITO-glass substrate was covered on the PDMS layer to form a pair of parallel plate electrodes. After overnight for cell attachment and stabilization in the incubator, an electric field induced by a signal generator (Model: DG1022; Rigol Technologies Inc., China) was applied across the ITO-glass substrates. Electric field was defined by the peak-to-peak voltage divided by the distance between two ITO-glass substrates. Different application conditions including frequency from 30 to 100 kHz, electric field from 0.37 to 0.71 V/cm, and duration were investigated for enhancing the proliferation of tenocytes. After stimulation, the co-culture device was transferred to the incubator for 7-day culture. After the culture course, cell proliferation was investigated by the bio-assay.
2.5 Tenocytes stimulated by the PRP
Cells at a density of 2 × 104 cells/cm2 suspended in 120 μL culture medium were respectively applied to two of three sub-chambers (cell chamber) of the co-culture device. Then, 10 μL PRP in liquid form was applied to the remaining sub-chamber (PRP chamber). It is noted that the level of solution was not added over the height of the barrier. Then, the co-culture device was placed in the incubator overnight. The next day, cells attached on the surface of the cell chamber and PRP became gel form in the PRP chamber. Another 500 μL culture medium was applied to the PRP chamber and flew over the PDMS barrier, causing exchange of culture medium between cell chamber and PRP chamber. Hence, tenocytes cultured in the cell chamber could receive platelet-derived growth factors by diffusion, as shown in Fig. 2(c). Finally, cells were cultured in the incubator for 7 days. After the culture course, cell proliferation was investigated by the bio-assay.
2.6 Quantification of cell number by the bio-assay
In this study, cell number was quantified by the conventional bio-assay, i.e., WST-1 assay (Roche Applied Science, USA). It is a colorimetric assay for the nonradioactive quantification of cell proliferation, cell viability, and cytotoxicity. After the culture course, culture medium was first removed manually. Then, the reagent of WST-1 assay in the dilution of 1:10 was added to each sub-chamber for reaction. After incubation at 37 °C for 2 h, the supernatant was collected and transferred to an enzyme-linked immunosorbent assay (ELISA) plate. Color intensity of the reacted product represented by optical density (OD) was quantified by a microplate reader (Model: ELx800; BioTek Instruments, Inc., USA) at an absorbance of 440 nm with a reference wavelength of 660 nm. Proliferation ratio was defined as the OD value of the experimental group divided by the OD value of the control group at the end of the culture course (day 7).
2.7 Quantification of TGF-β1 by enzyme-linked immunosorbent assay
To quantify the concentration of TGF-β1, the supernatant was collected and analyzed by using a commercial immunoassay kit (Quantikine® TGF-β1 ELISA kit; R&D Systems, USA) according to the manufacturer’s recommended protocol. Briefly, the sample was pipetted in a kit supplied 96-well microplate pre-coated with antibody incubated for 3 h at room temperature. Then, the wells were washed using the provided buffer and incubated with the detection antibody for 1.5 h at room temperature. Next, the substrate solution was added and incubated for 20 min. After the color development, the OD value of the reacted solution was quantified by a microplate reader at an absorbance of 440 nm with a reference wavelength of 660 nm. Moreover, a serial dilution of the provided standard TGF-β1 solution was used to construct the calibration curve. The concentration of TGF-β1 could be calculated according to the calibration curve.
2.8 Statistical analysis
The data presented as mean ± standard deviation (SD) were collected from at least three measurements independently. The results were analyzed by one way analysis of variance (ANOVA). Statistical significance was indicated as * for p < 0.05, ** for p < 0.01, and *** for p < 0.005.
3 Results and discussions
3.1 Proliferation of human tenocytes in response to the stimulation of electric field
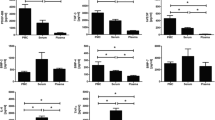
Electric field at intermediate frequency was investigated to stimulate the proliferation of human tenocytes. Different frequencies of 30, 60, 70, and 100 kHz were respectively applied to the culture wells to determine the optimal frequency for tenocytes proliferation. Electric field was set to 0.7 V/cm and applied for 60 min. Cell proliferation was studied after 7-day culture. Result is shown in Fig. 3(a) and indicated that the electric field at 50 and 70 kHz could significantly enhance the proliferation of tenocytes. Next, different electric fields of 0.37, 0.42, 0.57, and 0.71 V/cm were applied for 60 min and the frequency was set to be 70 kHz. The proliferation of tenocytes after 7-day culture was studied and result is shown in Fig. 3(b). The optimal electric field strength was shown to be 0.57 V/cm. The above studies confirmed that the proliferation of tenocytes could be enhanced by the electric field at intermediate frequency. Furthermore, different application durations were investigated to provide some guidelines for rehabilitation therapy. Normally, patient receives microcurrent therapy for muscle and fracture healing in rehabilitation clinic within 1 h every day because it increases concentrations of TGF-β1 and insulin-like growth factor II, which are associated with bone cell proliferation (Fitzsimmons et al., 1992; Zhuang et al., 1997). Therefore, four experimental groups were designed that are applying electric field for 30 min (30), applying electric field for 60 min (60), applying electric field for 30 min and resting for 10 min for 3 cycles (30 × 3), and applying electric field for 60 min and resting for 24 h for 3 cycles (60 × 3). The electric field was set to be 0.57 V/cm and 70 kHz. Result is shown in Fig. 3(c). Significant promotion of cell proliferation was shown among four groups. It was observed that longer stimulation duration, i.e., 60 min, could have higher efficacy than shorter duration, i.e., 30 min. Longer resting time, i.e., 24 h, could promote the proliferation of tenocytes than shorter resting time, i.e., 10 min.
Proliferation of human tenocytes in response to the stimulation of electric field. Control was the culture without the stimulation of electric field. (a) Investigation of different frequencies of 30, 60, 70, and 100 kHz. Electric field was set to 0.7 V/cm and applied for 60 min. The tenocytes were isolated from a female patient of 64 years old. (b) Investigation of different electric fields of 0.37, 0.42, 0.57, and 0.71 V/cm. The applied frequency was 70 kHz and the duration was 60 min. The tenocytes were isolated from a female patient of 73 years old. (c) Investigation of different application durations. Four experimental groups were designed including 30 (applied electric field for 30 min), 60 (applied electric field for 60 min), 30 × 3 (applied electric field for 30 min and rested for 10 min; 3 cycles), and 60 × 3 (applied electric field for 60 min and rested for 24 h; 3 cycles). The electric field was set to be 0.57 V/cm and 70 kHz. The tenocytes were isolated from a female patient of 73 years old)
3.2 Proliferation of human tenocytes in response to the stimulation of PRP
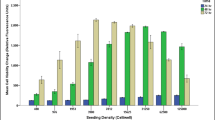
Investigation of the direct application of the PRP in the standard multi-well plates and co-culturing PRP in the current co-culture device was conducted. The multi-well plates of 96-well, 24-well, and 12-well plates were used and 10, 60, and 120 μL PRP was applied to each well respectively. Also, 10 μL PRP was directly applied to the PRP chamber of the co-culture device. And 10 μL PRP was co-cultured with the tenocytes based on the above described protocol. Result of proliferation ratio after the culture course is shown in Fig. 4. It revealed that PRP induced gelling of cells when using small culture wells, i.e., 96-well and 24-well plates. Gelling of cells may lead to cell apoptosis and the result could not reasonably elaborate the efficacy of the platelet-derived growth factors. Same situation was found when directly applying PRP to the cell chamber. Therefore, in order to eliminate the gelling effect, large culture wells, e.g., 12-well and 6-well plates, were normally used in the previous studies (Boswell et al., 2014; Mazzocca et al., 2012a). Herein, significant proliferation of tenocytes was also observed when using 12-well plates, agreeing with the previous studies (Boswell et al., 2014; Mazzocca et al., 2012a). Result revealed that significant proliferation of tenocytes co-culturing with PRP was shown and the co-culture device had the same efficacy compared to that in 12-well plates. However, by using the co-culture device, the amount of tenocytes and PRP could be reduced 6.5-fold and 12-fold, respectively, reducing the need for culture expansion of cells and phenotypic drift.
Investigation of the direct application of PRP in standard multi-well plates and co-culturing PRP in the co-culture device. The multi-well plates of 96-well, 24-well, and 12-well plates were used and 10, 60, and 120 μL PRP was applied to each well respectively. Also, 10 μL PRP was directly applied to the sub-chamber of the co-culture device and co-cultured with the tenocytes. Control was the culture without the stimulation of PRP. The tenocytes were isolated from a female patient of 63 years old. The platelet ratio in PRP was 6.79
Although clinical evaluations showed some evidences of positive promotion of soft tissue and bone healing process by the use of autologous PRP (Everts et al., 2007; Gosens et al., 2011; Wrotniak et al., 2007), the efficacy of PRP application during arthroscopic rotator cuff repair has not been clearly demonstrated (Jo et al., 2011). Here, co-culturing tenocytes and PRP using the co-culture device was performed based on the tenocytes obtained from 12 patients and the PRP collected from individual during surgery or volunteer. The correlation between the proliferation ratio and the platelet ratio in PRP is shown in Fig. 5. Generally, higher platelet ratio in PRP could promote higher proliferation of tenocytes with a linear correlation of R 2 = 0.6279. Result confirmed that higher platelet ratio in PRP can induce higher platelet-derived growth factors for the promotion of cell proliferation.
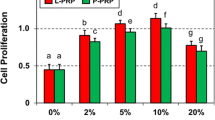
Dexamethasone injection to treat inflammatory conditions has remained one of the most common procedures for practicing orthopaedic surgeons, rheumatologists, and primary care physicians. Early treatment with intra-articular dexamethasone injections may reduce synovitis, thus shortening the natural history of the disease (Hannafin & Chiaia, 2000). However, in vitro studies demonstrated that dexamethasone administration (0.1 nM to 10 μM) in cell cultures from tendons particularly affect tenocyte viability, proliferation, and proteoglycan synthesis (Scutt et al., 2006). There were several publications discussed about the decreased cytotoxic effect of PRP against dexamethasone. Wong et al. described platelet-derived growth factor was a protective agent and compensated the negative effects of dexamethasone (1 nM to 100 μM) on the culture of human tenocytes (Wong et al., 2003). Zargar Baboldashti et al. showed protective effects on tenocytes with the addition of PRP under the 1 μM dexamethasone administration (Zargar Baboldashti et al., 2011). Therefore, combination therapies with PRP and steroids may serve as an interesting research topic. Autologous PRP administered before or immediately after injections of corticosteroids or local anesthetics may play an important role in cell viability. Therefore, short-term symptomatic benefits of these anti-inflammatory and pain-reducing agents can be obtained while improving the maintenance of tenocytes. In this work, co-culturing tenocytes and PRP in the culture medium containing dexamethasone in the concentrations of 2.5 and 5 mg/mL (6.37 and 12.74 μM) was studied. The reason of choosing these concentrations was that 5 mg/mL dexamethasone is a commonly used dose in clincal practice. Therefore, 100% (5 mg/mL) and 50% (2.5 mg/mL) dilution of clincial dose were used in this study. The result is shown in Fig. 6 and revealed that higher concentration of dexamethasone induced higher cell apoptosis. Significant difference between 5 mg/mL dexamethasone and control was observed. However, result confirmed that PRP could significantly compensate the cytotoxic effect of dexamethasone. These in vitro results suggested that a combined treatment with dexamethasone and PRP would have synergistic anti-inflammatory effects while avoiding the deleterious effects of dexamethasone.
Investigation of the co-culture of the tenocytes and the PRP in the culture medium containing dexamethasone in 2.5 and 5 mg/mL. Control was the culture without the stimulation of PRP. (a) Results quantified by WST-1 assay. (b) Microscopic images. The tenocytes were isolated from a male patient of 20 years old. The platelet ratio in PRP was 11.17
3.3 Proliferation of human tenocytes in response to the combined stimulation
The combined stimulation of electric field and PRP was studied using the co-culture device, as shown in Fig. 7. Three experimental groups were designed including simply applying electric field (EF), co-culturing with PRP (co-culture PRP), and combined stimulation (EF + co-culture PRP). The electric field was set to be 0.57 V/cm and 70 kHz. It indicated that the electric field and the PRP could promote the cell proliferation that agrees with the above results. Also, superposition effect was observed when tenocytes were under the combined stimulation. Significant differences were found between the groups of EF and EF + co-culture PRP and the groups of co-culture PRP and EF + co-culture PRP. Moreover, at the end of the culture course, the supernatant was collected and the concentration of TGF-β1 was analyzed by immunoassay. TGF-β1 is a type of cytokine that controls cell proliferation, differentiation, and immunosuppressive functions. The result is shown in Fig. 8. In the group of EF, the TGF-β1 could be significantly induced by simply applying electric field. In the group of co-culture PRP, a large amount of TGF-β1 was released from the PRP and induce cell proliferation (Fig. 7). Moreover, superposition effect was observed when tenocytes were under the combined stimulation.
Investigation of the combined stimulation of electric field and PRP. Control was the culture without any stimulation. Three experimental groups were designed including simply applying electric field (EF), co-culturing with PRP (co-culture PRP), and combined stimulation (EF + co-culture PRP). The tenocytes were isolated from a female patient of 60 years old. The platelet ratio in PRP was 3.2
Investigation of the TGF-β concentration in the culture medium at the end of the culture course (day 7) after tenocytes were treated by the combined stimulation. Control was the culture without any stimulation. Three experimental groups were designed including simply applying electric field (EF), co-culturing with PRP (co-culture PRP), and combined stimulation (EF + co-culture PRP). The TGF-β concentration was analyzed by ELISA and represented by the OD value. The TGF-β concentration ratio was defined as the OD value of the experimental group divided by the OD value of the control group. The tenocytes were isolated from a male patient of 81 years old. The platelet ratio in PRP was 2.8
On tendon lesions, although PRP improved biomechanical, collagen fiber orientation, metabolic activity properties, and extracellular matrix gene expression with a decrease of inflammatory cell number, vascularity, insulin-like growth factor 1 (IGF-1), and TGF-β1 (Salamanna et al., 2015), there are still concerns regarding the optimized preparation protocol of PRP for each patient as he or she may have different stages of tendon injuries. For example, the best activation methods of PRP preparation should be determined before injection. Moreover, there are controversies about the best activation methods, like bovine thrombin (Foster et al., 2009), calcium chloride (Foster et al., 2009), type I collagen (Fufa et al., 2008), chitosan (Salamanna et al., 2015), and electric pulse stimulation (Torres et al., 2014). For example, Torres et al. used nanosecond pulsed electric fields as an alternative non-biochemical platelet activation method to activate platelet, thereby avoiding exposure to xenogeneic thrombin and associated risks (Torres et al., 2014). In the present study, electric field was applied as an activation method of PRP and combined stimulation (EF + co-culture PRP) shown in Fig. 7. Significant differences were found between the groups of EF and EF + co-culture PRP and the groups of co-culture PRP and EF + co-culture PRP, which implied combined stimulation of electric field and PRP may have synergistic effect in stimulation of tenocytes proliferation.
The clinical application of this co-culture and electric field device is to provide an in vitro platform to screen the optimized stimulation combination regarding each patient’s tenocytes. Although the in vitro screening may not completely confirm the success rate of tissue healing, this platform may provide useful information once combination treatments is warranted. For example, a special suture anchor with the ability to provide local electric field induced by embedded electric stimulator may be developed to increase tenocytes proliferation during surgery. On the other hand, the optimized co-culture condition of electric field and PRP may be determined by this platform and which information may provide guidance of these combined therapy in clinical scenario.
4 Conclusions
In the current work, a novel co-culture device was developed for the study of tenocytes proliferation under the stimulations of electric field and PRP. The device could reduce the usage of primary cultures and eliminate the PRP gelling effect by partitioning tenocytes and PRP in different sub-chambers and culturing under the interlinked medium. Results showed that the tenocytes under the stimulation of AC electric field of 0.57 V/cm and 70 kHz could proliferate significantly compared to the control group. For PRP stimulation, increasing platelet ratio in PRP showed positive proportion to cell proliferation. Also, PRP could compensate the cytotoxic effect of drug. Moreover, superposition effect was found when cells were cultured under the combined stimulation. In clinical scenario, situation of each patient may not be identical such as patient age, platelet ratio in autologous PRP, and stages of tendon injuries. A precise in vitro screening procedure for determining the optimized conditions of PRP and electric field provides useful reference for PRP administration during surgery and subsequent rehabilitation therapy.
References
A.J. Almarza, S.M. Augustine, S.L. Woo, Changes in gene expression of matrix constituents with respect to passage of ligament and tendon fibroblasts. Ann. Biomed. Eng. 36, 1927–1933 (2008)
H. Bai, J.V. Forrester, M. Zhao, DC electric stimulation upregulates angiogenic factors in endothelial cells through activation of VEGF receptors. Cytokine 55, 110–115 (2011)
S.G. Boswell, L.V. Schnabel, H.O. Mohammed, E.A. Sundman, T. Minas, L.A. Fortier, Increasing platelet concentrations in leukocyte-reduced platelet-rich plasma decrease collagen gene synthesis in tendons. Am. J. Sports Med. 42, 42–49 (2014)
J. Brook, D.M. Dauphinee, J. Korpinen, I.M. Rawe, Pulsed radiofrequency electromagnetic field therapy: A potential novel treatment of plantar fasciitis. J. Foot Ankle Surg. 51, 312–316 (2012)
D.R. Carter, P.R. Blenman, G.S. Beaupré, Correlations between mechanical stress history and tissue differentiation in initial fracture healing. J. Orthop. Res. 6, 736–748 (1988)
B.J. Cole, N.S. ElAttrache, A. Anbari, Arthroscopic rotator cuff repairs: an anatomic and biomechanical rationale for different suture-anchor repair configurations. Arthroscopy 23, 662–669 (2007)
J.M. DeLong, R.P. Russell, A.D. Mazzocca, Platelet-rich plasma: the PAW classification system. Arthroscopy 28, 998–1009 (2012)
H. El-Azab, S. Buchmann, K. Beitzel, S. Waldt, A.B. Imhoff, Clinical and structural evaluation of arthroscopic double-row suture-bridge rotator cuff repair: early results of a novel technique. Knee Surg. Sports Traumatol. Arthrosc. 18, 1730–1737 (2010)
E. Elson, in The biomedical engineering handbook, ed. by J. D. Bronzino. Biologic effects of radiofrequency and microwave fields: in vivo and experimental results (CRC Press, Inc, Boca Raton, 1995), pp. 1417–1423
P.A. Everts, E.P. Overdevest, J.J. Jakimowicz, C.J. Oosterbos, J.P. Schönberger, J.T. Knape, et al., The use of autologous platelet-leukocyte gels to enhance the healing process in surgery, a review. Surg. Endosc. 21, 2063–2068 (2007)
M. Fini, G. Giavaresi, A. Carpi, A. Nicolini, S. Setti, R. Giardino, Effects of pulsed electromagneticfields on articular hyaline cartilage: Review of experimental and clinical studies. Biomed Pharmacother 59, 388–394 (2005)
R.J. Fitzsimmons, D.D. Strong, S. Mohan, D.J. Baylink, Low-amplitude, low-frequency electric field-stimulated bone cell proliferation may in part be mediated by increased IGF-II release. J. Cell. Physiol. 150, 84–89 (1992)
T.E. Foster, B.L. Puskas, B.R. Mandelbaum, M.B. Gerhardt, S.A. Rodeo, Platelet-rich plasma: from basic science to clinical applications. Am. J. Sports Med. 37, 2259–2272 (2009)
D. Fufa, B. Shealy, M. Jacobson, S. Kevy, M.M. Murray, Activation of platelet-rich plasma using soluble type I collagen. J. Oral Maxillofac. Surg. 66, 684–690 (2008)
L. de Girolamo, D. Stanco, E. Galliera, M. Vigano, A. Colombini, S. Setti, et al., Low frequency pulsed electromagnetic field affects proliferation, tissue-specific gene expression, and cytokines release of human tendon cell. Cell Biochem. Biophys. 66, 697–708 (2013)
C. Goldstein, S. Sprague, B.A. Petrisor, Electrical stimulation for fracture healing: Current evidence. J. Orthop. Trauma 24, S62–S65 (2010)
T. Gosens, J.C. Peerbooms, W. van Laar, B.L. den Oudsten, Ongoing positive effect of platelet-rich plasma versus corticosteroid injection in lateral epicondylitis: A double-blind randomized controlled trial with 2-year follow-up. Am. J. Sports Med. 39, 1200–1208 (2011)
J.A. Hannafin, T.A. Chiaia, Adhesive capsulitis. A treatment approach. Clin. Orthop. Relat. Res 372, 95–109 (2000)
S. Hoppe, M. Alini, L.M. Benneker, S. Milz, P. Boileau, M.A. Zumstein, Tenocytes of chronic rotator cuff tendon tears can be stimulated by platelet-released growth factors. J. Shoulder Elb. Surg. 22, 340–349 (2013)
C.H. Jo, J.E. Kim, K.S. Yoon, J.H. Lee, S.B. Kang, J.H. Lee, et al., Does platelet-rich plasma accelerate recovery after rotator cuff repair? Am. J. Sports Med. 39, 2082–2090 (2011)
C.H. Jo, J.E. Kim, K.S. Yoon, S. Shin, Platelet-rich plasma stimulates cell proliferation and enhances matrix gene expression and synthesis in tenocytes from human rotator cuff tendons with degenerative tears. Am. J. Sports Med. 40, 1035–1045 (2012)
T.E. Jorgensen, Electrical stimulation of human fracture healing by means of a slow pulsating, asymmetrical direct current. Clin. Orthop. Relat. Res. 124, 124–127 (1977)
T. Kanno, T. Takahashi, W. Ariyoshi, T. Tsujisawa, M. Haga, T. Nishihara, Tensile mechanical strain up-regulates Runx2 and osteogenic factor expression in human periosteal cells: Implications for distraction osteogenesis. J. Oral Maxillofac. Surg. 63, 499–504 (2005)
A.D. Mazzocca, M.B.R. McCarthy, D.M. Chowaniec, E.M. Dugdale, D. Hansen, M.P. Cote, et al., The positive effects of different platelet-rich plasma methods on human muscle, bone, and tendon cells. Am. J. Sports Med. 40, 1742–1749 (2012a)
A.D. Mazzocca, D. Chowaniec, M.B. McCarthy, K. Beitzel, M.P. Cote, W. McKinnon, et al., In vitro changes in human tenocytes cultures obtained from proximal biceps tendon: multiple passages result in changes in routine cell markers. Knee Surg. Sports Traumatol. Arthrosc. 20, 1666–1672 (2012b)
S.J. Nho, M.K. Shindle, S.L. Sherman, K.B. Freedman, S. Lyman, J.D. MacGillivray, Systematic review of arthroscopic rotator cuff repair and mini-open rotator cuff repair. J. Bone Joint Surg. Am. 89(Suppl 3), 127–136 (2007)
M. O'Brien, Structure and metabolism of tendons. Scand. J. Med. Sci. Sports 7, 55–61 (1997)
S. Pauly, K. Stahnke, F. Klatte-Schulz, B. Wildemann, M. Scheibel, S. Greiner, Do patient age and sex influence tendon cell biology and clinical/radiographic outcomes after rotaor cuff repair? Am. J. Sports Med. 43, 549–556 (2015)
C. Polk, in The biomedical engineering handbook, ed. by J. D. Bronzino. Therapeutic applications of low-frequency sinusoidal and pulsed electric and magnetic fields (CRC Press, Inc, Boca Raton, 1995), pp. 1404–1416
F. Salamanna, F. Veronesi, M. Maglio, E. Della Bella, M. Sartori, M. Fini, New and emerging strategies in platelet-rich plasma application in musculoskeletal regenerative procedures: general overview on still open questions and outlook. Biomed. Res. Int. 2015, 846045 (2015)
M. Sanchez, E. Anitua, J. Azofra, I. Andia, S. Padilla, I. Mujika, Comparison of surgically repaired Achilles tendon tears using platelet-rich fibrin matrices. Am. J. Sports Med. 35, 245–251 (2007)
N. Scutt, C.G. Rolf, A. Scutt, Glucocorticoids inhibit tenocyte proliferation and Tendon progenitor cell recruitment. J. Orthop. Res. 24, 173–182 (2006)
P. Sharma, N. Maffulli, Tendon injury and tendinopathy: Healing and repair. J. Bone Joint Surg. Am. 87, 187–202 (2005)
L. Tang, Z. Lin, Y.M. Li, Effects of different magnitudes of mechanical strain on osteoblasts in vitro. Biochem. Biophys. Res. Commun. 344, 122–128 (2006)
R.Z. Tashjian, Epidemiology, natural history, and indications for treatment of rotator cuff tears. Clin. Sports Med. 31, 589–604 (2012)
R.Z. Tashjian, A.M. Hollins, H.M. Kim, S.A. Teefey, W.D. Middleton, K. Steger-May, et al., Factors affecting healing rates after arthroscopic double-row rotator cuff repair. Am. J. Sports Med. 38, 2435–2442 (2010)
A.S. Torres, A. Caiafa, A.L. Garner, S. Klopman, N. LaPlante, C. Morton, et al., Platelet activation using electric pulse stimulation: growth factor profile and clinical implications. J. Trauma Acute Care Surg. 77, S94–S100 (2014)
B. Toussaint, E. Schnaser, J. Bosley, Y. Lefebvre, R. Gobezie, Early structural and functional outcomes for arthroscopic double-row transosseous-equivalent rotator cuff repair. Am. J. Sports Med. 39, 1217–1225 (2011)
P. Vavken, F. Arrich, O. Schuhfried, R. Dorotka, Effectiveness of pulsed electromagnetic field therapy in the management of osteoarthritis of the knee: A meta-analysis of randomized controlled trials. J. Rehabil. Med. 41, 406–411 (2009)
X. Wang, Y. Qiu, J. Triffitt, A. Carr, Z. Xia, A. Sabokbar, Proliferation and differentiation of human tenocytes in response to platelet rich plasma: an in vitro and in vivo study. J. Orthop. Res. 30, 982–990 (2012)
M.W. Wong, Y.Y. Tang, S.K. Lee, B.S. Fu, B.P. Chan, C.K. Chan, Effect of dexamethasone on cultured human tenocytes and its reversibility by platelet-derived growth factor. J. Bone Joint Surg. Am. 85-A, 1914–1920 (2003)
R. de Wreede, J.R. Ralphs, Deposition of collagenous matrices by tendon fibroblasts in vitro: a comparison of fibroblast behavior in pellet cultures and a novel three-dimensional long-term scaffoldless culture system. Tissue Eng. A 15, 2707–2715 (2009)
M. Wrotniak, T. Bielecki, T.S. Gazdzik, Current opinion about using the platelet-rich gel in orthopaedics and trauma surgery. Ortop. Traumatol Rehabil. 9, 227–238 (2007)
T. Yamaji, K. Ando, S. Wolf, P. Augat, L. Claes, The effect of micromovement on callus formation. J. Orthop. Sci. 6, 571–575 (2001)
L. Yao, C.S. Bestwick, L.A. Bestwick, N. Maffulli, R.M. Aspden, Phenotypic drift in human tenocyte culture. Tissue Eng. 12, 1843–1849 (2006)
N. Zargar Baboldashti, R.C. Poulsen, S.L. Franklin, M.S. Thompson, P.A. Hulley, Platelet-rich plasma protects tenocytes from adverse side effects of dexamethasone and ciprofloxacin. Am. J. Sports Med. 39, 1929–1935 (2011)
W. Zhai, N. Wang, Z. Qi, Q. Gao, L. Yi, Platelet-rich plasma reverses the inhibition of tenocytes and osteoblasts in tendon-bone healing. Orthopedics 35, e520–e525 (2012)
M. Zhao, H. Bai, E. Wang, J.V. Forrester, C.D. McCaig, Electrical stimulation directly induces pre-angiogenic responses in vascular endothelial cells by signaling through VEGF receptors. J. Cell Sci. 117, 397–405 (2004)
H. Zhuang, W. Wang, R.M. Seldes, A.D. Tahernia, H. Fan, C.T. Brighton, Electrical stimulation induces the level of TGF-beta1 mRNA in osteoblastic cells by a mechanism involving calcium/calmodulin pathway. Biochem. Biophys. Res. Commun. 237, 225–229 (1997)
Acknowledgements
The authors would like to thank for Mr. Yuan-Sheng Chen for his technical support. This study was supported by Linkou Chang Gung Memorial Hospital, Taiwan (Project no. CMRPG5F0031 and BMRPC05).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Chiu, CH., Lei, K.F. & Yeh, WL. Development of a co-culture device for the study of human tenocytes in response to the combined stimulation of electric field and platelet rich plasma (PRP). Biomed Microdevices 19, 69 (2017). https://doi.org/10.1007/s10544-017-0214-z
Published:
DOI: https://doi.org/10.1007/s10544-017-0214-z