Abstract
For decades, low-level laser therapy (LLLT) has widespread applications in tendon-related injuries. Although the therapeutic effect of LLLT could be explained by photostimulation of target tissue and cells, how tenocytes sense photonic energy and convert them into cascades of cellular and molecular events is still not well understood. This study was designed to elucidate the effects of LLLT on cell proliferation and collagen synthesis by examining the associated second messengers including ATP, Ca2+, and nitric oxide using rat Achilles tenocytes. Moreover, proliferating cell nuclear antigen (PCNA) and transforming growth factor-β1 (TGF-β1) related to cell proliferation and matrix metabolism were also studied. The results showed that 904 nm GaAs laser of 1 J/cm2 could significantly increase the MTT activity and collagen synthesis of tenocytes. Second messengers including ATP and intracellular Ca2+ were increased after laser treatment. Quantitative PCR analysis of tenocytes treated with laser revealed up-regulated expression of PCNA, type I collagen, and TGF-β1. Besides, laser-induced TGF-β1 expression was significantly inhibited by extracellular signal-regulated kinase (ERK) specific inhibitor (PD98059). The findings suggested that LLLT stimulated ATP production and increased intracellular calcium concentration. Directly or indirectly via production of TGF-β1, these second messengers mediated the proliferation of tenocytes and synthesis of collagen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Laser technologies are rapidly advancing, and their usage in medicine is constantly growing. They are available in high or low-level (power). Low-level (HeNe and GaAs) lasers have power less than 60 mW and cause non-thermal effects. The clinical therapeutic effects attributed to low-level laser therapy (LLLT) include accelerated tissue healing and reduction of inflammation or pain [1]. For decades, LLLT has widespread applications in tendon-related injuries. Some controlled clinical studies were performed using different laser types and dosages to delineate the role of laser phototherapy in the management of tendon injuries, such as lateral elbow and Achilles tendinopathy [2]. Several studies using animal models showed that LLLT could improve healing of tendons by improving organization or synthesis of collagen, increasing matrix metalloproteinase (MMP) activity, and preventing oxidative stress [3–6]. Although those studies have demonstrated the potential of LLLT in the facilitation of the tendon healing process, the understandings of the underlying molecular and biochemical mechanisms remain to be limited. Many theories exist as to the mechanism of action for LLLT but, simply put, photonic energy is absorbed by photon acceptor sites on the cell membrane and triggers secondary messengers to initiate a cascade of intracellular signals [7]. Therefore, in order to understand the cellular and molecular reactions of tenocytes in response to low-level laser, it is important to determine firstly, if there is an effect on the second messengers including intracellular molecules (ATP/cyclic AMP, nitric oxide) or ions (calcium ions). Secondly, it is necessary to investigate if that effect can be linked to the change of cell viability or proliferation.
During tendon healing, the regenerative phase begins a few days after injury. In the regenerative phase, tenocyte migrate into the injury site and proliferate actively. Proliferating cell nuclear antigen (PCNA), as an auxiliary protein of DNA polymerase, is essential for the coordinated synthesis of DNA strands during cell proliferation. Therefore, in order to evaluate the effect of low-level laser on tendon repair, PCNA can be used as a potential indicator to assess the proliferation of tenocytes.
A number of different growth factors have been shown to promote biological processes involved in tendon healing. Transforming growth factor-β1 (TGF-β1) is the one often associated with the stimulation of collagen production during tendon healing. Several studies have showed that TGF-β1 can increase the secretion of collagen and stimulate the expression of extracellular matrix proteins [8]. Our previous study also revealed that the proliferation of tenocytes is mediated by up-regulation of TGF-β1 gene expression [9]. Therefore, it is imperative to investigate the effects of low-level laser on the expression of TGF-β1 and the association between TGF-β1 and second messengers. Besides, it is well described that mitogen-activated protein kinases (MAPKs) constitute key steps in many intracellular signaling pathways. They usually function as final effectors of signal transduction pathways directly to activate transcription factors in the cytoplasm and nucleus. Subsets of MAPKs such as extracellular signal-regulated kinase (ERK), MAPK-p38, and stress-activated protein kinase/c-jun NH2-terminal kinase (SAPK/JNK) can be activated by mechanical stress and biochemical signals and involved in the regulation of proliferation and intracellular metabolism of several cell types [10, 11]. One of our previous studies also showed that the crosstalk between TGF-β1 and MAPKs signaling pathways plays a role in tenocyte proliferation and regulates their matrix metabolism [12]. In this study, we aimed to elucidate the effects of LLLT on cultured tenocytes, and to further investigate the biochemical mechanisms of LLLT promoting tenocyte proliferation and regulating collagen synthesis. Therefore, we first investigated the effects of LLLT on cell proliferation and collagen synthesis using cultured tenocytes harvested from rat Achilles tendon. Second messengers such as ATP, intracellular Ca2+, and NO by which low-level laser may modulate tenocyte proliferation and regulate collagen synthesis were then examined. Next, PCNA mRNA expression, as a marker of cell proliferation, was also studied. Finally, the MAPKs pathways and their association with TGF-β1, type I, and type III collagen expression in tenocytes irradiated with low-level laser were evaluated using quantitative PCR and specific ERK, p38, and JNK inhibitors.
Materials and methods
Primary culture of rat Achilles tendon cells
The Achilles tendons from Sprague–Dawley rats (6 weeks old, weighing 200–250 g, from the animal laboratory of National Yang-Ming University) were excised and washed twice in phosphate-buffered saline (PBS). Each tendon was then cut into small pieces and these pieces were individually placed in six-well culture plates; 1 ml of Dulbecco’s Modified Eagle Medium (DMEM), with 10 % fetal bovine serum (FBS), 100 U/mL penicillin, and 100 μg/mL streptomycin were added to each well. The culture was incubated at 37 °C in a humidified atmosphere of 5 % CO2. Tenocytes under passages 3–5 with normal fibroblast shape were used in the following experiments. All study procedures received approval of the Animal Care and Use Committee of National Yang-Ming University.
Low-level laser treatment
Tenocytes derived from rat Achilles tendon were subjected to laser treatment as described above. An infrared GaAs diode laser (Roland Series IR 27; Electronica Pagani, Milano, Italy) with a wavelength of 904 nm, frequency range of 5000–7000 Hz, pulse duration of 200 nsec, maximum power output of 27 W, average power of 2.4 mW, and spot size of 0.07 cm2 was used for our study. The experiment was done using four different energy levels (0.5, 1, 2, 4 J/cm2). Tenocytes in the 96-well plate without treatment of low-level laser were used as the control group.
Colorimetric MTT (tetrazolium) assay for tenocyte viability
The mitochondria activity of the tenocytes after low-level laser irradiation treatment was determined by colorimetric assay, which detects the conversion of 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT, Sigma catalog no. M2128, Sigma Co., St. Louis, MO, USA) to formazan. Tenocytes were cultured in a 96-well plate with 104 cells/well and 200 μL culture medium; 24 and 48 h after low-level laser treatment, MTT assay was performed. Culture medium of each well was discarded at the end of culture, and culture wells were washed twice with PBS; 25 μl MTT solution was added to each well, and the plate was incubated at 37 °C for four additional hours. MTT solution was then discarded, and dark blue crystals of formazan were dissolved by adding 100 μL 0.04 N hydrochloride (HCl) to each well. As the regent turned purple, the plate was read by a Spectra MAX340 ELISA reader (Molecular Device, Sunnyvale, CA, USA) at 570 nm for optical density.
Sircol collagen assay for total collagen in culture medium
The Sircol Collagen Assay (Biocolor, Newtownabbey, Northern Ireland) is a quantitative dye-binding method designed for the analysis of collagens released into culture medium in vitro. To evaluate the amount of total collagen in medium, fetal bovine serum (FBS) was changed to 2 % to minimize the interference with the assays. Tenocytes were seeded in 96-well culture plates at a density of 2.5 × 104 cells/well with 200 μL culture medium in each well and incubated for 4 days. At the end of incubation, 200 μL of sample medium in each group was collected into a 1.5-mL centrifuge tube; 1.0 ml Sircol dye reagent was added into each tube. The mixed contents were kept at room temperature for 30 min. The tubes were then centrifuged at 10,000 rpm for 10 min. The supernatants were discarded. The remaining pellets were mixed with 1.0 mL alkali reagent; 200 μL aliquots of the alkali dye solutions were transferred to a 96-well plate, and the plate was read with a spectrophotometer at 540 nm. For collagen quantification, 0.78, 1.56, and 3.12 μL collagen standards were used to create a standard curve.
Enzyme-linked immunosorbent assay for TGF-β1
Tenocytes treated with low-level laser irradiation were seeded in a 96-well culture plate at a density of 2.5 × 104 cells/well with 200 μL culture medium in each well and incubated for 12, 24, 48, and 72 h. TGF-β1 in the medium was determined by ELISA assays (Quantikine, R&D Systems, Minneapolis, MN); 50 μL of acid-activated culture supernatant and 50 μL assay diluent were added to each 96-well polystyrene microplate coated with a monoclonal antibody specific for TGF-β1. The plate was incubated for 2 h at room temperature. After four washes with wash buffer, 100 μL of TGF-β1 conjugate was added to each well and further incubated for 2 h at room temperature. After another four washes with PBS, 100 μL of substrate solution was added to each well and incubated for 30 min at room temperature; 100 μL of stop solution was added to each well. The plate was then read with a spectrophotometer at 450 nm. Recombinant TGF-β1 was serially diluted ranging from 0 to 2000 pg/ml, and the readings were used to create a standard curve.
ATP colorimetric assay
ATP Colorimetric Assay (BioVision, USA) utilizes the phosphorylation of glycerol to generate a product that can be quantified by colorimetric methods. After low-level laser irradiation, tenocytes were cultured in a 96-well plate with 2.5 × 104 cells and 200 μL culture medium in each well. Cells were cultured for 15, 30 min and 1, 2, 4, and 8 h. Culture medium of each well was then discarded at the end of cultivation, and the plate was washed twice with PBS. Cells were lysed with 300 μL of ATP assay buffer, and then centrifuged at 15,000×g for 2 min to pellet insoluble materials; 50 μL of supernatant was collected and added to a 96-well plate; 50 μL of the reaction mix was added to the well containing the ATP standard or test samples. After incubation at room temperature for 30 min with protection from light, the plate was read for optical density with a microplate reader at 570 nm.
Greiss reaction for nitric oxide in medium
After low-level laser irradiation, tenocytes were cultured for 15, 30 min, 1, 2, 4, 8, 12, and 24 h. Culture medium was collected and evaluated for the synthesis of nitric oxide. Nitrite (NO2 −), the stable end production of nitric oxide, was measured in the culture media utilizing the spectrophotometric method based on the Greiss reaction. The Greiss reagent consisted of a 1:1 solution of 0.1 % (w/v) N-(1-Naphthyl)-ethylenediamine (Sigma Co., St. Louis, MO, USA) in water and 0.1 % sulphanilamide (Sigma Co., St. Louis, MO, USA) in 5 % phosphoric acid; 100 μL of the Greiss reagent was added to 100 μL of sample media in a 96-well plate and the plate was then read spectrophotometrically at 550 nm. For nitrite quantification, serial dilutions of NaNO2 dissolved in DMEM were used to create a standard curve.
Fluo-3AM fluorescent intensity for intracellular calcium concentration
Changes in cytosolic Ca2+ concentrations were monitored with the green fluorescent probe, Fluo-3AM (BioVision, USA), by measuring the optical absorption at 575 nm. Tenocytes were seeded in 96-well culture plates at a density of 2.5 × 104 cells/well with 200 μL culture medium in each well and incubated for 15 and 30 min. Culture medium of each well was discarded at the end of cultivation, and the wells were washed twice with (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) (HEPES). Tenocytes were loaded with 5 μL Fluo-3AM containing 1 μM pluronic acid F-127 in a humidified CO2 incubator (5 % CO2) at 37 ° C for 40 min. Nonhydrolyzed Fluo-3AM was removed by washing the cells with HEPES just before fluorescence acquisition.
Ribonucleic acid (RNA) isolation and real-time polymerase chain reaction (PCR) of proliferating cell nuclear antigen (PCNA), type I collagen, type III collagen, and TGF-β1
Tenocytes after low-level laser treatment were seeded in 96-well culture plate at 2.5 × 104 cells/well cell density with 200 μL culture medium in each well. After a predetermined time, the RNA was isolated from tenocytes by an acid guanidine method using TRIzol Reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) and reversely transcribed to cDNA. Real-time PCR reactions were carried out using the Roche Light Cycler, utilizing SYBR Green reagents (Light Cycler-DNA Master SYBR Green I kit; Roche, Basel, Switzerland), according to the manufacturer’s instructions. The reactions were carried out in 20-μL capillaries containing 2.5 mM MgCl2, 0.2 μM concentrations of each forward and backward primer, 1 μL DNA Master SYBR Green and 1 μL of cDNA. The amplification program consisted of one cycle at 95 ° C for 10 min, followed by 40 cycles with a denaturing phase at 95 ° C for 15 s, an annealing phase of 5 s at 60 ° C, and an elongation phase at 72 ° C for 15 s. A melting curve analysis was done after amplification to verify the accuracy of the amplicon. Amplification of PCR products was quantified during PCR by measurement of fluorescence associated with binding of double-stranded DNA to the SYBR Green dye incorporated in the reaction mix. Oligonucleotide sequences for the specific primers used in this study are summarized in Table 1.
Inhibitors of MAPK pathway
The inhibitory effects of the three MAPK pathways-specific inhibitors on TGF-β1expression induced by LLLT were evaluated. Tenocytes were pretreated with ERK inhibitor (PD98059, 30 μM), p38 inhibitor (SB203580, 10 μM), or JNK inhibitor (SP600125, 30 μM) (Sigma Co., St. Louis, MO, USA) for 1 h, respectively. Then, the cells were treated with low-level laser irradiation. The expressions of TGF-β1 were determined at 24 h after treatment.
Statistical analysis
All data were expressed as mean ± standard error of the mean (SEM). Comparisons between the LLLT groups and the control group were performed using Student’s t test, and the level of statistical significance was defined as p < 0.05.
Results
Low-level laser promoted tenocyte viability
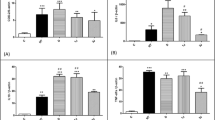
Tenocytes were exposed to GaAs diode laser of four different energy levels (0.5, 1, 2, 4 J/cm2). The control group was treated under the same preparation without stimulation of low-level laser. The OD value for control group and tenocytes treated with 0.5, 1, 2, and 4 J/cm2 laser was 0.06 ± 0.003, 0.068 ± 0.007, 0.073 ± 0.011, 0.065 ± 0.008, and 0.064 ± 0.004 at 24 h and was 0.093 ± 0.011, 0.103 ± 0.006, 0.106 ± 0.012, 0.104 ± 0.012, and 0.100 ± 0.011 at 48 h. The results showed laser of 0.5 and 1 J/cm2 significantly increased the cell viability at 24 and 48 h (Fig. 1a). On the other hand, low-level laser with energy of 2 and 4 J/cm2 showed higher average viability. However, the difference was not statistically significant in comparison with the control group.
a Using MTT assay, tenocytes treated with low-level laser of 0.5 and 1 J/cm2 showed significant increase in cell viability in comparison with the control group after 24 and 48 h. b Tenocytes treated with low-level laser treatment of 1 J/cm2 showed significant increase in the collagen synthesis than the control group. c TGF-β1 concentration of conditioned medium of tenocytes treated with laser (1 J/cm2) increased significantly at 12, 48, and 72 h (n = 8; *P < 0.05, **P < 0.01)
Low-level laser stimulated collagen synthesis by tenocytes
According to the MTT assay, low-level laser with 1 J/cm2 energy showed significant stimulatory effects on cell viability. Therefore, the energy level of 1 J/cm2 was further employed for subsequent experiments. The level of total collagen in medium for laser irradiation on the fourth day was measured by Sircol collagen assay kit. The results showed that low-level laser with 1 J/cm2 energy significantly increased collagen synthesis of tenocytes (20.09 ± 1.97 μg/mL vs. control [14.44 ± 0.35 μg/mL]) (Fig. 1b).
Low-level laser-stimulated TGF-β1 production
The results of TGF-β1 production showed that low-level laser significantly stimulated the release of TGF-β1 into culture medium at 12 h (7.84 ± 4.48 pg/mL for laser treatment group and 3.07 ± 2.71 pg/mL for control group, p < 0.05), 48 h (8.72 ± 3.14 pg/mL for laser treatment group and 5.22 ± 2.44 pg/mL for control group, p < 0.05), and 72 h (10.77 ± 5.82 pg/mL for laser treatment group and 4.51 ± 3.80 pg/mL for control group, p < 0.05) (Fig. 1c). The concentrations of TGF-β1 in culture medium 24 h after laser stimulation were 5.70 ± 3.23 and 7.89 ± 4.38 pg/mL for control and experimental groups, respectively.
Low-level laser increased ATP level
After 1 J/cm2 low-level laser treatment, the viable cells were further cultured for 15, 30 min, 1, 2, 4, and 8 h for ATP level. Irradiated tenocytes showed a significant increase in ATP level when compared with the control group (non-irradiated tenocytes) at 15, 30 m, and 4 h after laser irradiation. No further increment of ATP after 4 h was noted (Fig. 2a).
a Laser-treated tenocytes showed a significant increase in ATP level when compared with the control group at 15, 30 m, and 4 h (n = 5) (*p < 0.05). b The effect of low-level laser irradiation on NO production of tenocytes. Although greater NO production was shown in laser-treated group at 12 and 24 h, the difference was not statistically significant (n = 10)
Effects of low-level laser on NO release
NO released into cell culture medium in each group was measured by Greiss reaction assay. The concentrations of NO were 2.58 ± 0.36, 2.56 ± 0.26, 2.68 ± 0.34, 2.46 ± 0.13, 3.29 ± 0.14, 3.45 ± 0.08, and 3.69 ± 0.17 μM at 15, 30 min, 1, 2, 4, 8, 12, and 24 h in control groups. The concentrations of NO were 2.56 ± 0.28, 2.61 ± 0.33, 2.66 ± 0.25, 2.48 ± 0.25, 3.23 ± 0.12, 3.54 ± 0.14, and 3.82 ± 0.23 μM at 15, 30 min, 1, 2, 4, 8, 12, and 24 h in laser treatment groups. Concentrations of NO measured in the culture medium from control and laser groups were similar at 15, 30 min, 1, 2, 4, and 8 h. Although greater NO production was shown in laser-treated group at 12 and 24 h, the difference was not statistically significant (Fig. 2b).
Low-level laser increased intracellular calcium release
The effect of 904 nm GaAs laser irradiation on tenocytes calcium was investigated through fluorescent calcium indicator, Fluo-3AM. Fluo-3AM is a sensitive fast-responding Ca2 probe, which is not fluorescent except in its calcium chelates. Figure 3A1 and B1 depicts the Fluo-3AM fluorescence in the control state. Figure 3(A2 and B2) show the increase in the Fluo-3AM fluorescence induced by 1 J/cm2 low-level laser. An immediate increase in intracellular calcium levels was observed after laser stimulation for 15 (Fig. 3(A2)) and 30 min (Fig. 3(B2)).
Low-level laser stimulated the expression of PCNA, type I collagen, and TGF-β1 at transcriptional level
PCNA is essential for DNA replication and repair [13]. PCNA was evaluated to reflect tenocyte proliferation in our study. After laser treatment, up-regulation of PCNA expression was noted. At 24 h after laser stimulation, the expression of PCNA mRNA was significantly increased (137.5 % ± 30.4 % of control group, p = 0.029) (Fig. 4a). However, after 48 h, there was no significant stimulatory effect observed in the laser-treated group. Apart from PCNA, the results also showed that mRNA expressions of TGF-β1 were enhanced at 72 h (149.8 % ± 27.2 % of control group, p = 0.006) (Fig. 4b). Although the trend of increased expression of type III collagen (116 % at 24 h, 145 % at 48 h) did not reach statistical significance during the first 48 h after laser treatment, our results showed that low-level laser significantly up-regulated mRNA expression of type I collagen at 24 h (137.5 % ± 30.4 % of control group, p = 0.029). However, the expression of type I collagen stimulated by laser decreased after 48 h (Fig. 4c, d).
Effects of low-level laser irradiation on gene expression of PCNA, TGF-β1, type I collagen, and type III collagen. a When stimulated by low-level laser, PCNA gene expression was significantly stronger than the control group at 24 h (p < 0.05). b TGF-β1 mRNA expression after laser treatment showed gradual increase with time and reached a significant difference at 72 h (p < 0.01). c After laser treatment, type I collagen mRNA expression significantly increased at 24 h. d The expression of type III collagen did not significantly increase with low-level laser stimulation (n = 6; *p < 0.05, **p < 0.01)
Laser-induced TGF-β1 production through ERK pathway
Laser exposure in tenocytes increased expression of TGF-β1 in both protein and mRNA levels (Fig. 1c and 4b). In order to understand the underlying mechanisms, tenocytes were pretreated with PD98059 (an ERK inhibitor), SP600125 (a JNK inhibitor), or SB203580 (a p38 inhibitor). The results showed laser-induced TGF-β1 expression (6.69 ± 2.28 pg/mL) was significantly inhibited in tenocytes pretreated with PD98059 (3.59 ± 1.42 pg/mL), whereas TGF-β1 expression was not suppressed with SP600125 (8.20 ± 4.29 pg/mL) and SB203580 (9.05 ± 2.75 pg/mL) (Fig. 5).
Discussion
The effect of LLLT on cell proliferation has been described in various cell types. For tenocytes, it has been reported that proliferation could be enhanced, in a dose-dependent manner, by 660-nm laser with energy densities of 1.0, 1.5, 2.0, and 2.5 J/cm2 [14]. Interestingly, using GaAs laser with a wavelength of 904 nm, our results showed viability of tenocytes increased significantly at the energy density of 0.5 and 1 J/cm2. However, viability of tenocytes did not improve with higher energy density (2 and 4 J/cm2). As we know, effects of LLLT on proliferation of cell cultures may depend on consumed energy density, total energy, number of irradiated points, and diameter of beam or irradiated area, type of laser, and sometimes on the type of cells as well [15]. Therefore, it is not surprising that a unique dose–frequency regime may exist for tenocytes and the determination of that treatment paradigm is necessary in order to achieve maximal stimulation of cell proliferation. Nevertheless, from the results, we believed that the proliferation rate of tenocytes can be increased with the appropriate use of LLLT, which would be very useful in tendon tissue engineering and regenerative medicine. In addition, mRNA expression of PCNA at 24 h in this study was also consistent with the results of cell proliferation at 24 and 48 h after low-level laser treatment. These findings suggested that the effects of low-level laser on proliferation of tenocytes might be modulated through PCNA-related pathway.
Current research about the mechanisms of LLLT effects suggested the involvement of mitochondria. Mitochondria play an important role in energy generation and metabolism. Alteration in the activity of cytochrome c oxidase in the mitochondria could then result in increased production of ATP. In our study, irradiated tenocytes showed a significant increase in ATP level when compared with the control group (non-irradiated tenocytes) at 15, 30 min, and 4 h after laser irradiation. As a result, ATP can then be turned into cyclic AMP (cAMP) under the presence of adenylyl cyclase and has several metabolic functions as being involved in phosphorylation reactions, allosteric regulation, assembly of several cofactors including RNA, and metabolic control by covalent modification. Furthermore, a hallmark of cAMP is its cell type-specific effects on the mitogen-activated protein (MAP) kinase (also called extracellular signal-regulated kinase, or ERK) cascade, which account for proliferation of certain types of cells [16]. Therefore, our findings might indicate that laser treatment on tenocytes increased ATP production that would trigger a downstream messenger to initiate a cascade of intracellular signals regulating cellular behavior in metabolism and proliferation.
It is known that a number of chemical and physical stimuli mediate their effects via transient increases in the concentration of intracellular free Ca2+. Laser irradiation has been shown to induce changes in intracellular Ca2+ concentrations in macrophages, hepatocytes [17], and osteoblasts [18] in vitro. In this study, we first demonstrated an immediate increase in intracellular calcium levels 15 and 30 min after 1 J/cm2 laser stimulation in tenocytes. As intracellular concentration of calcium increases, calcium can modulate the protein interaction properties of ERKs, which further affect the subcellular localization of the latter and as a consequence also the distribution of their targets. These effects of calcium also play an important role in the regulation of ERK-dependent cellular processes [19]. Several mechanisms can contribute to the increase of intracellular calcium levels. Cohen et al. reported HeNe laser irradiation activates the redox reactions in the respiratory chain, possibly leading to changes in the intracellular calcium concentration [20]. On the other hand, the receptors on the cell surface can also be triggered by photons and cause the influx of calcium ions. Further, it has been shown that nitric oxide (NO) works on its target cells by activating soluble guanylate cyclase (GC) to make cGMP. cGMP can activate ADP ribosyl cyclase, which acts on NAD+ to form cyclic ADP ribose, which then allows calcium to flow into the cytoplasm and raises the intracellular concentration of calcium [7]. However, in spite of increased concentration of calcium in this study, concentrations of NO measured in the culture medium from control and laser groups were similar at 15, 30 min, 1, 2, 4, and 8 h. Although more NO production was noticed in laser-treated group at 12 and 24 h, the difference was not statistically significant. Previously, NO was reported to play a role in several aspects of tendon healing. Murrell et al. reported that inhibition of nitric oxide synthase resulted in a significant reduction in cross-sectional area and failure load of healing Achilles tendon constructs [21]. However, their results showed that the various isoforms of nitric oxidase synthases peak from the fourth day to the 21st day. This might partially explain why NO did not increase significantly 24 h after laser treatment in the present study. In addition, a previous study showed that the level of NO in tenocytes induced by low-level laser increases in a dose-dependent manner [14]. In this study, only low-level laser of 1 J/cm2 used for the stimulation of tenocytes might contribute the limited increase in NO production.
During tendon healing, tenocytes migrate to the lesion site and proliferate [22]. Then, type I and type III collagen synthesis increases [23]. Thus, both tenocyte proliferation and collagen synthesis are important cellular responses in tendon injury and fundamental to the healing process. The results of this study revealed that low-level laser enhanced mRNA expression of type I collagen at 24 h (Fig. 4c) and total collagen synthesis at 4 days (Fig. 1b). Our results agreed with several previous reports, which showed low-level laser might improve tendon healing through increase of collagen synthesis [24]. Nonetheless, the trend of increased expression of type III collagen (116 % at 24 h, 145 % at 48 h) did not reach statistical significance during the first 48 h after laser treatment. These findings were different from some of the previous reports. For instance, Guerra et al. found increased type III collagen production in rat Achilles tendons 8 days after stimulation with pulsed low-level laser [6]. However, on the contrary, Casalechi et al. reported type I collagen predominated over type III collagen in rat Achilles tendon 7 and 14 days after low-intensity laser treatment [3]. Wood et al. also found that ultrasound, LLLT, and the combined use of LLLT and ultrasound resulted in greater synthesis of type I collagen (but not type III collagen) [25]. Moreover, different power settings of low-level laser treatment might also contribute to the significantly differential expressions of type I and type III collagen [26]. Thus, we believed, under certain settings of laser parameters, low-level laser could predominantly affect the synthesis of type I collagen from tenocytes.
TGF-β1 mRNA expression has been found to dramatically increase a short time after tendon injury. Several groups have demonstrated that TGF-β1 can increase the production of collagen and stimulate the expression of extracellular matrix proteins in vitro and in vivo [8, 27]. In the present study, we noticed that TGF-β1 production from tenocytes significantly increase at 12, 48, and 72 h at the presence of 1 J/cm2 low-level laser. However, mRNA expression of TGF-β1 increased significantly only 72 h after laser treatment. Therefore, a possible explanation is that laser treatment induced a biphasic increase of TGF-β1 by stimulating the initial release of intracellular TGF-β1 and the subsequent increase of TGF-β1 production resulting from the latent expression of TGF-β1 gene. Because TGF- β1 is a naturally secreted latent complex, there is also evidence that LLLT can modulate latent TGF-β1 gene expression [28]. As LLLT induced the expression of TGF-β1, the increased TGF-β1 could further modulate the synthesis of collagen and improves the healing of tendon [29].
Previously, LLLT was found to activate cellular responses through the MAPK/ERK signaling pathway in skeletal muscle cells [30] and human dental pulp cells [31]. For tenocytes, we demonstrated in this study that low-level laser also affected the expression of TGF-β1 by specifically activating ERK cascade, but not the JNK or p38 MAPK pathways, in tenocytes (Fig. 5). As mentioned above, ATP/cAMP system has cell type-specific effects on the ERK cascade, which may account for the regulation of TGF-β1 expression and consequently affect proliferation and collagen synthesis of tenocytes. Besides, calcium can also modulate the protein interaction properties of ERKs and play an important role in the regulation of ERK-dependent cellular processes [19]. Although the detailed mechanisms still remain unclear, our results implied that LLLT increased ATP and intracellular Ca2+ concentration that then activated ERK signaling and stimulated TGF-β1 production. By direct activation of ERK cascade or indirect influences through TGF-β1 production, LLLT further enhanced proliferation and collagen synthesis of tenocytes.
Conclusion
This in vitro study demonstrated that low-level laser of 1 J/cm2 can stimulate proliferation and collagen synthesis of tenocytes. The associated tenocyte proliferation is mediated by up-regulation of PCNA. This study suggests that photons of LLLT absorbed by the mitochondria stimulate ATP production and increase concentration of intracellular calcium. Increased ATP and calcium concentration then activated the ERK pathway to directly or indirectly, through TGF-β1 production, enhance proliferation of tenocytes and synthesis of collagen. Although the detailed molecular and cellular pathways still need to be investigated, our findings provide the basis underlying LLLT for treating tendon injury.
References
Hawkins D, Abrahamse H (2006) Effect of multiple exposures of low-level laser therapy on the cellular responses of wounded human skin fibroblasts. Photomed Laser Surg 24:705–714. doi:10.1089/pho.2006.24.705
Bjordal JM, Lopes-Martins RA, Joensen J et al (2008) A systematic review with procedural assessments and meta-analysis of low level laser therapy in lateral elbow tendinopathy (tennis elbow). BMC Musculoskelet Disord 9:75. doi:10.1186/1471-2474-9-75
Casalechi HL, de Farias Marques AC, da Silva EAP et al (2014) Analysis of the effect of phototherapy in model with traumatic Achilles tendon injury in rats. Lasers Med Sci 29:1075–1081. doi:10.1007/s10103-013-1468-1
Oliveira FS, Pinfildi CE, Parizoto NA et al (2009) Effect of low level laser therapy (830 nm) with different therapy regimes on the process of tissue repair in partial lesion calcaneous tendon. Lasers Surg Med 41:271–276. doi:10.1002/lsm.20760
Fillipin LI, Mauriz JL, Vedovelli K et al (2005) Low-level laser therapy (LLLT) prevents oxidative stress and reduces fibrosis in rat traumatized Achilles tendon. Lasers Surg Med 37:293–300. doi:10.1002/lsm.20225
Guerra FDR, Vieira CP, Almeida MS et al (2013) LLLT improves tendon healing through increase of MMP activity and collagen synthesis. Lasers Med Sci 28:1281–1288. doi:10.1007/s10103-012-1236-7
Evans DH, Abrahamse H (2009) A review of laboratory-based methods to investigate second messengers in low-level laser therapy (LLLT). Med Laser Appl 24:201–215. doi:10.1016/j.mla.2009.05.003
Fujita T, Shiba H, Sakata M et al (2002) Effects of transforming growth factor-beta 1 and fibronectin on SPARC expression in cultures of human periodontal ligament cells. Cell Biol Int 26:1065–1072
Chao Y-H, Tsuang Y-H, Sun J-S et al (2008) Effects of shock waves on tenocyte proliferation and extracellular matrix metabolism. Ultrasound Med Biol 34:841–852. doi:10.1016/j.ultrasmedbio.2007.11.002
Kjaer M (2004) Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev 84:649–698. doi:10.1152/physrev.00031.2003
Cowan KJ, Storey KB (2003) Mitogen-activated protein kinases: new signaling pathways functioning in cellular responses to environmental stress. J Exp Biol 206:1107–1115
Chao Y-H, Tsuang Y-H, Sun J-S et al (2011) The cross-talk between transforming growth factor-beta1 and ultrasound stimulation during mechanotransduction of rat tenocytes. Connect Tissue Res 52:313–321. doi:10.3109/03008207.2010.525673
Prelich G, Stillman B (1988) Coordinated leading and lagging strand synthesis during SV40 DNA replication in vitro requires PCNA. Cell 53:117–126
Tsai W-C, Cheng J-W, Chen J-L et al (2014) Low-level laser irradiation stimulates tenocyte proliferation in association with increased NO synthesis and upregulation of PCNA and cyclins. Lasers Med Sci 29:1377–1384. doi:10.1007/s10103-014-1528-1
AlGhamdi KM, Kumar A, Moussa NA (2012) Low-level laser therapy: a useful technique for enhancing the proliferation of various cultured cells. Lasers Med Sci 27:237–249. doi:10.1007/s10103-011-0885-2
Stork PJS, Schmitt JM (2002) Crosstalk between cAMP and MAP kinase signaling in the regulation of cell proliferation. Trends Cell Biol 12:258–266
Vacca RA, Moro L, Petragallo VA et al (1997) The irradiation of hepatocytes with He-Ne laser causes an increase of cytosolic free calcium concentration and an increase of cell membrane potential, correlated with it, both increases taking place in an oscillatory manner. Biochem Mol Biol Int 43:1005–1014
Coombe AR, Ho CT, Darendeliler MA et al (2001) The effects of low level laser irradiation on osteoblastic cells. Clin Orthod Res 4:3–14
Chuderland D, Marmor G, Shainskaya A, Seger R (2008) Calcium-mediated interactions regulate the subcellular localization of extracellular signal-regulated kinases. J Biol Chem 283:11176–11188. doi:10.1074/jbc.M709030200
Cohen N, Lubart R, Rubinstein S, Breitbart H (1998) Light irradiation of mouse spermatozoa: stimulation of in vitro fertilization and calcium signals. Photochem Photobiol 68:407–413
Murrell GA, Szabo C, Hannafin JA et al (1997) Modulation of tendon healing by nitric oxide. Inflamm Res Off J Eur Histamine Res Soc Al 46:19–27
Sharma P, Maffulli N (2005) Tendon injury and tendinopathy: healing and repair. J Bone Joint Surg Am 87:187–202. doi:10.2106/JBJS.D.01850
Abrahamsson SO (1991) Matrix metabolism and healing in the flexor tendon. Experimental studies on rabbit tendon. Scand J Plast Reconstr Surg Hand Surg Suppl 23:1–51
Chen C-H, Tsai J-L, Wang Y-H et al (2009) Low-level laser irradiation promotes cell proliferation and mRNA expression of type I collagen and decorin in porcine Achilles tendon fibroblasts in vitro. J Orthop Res Off Publ Orthop Res Soc 27:646–650. doi:10.1002/jor.20800
Wood VT, Pinfildi CE, Neves MAI et al (2010) Collagen changes and realignment induced by low-level laser therapy and low-intensity ultrasound in the calcaneal tendon. Lasers Surg Med 42:559–565. doi:10.1002/lsm.20932
Neves MAI, Pinfildi CE, Wood VT et al (2011) Different power settings of LLLT on the repair of the calcaneal tendon. Photomed Laser Surg 29:663–668. doi:10.1089/pho.2010.2919
Lijnen PJ, Petrov VV, Fagard RH (2000) Induction of cardiac fibrosis by transforming growth factor-beta(1). Mol Genet Metab 71:418–435. doi:10.1006/mgme.2000.3032
Aliodoust M, Bayat M, Jalili MR et al (2014) Evaluating the effect of low-level laser therapy on healing of tentomized Achilles tendon in streptozotocin-induced diabetic rats by light microscopical and gene expression examinations. Lasers Med Sci 29:1495–1503. doi:10.1007/s10103-014-1561-0
Molloy T, Wang Y, Murrell G (2003) The roles of growth factors in tendon and ligament healing. Sports Med Auckl NZ 33:381–394
Shefer G, Oron U, Irintchev A et al (2001) Skeletal muscle cell activation by low-energy laser irradiation: a role for the MAPK/ERK pathway. J Cell Physiol 187:73–80. doi:10.1002/1097-4652(2001)9999:9999<::AID-JCP1053>3.0.CO;2-9
Miyata H, Genma T, Ohshima M et al (2006) Mitogen-activated protein kinase/extracellular signal-regulated protein kinase activation of cultured human dental pulp cells by low-power gallium-aluminium-arsenic laser irradiation. Int Endod J 39:238–244. doi:10.1111/j.1365-2591.2006.01080.x
Acknowledgments
Support for this research was provided through the National Taiwan University Hospital Hsin-Chu Branch from Grants HCH 102-31.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, MH., Huang, YC., Sun, JS. et al. Second messengers mediating the proliferation and collagen synthesis of tenocytes induced by low-level laser irradiation. Lasers Med Sci 30, 263–272 (2015). https://doi.org/10.1007/s10103-014-1658-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-014-1658-5