Abstract
The treatment of muscle injuries is a common practice at rehabilitation centers. Low-level laser therapy (LLLT) has demonstrated positive effects regarding the modulation of the inflammatory response, the enhancement of the tissue repair process and the prevention of fibrosis. The aim of the present study was to evaluate the effects of LLLT on morphological aspects of muscle tissue, collagen remodeling and activity of matrix metalloproteinase 2 (MMP-2) in rat skeletal muscle following acute injury. Wistar rats were divided into five groups: (1) control group (n = 10), (2) sham group (n = 10), (3) LLLT group (n = 30), (4) non-treated injury group (n = 30) and (5) injury + LLLT group (n = 30). Cryoinjury was performed on the belly of the tibialis anterior (TA) muscle. LLLT was performed daily with an AlGaAs laser (780 nm; beam spot of 0.04 cm2, output power of 40 mW, power density of 1 W/cm2, energy density of 10 J/cm2 and 10-s exposure time). Animals were euthanized at 1, 3 and 7 days. The TA muscles were removed and weighed. Morphological aspects were evaluated using H & E staining. The amount and distribution of collagen fibers were evaluated by picrosirius staining. Characterization and activity of MMP-2 were evaluated by zymography and Western blot techniques, respectively. The results revealed that LLLT induced a reduction in inflammatory infiltrate and myonecrosis after 1 day, an increase in the number of blood vessels after 3 and 7 days as well as an increase in the number of immature muscle fibers and MMP-2 gelatinase activity after 7 days. In conclusion, LLLT has a positive effect on the inflammatory process, MMP2 activity and collagen organization and distribution in the repair process of rat skeletal muscle.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Muscle injuries can occur by either direct causes (contusions and lacerations) or indirect causes (ischemia and neurological dysfunction) [1–3]. The major goals of the rehabilitation process following such injuries are the fast, efficient regeneration of the muscle tissue and the prevention of fibrotic scar tissue [4].
The muscle repair process consists of several interdependent phases: degeneration and inflammation, regeneration, fibrosis/scarring and remodeling [5]. The initial phase of this process is characterized by the migration of inflammatory cells, muscle fiber necrosis (myonecrosis) and the phagocytosis of cell debris, leading to the activation of myogenic precursor (stem) cells known as satellite cells [6, 7]. These cells then proliferate and differentiate into myoblasts, subsequently forming new muscle fibers [2, 3, 6, 8]. Throughout the repair process, different cell types are activated, along with the synthesis and degradation of intracellular proteins and components of the extracellular matrix (ECM) [4, 9–11]. In normal muscle tissue, the ECM is composed primarily of collagen types I, III and IV, laminin, fibronectin, tenascin and proteoglycans, which surround the muscle fibers and play an important role in maintaining contractile function [9, 11, 12].
Scar tissue develops as a result of the excessive deposition of ECM and constitutes a mechanical barrier to cell migration and fusion, limiting infusion at the site of vascular injury and preventing normal regeneration following tissue injury [9, 13, 14]. The remodeling of the ECM is mainly performed by a family of matrix metalloproteinases (MMPs), which are dependent on calcium and zinc and are capable of degrading one or several ECM proteins [13, 15]. MMP-2 plays an important role in the formation of new fibers by promoting the degradation of basement membrane collagen IV and other ECM components [13]. The degradation of basement membrane components following an injury facilitates the migration, proliferation and fusion of myoblasts as well as the formation of new blood vessels (angiogenesis) [10, 13, 16, 17]. Angiogenesis plays an important role in the success of muscle regeneration [18].
Low-level laser therapy (LLLT) has demonstrated positive effects in modulating the inflammatory response, especially in relation to muscle regeneration following an injury [19–21], muscle tissue repair and the prevention of fibrosis [22–26]. However, the mechanisms responsible for these findings are still under investigation.
The aim of the present study was to evaluate the effects of LLLT on the repair of skeletal muscle in rats following acute injury through an analysis of morphological aspects and the quantification of inflammatory infiltrate, blood vessels, new immature muscle cells, collagen fibers and the gelatinolytic activity of MMP-2.
Methods
The experiments were carried out in accordance with the guidelines of the Brazilian National Council for the Control of Animal Experimentation (CONCEA) and of the Animal Research Ethics Committee of the Universidade Nove de Julho (Brazil) under process number AN12/2012.
Male Wistar rats (age, 12 weeks; body mass, 200 ± 11.9 g) were kept in plastic boxes at an appropriate temperature (22 °C) and humidity (40 %), with controlled lighting (12-h light/dark cycle) and food and water ad libitum. The animals were divided into five groups: (1) control group (n = 10); (2) sham group, subjected only to exposure of the TA muscle (n = 10); (3) LLLT group (n = 30); (4) non-treated injury group (n = 30) and (5) injury + LLLT group (n = 30). The control group was euthanized on the first day after the start of the experiment. Groups 3, 4 and 5 were analyzed on days 1, 3 and 7 following the induction of acute injury. Five samples from each group were destined for histological analysis with hematoxylin-esosin (HE) and Picrosirius red staining and five samples were destined for zymography and Western blot analysis.
Cryoinjury procedure
The surgical procedures are described elsewhere [22, 23, 27]. Briefly, animals were anesthetized with an intraperitoneal administration of 1 mL/kg of 1 % 2 % xylazine and ketamine. The right TA muscle was surgically exposed and subjected to the cryoinjury procedure, which consisted of applying the flat end of a metal rod (3 mm in diameter) previously cooled in liquid nitrogen directly onto the surface of the exposed muscle for 10 s, followed by a second application to the same area for another 10 s. The wounds were then sutured and the animals were kept in plastic boxes at room temperature to prevent hypothermia.
LLLT procedure
For LLLT, an aluminum-gallium-arsenide Twin Laser® (MM Optics, São Carlos, SP, Brazil) was used with the following parameters: wavelength 780 nm, output power 40 mW, density power 1 W/cm2, beam area 0.04 cm2, energy density 10 J/cm2, and exposure time 10 s.
The transducer was covered with a transparent protective film. The laser beam was applied in contact with the surface of the skin at the incision and around the injured area at an angle of 90° between the emitter and skin to prevent refraction. Irradiation was applied to eight points within the injured area [22, 23, 28, 29]. The energy per point was 0.4 J, totaling 3.2 J per treatment. A power meter (Laser Check, MM Optics, São Carlos, Brazil) was used to determine the output power of the equipment. The experiments were performed with standard procedures based on previous studies conducted by our research group [22, 23, 28, 29]. LLLT was initiated 2 h following injury and was performed daily, with a 24-hour interval between sessions, for a total of one, two and six sessions for the groups evaluated at 1, 3 and 7 days, respectively.
After the experimental period for each group, the animals were euthanized using a CO2 chamber and subsequently weighed, followed by the removal and weighing of the TA muscle. The muscles were stored in 10 % buffered formalin for subsequent histological analysis. Samples destined for the analysis of gelatinolytic activity were stored in a freezer at −80 °C.
Morphological analysis
Muscle samples were fixed in 10 % buffered formalin and embedded in paraffin. The specimens were cut into 10-μm cross sections using a microtome (Leica RM2125, Nussloch, Germany). Tissue specimens were stained with HE for the visualization of general morphology using a conventional light microscope (Zeiss Axioplan 2, Germany). The qualitative analysis of histological sections stained with HE included a description of the stages of tissue repair and involved the presence and type of inflammatory infiltrate, edema, myonecrosis, new immature muscle fibers and angiogenesis.
For the quantitative analysis, five areas corresponding to the injury area were photographed using a conventional light microscope (Zeiss Axioplan 2, Germany). The images were analyzed by an experienced pathologist without prior knowledge of the experimental groups, using the Count Cells plugin of the ImageJ software program (National Institutes of Health, USA). The total amount of inflammatory cells, myonecrosis, blood vessels and immature (new) muscle fibers were counted by area. At least three slides from each animal were examined and the data were statistically analyzed.
Analysis of collagen
Additional sections were stained with Picrosirius Red (Sigma, St. Louis, MO, USA) following the method described by Junqueira et al. [30] and were examined with a polarized light microscope (Pol-Interferential Photomicroscope, Model 61 282, Carl Zeiss, Germany). The images were also analyzed using the ImageJ program (National Institutes of Health, USA) and the area occupied by collagen was calculated relative to the total area of the cut, as described by Hadi et al. [31].
Zymography
The tissue extract of the TA muscle was tested for the presence of gelatinolytic activity, as described by Cleutjens [32]. Muscle samples of approximately 50 mg were washed twice in saline and then homogenized in 2 ml of extraction buffer (10 mM cacodylic acid, pH 5.0, 150 mM NaCl, 1 mM ZnCl2, 20 mM CaCl2, 1.5 mM NaN3, and 0.01 % Triton X-100), with continuous homogenization on ice for 10 min. The total protein content was estimated using the Bradford reagent (BioRad Laboratories, Hercules, CA, USA) following the method described by Bradford [33].
For the enzyme assay, 100 μg of protein were resolved by polyacrylamide gel electrophoresis (10 %) with 1 mg/ml gelatin. After the run, the gel was washed twice for 30 min in a 2.5 % Triton X-100 solution to remove the sodium dodecyl sulfate (SDS) and incubated in substrate buffer (50 mM Tris–HCl, pH 8.0, 5 mM CaCl2, 10 mM ZnCl2 and 0.02 % NaN3) at 37 °C for approximately 20 h. The gel was then stained with Coomassie Blue for 60 min and destained with acetic acid, methanol and water (1:4:5) for the visualization of band activity. Three gels were scanned and analyzed for documentation. The ImageJ program (National Institutes of Health, USA) was used for the quantification of the bands of proteolytic activity by densitometry (amount in pixels). These values were then converted into arbitrary units from the value obtained in the control group.
Western blot analysis
Extracted muscle tissue was also used to characterize MMP-2 using Western blot analysis. For such, 100 μg of reduced samples were resolved by SDS-PAGE (10 %) and transferred to a nitrocellulose membrane. After blocking with 5 % skim milk, the membrane was incubated with primary anti-MMP-2 (Santa Cruz, California, USA; sc-13 595) for 3 h at room temperature. The membrane was washed and incubated with secondary anti-mouse IgG conjugated to alkaline phosphatase (Santa Cruz, California, USA; sc- 2047). Detection was performed using the BCIP and NBT substrate (Sigma-Aldrich, St. Louis, USA; B5655) and the membrane was photographed for documentation.
Statistical analysis
The data were analyzed using the BioStat 5.0 program (PA, Brazil). The Shapiro-Wilk test was used to determine the distribution of the data. Data with parametric distribution were tested using One-Way ANOVA, followed by Tukey’s test for comparisons between groups. Confidence levels were adjusted to 95 % (p < 0.05).
Results
Qualitative morphological analysis
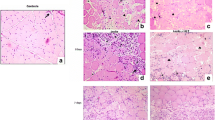
In the morphological analysis, the muscles in the control group exhibited a normal histological appearance, with the presence of fibers with peripheral nuclei and no signs of inflammation or injury (Fig. 1a). These results were similar to those found in the LLLT group at all evaluation periods.
Photomicrographs of histological sections of muscles stained with hematoxylin and eosin (original magnification × 100); a Control muscle, showing normal morphology; b Sham muscle, showing mild inflammatory infiltrate in perimysium (arrow); c Injury group after 1 day and d Injury + LLLT group after 1 day, both showing edema (asterisk) and myonecrosis; e Injury group after 3 days, showing edema (asterisk), myonecrosis and adjacent inflammatory infiltrate (arrow); f Injury + LLLT group after 3 days, showing edema (asterisk), myonecrosis and leukocyte infiltration (arrow); g Injury group after 7 days, showing collagen fibers (circle) and immature fibers (arrow); h Injury +LLLT group after 7 days, showing minor amounts of collagen fibers (circle), immature fibers (arrow) and blood vessels (triangle)
The sham group exhibited mild mononuclear inflammatory infiltrate, predominantly in the surface region of the surgically exposed muscle (Fig. 1b). After 1 and 3 days, the injury group without treatment exhibited edema between muscle fibers, moderate inflammatory infiltrate with neutrophils and lymphocytes scattered among the fibers, the majority of which were undergoing myonecrosis (Fig. 1c, e). In these same evaluation periods, the injury + LLLT group exhibited a reduction in both inflammatory infiltrate and myonecrosis (Fig. 1d, f).
At 7 days, the injury group without treatment exhibited a reduction in inflammation, scarce myonecrosis and the emergence of immature (new) muscle fibers (Fig. 1g). However, the muscle fibers in the injury + LLLT group had a greater degree of maturation (polygonal fibers with a peripheral nucleus) and exhibited a decrease in both edema and myonecrosis (Fig. 1h).
Quantitative morphological analysis
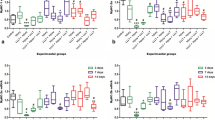
The quantitative analysis revealed that the cryoinjury group submitted to LLLT exhibited a significant reduction in the total amount of inflammatory cells and myonecrosis after 1 day in comparison to the non-treated cryoinjury group in the same evaluation period (p < 0.05; Fig. 2a, b ). Furthermore, the cryoinjury group submitted to LLLT exhibited a significant increase in the number of blood vessels after 3 and 7 days (p < 0.01; Fig. 2c) as well as an increase in the number of immature (new) muscle fibers after 7 days (p < 0.01; Fig. 2d). The group without laser or injury exhibited aspects similar to those in the control group in the three evaluation periods (data not shown).
Effects of LLLT on total number of inflammatory cells (a), amount of myonecrosis (b), number of blood vessels (c) and number of immature muscle fibers (d); Values expressed as mean ± standard deviation (ANOVA/Tukey); * p < 0.05 compared to non-treated injury group; *** p < 0.01 compared to non-treated injury group
Analysis of collagen
The control group exhibited normal tissue architecture with organized collagen in the region of the endomysium and perimysium (Fig. 3a). The LLLT and sham groups exhibited collagen organization similar to that of the control group in all evaluation periods (data not shown). At 1 and 3 days, the distribution of collagen was similar in the injury groups with and without LLLT, with dispersed fibers in the region of the inflammatory infiltrate and necrotic fibers in the cell spaces (Fig. 3b–e).
Photomicrographs of histological sections of muscles stained by Picrosirius Red with and without polarized light (original magnification × 400); Figures illustrating images used to quantify percentage area of collagen fibers using polarized light microscopy; a Control muscle, showing normal morphology; b Injury group after 1 day; c Injury + LLLT group after 1 day; d Injury group after 3 days; e Injury + LLLT group after 3 days; f Injury group after 7 days; g Injury + LLLT group after 7 days, showing better organization of collagen in endomysium in comparison to non-treated group in same evaluation period
At 7 days, collagen fibers began to demonstrate greater organization in bundles. In the non-treated injury group, diffuse and disorganized collagen was observed predominantly in the regions of the endomysium and perimysium (Fig. 3f). The injury + LLLT group exhibited greater organization of collagen bundles arranged in a compact form, especially in the region of the perimysium (Fig. 3g).
Collagen quantification from images analyzed under polarized light revealed no differences among groups after 1 and 3 days (p ≥ 0.05). However, a significant increase in collagen concentration was found in the non-treated injury group (p < 0.01) and injury + LLLT group (p < 0.05) after 7 days in comparison to the other groups. Moreover, no difference was found in the concentration of collagen fibers between injury + LLLT group (12.88 ± 2.04 %) and the non-treated injury group (17.15 ± 3.33 %) after 7 days (p ≥ 0.05; Fig. 4).
Quantification of area of collagen fibers in TA relative to total muscle area; Values expressed as mean and standard deviation (ANOVA/Tukey); *** p < 0.01 in comparison to control group and experimental groups evaluated after 1 and 3 days; * p < 0.05 in comparison to control group and experimental groups evaluated after 1 and 3 days
Analysis of gelatinase activity of MMP-2
The activity of MMP-2 was detected in the TA muscle extract as two lytic bands of approximately 72 kDa (pro-MMP-2) and 64 kDa (active MMP-2), suggesting the presence of MMP-2 (Fig. 5a), as confirmed by Western blot analysis (Fig. 5b).
Illustrative image of gelatinase activity in rat TA muscle evaluated by zymography; a Activity of MMP-2, pro and active forms (~72 and 64 kDa, respectively), was detected as clear bands on dark background. Each line corresponds to one experimental group, in which the same amount of protein (100 μg) was resolved on an SDS polyacrylamide gel (10 %) containing gelatin. b Detection of MMP-2 by Western blot analysis; Data expressed as mean ± standard deviation (ANOVA/Tukey); c Graph representing lytic bands quantified by densitometry in control and sham groups after 1 day; d Graph representing lytic bands in control and sham groups after 3 days; e Graph representing lytic bands in control and sham groups after 7 days; * p < 0.05 compared to control group; *** p < 0.01 compared to non-treated injury group; # p < 0.01 compared to control, sham and LLLT groups
No significant difference in pro-MMP-2 activity was found between the control and sham groups (p ≥ 0.05). Furthermore, only one lytic band was found after 1 day, corresponding to pro-MMP-2, with no statistically significant difference (p ≥ 0.05) among the groups (Fig.5a).
Interestingly, two lytic bands of MMP-2 were found after 3 days in the treated and non-treated injury groups, corresponding to pro and active MMP-2. A 13 % increase in pro-MMP-2 was detected in the non-treated injury group (p < 0.05) and an 11 % increase was detected in the injury + LLLT group (p < 0.05) in comparison to the control group. No significant difference between the injury groups with and without LLLT was found regarding active MMP-2 (p ≥ 0.05) (Fig. 5b). However, after 7 days, LLLT led to a 75 % increase in pro-MMP-2 (p < 0.01) and a 91 % increase in active MMP-2 (p < 0.01) in comparison to the non-treated injury group (Fig. 5c). There was also a significant increase in pro-MMP-2 in the injury groups with and without LLLT (p < 0.01) after 7 days in comparison to the control, sham and LLLT groups (Fig. 5c).
Discussion
The therapeutic advantages of LLLT regarding the regulation of the inflammatory process [22, 34] and the regeneration of skeletal muscle have been evaluated in a number of studies [20, 24, 26]. However, the mechanisms behind these effects have not been fully identified.
The results of the present study showed that LLLT induced a reduction in inflammatory infiltrate and myonecrosis after 1 day, an increase in the number of blood vessels after 3 and 7 days as well as an increase in the number of immature muscle fibers and MMP-2 gelatinase activity after 7 days. Furthermore, the animals in the injury + LLLT group exhibited better organization and distribution of collagen bundles, especially in the region of endomysium and perimysium after 7 days.
These results are in agreement with findings described previously by our research group. Souza et al. [24] found that LLLT (wavelength 660 nm, output power 20 mW, energy density 5 J/cm2) enhanced the regeneration and fibrosis phases of the skeletal muscle repair process, leading to the promotion of angiogenesis, a reduction in myonecrosis and the induction of the synthesis of types I and III collagen in the TA muscle of rats following cryoinjury. Analyzing the amount and distribution of type IV collagen in the TA muscle of rats during the repair process following cryoinjury, Baptista et al. [23] concluded that LLLT (wavelength 660 nm, output power 20 mW, energy density 5 J/cm2) induced an increase in type IV collagen 7 days following the induction of injury, but did not alter the duration of the repair process, as complete muscle regeneration was observed after 21 days.
In the present study, the animals in the injury + LLLT group exhibited better collagen organization and deposition between muscle fibers after 7 days. This organization of the collagen fibers may have occurred mainly due to the increased gelatinolytic activity of MMP-2, which was also found after 7 days and plays an important role in the formation of new fibers, promoting the degradation of basement membrane collagen IV and other ECM components [13].
After an injury, coordinated activity occurs between the degradation and synthesis of ECM components, especially collagen [9, 13, 17, 35]. The degradation of these components facilitates the migration, proliferation and fusion of myoblasts and the formation of new blood vessels [10, 13, 16, 17]. Interestingly, the quantitative morphological analysis revealed an increase in blood vessels after 3 and 7 days as well as immature fibers after 7 days, which could have been facilitated by the increase in gelatinolytic activity of MMP-2 [36, 37]. The effects of LLLT on collagen and procollagen synthesis have been demonstrated in the literature [23, 24, 26, 38–41]. However, the effects on collagen metabolism have produced divergent results. Some authors have demonstrated that LLLT increases collagen synthesis [23, 24, 38], whereas other authors report a decrease in collagen synthesis upon irradiation [26, 37, 39]. In the present study, a reduction in collagen was found, with better distribution and organization after 7 days in the injury group submitted to LLLT. However, the difference did not achieve statistical significance.
In the skeletal muscle, collagen plays a key role in maintaining the functional integrity of the fibers for the transmission of force during muscle contraction. Type I collagen, which is most commonly found in dense connective tissue, is necessary for the stabilization of tissue architecture, while type III collagen, which is most commonly found in the loose connective tissue, plays an important role in tissue elasticity [42–44]. Thus, better collagen organization and distribution could directly affect the functional aspects of skeletal muscle tissue [45].
Our research group has previously demonstrated the effects of LLLT on the expression of growth factors that modulate the synthesis and degradation of ECM and on the muscle regeneration process using the same experimental injury model, showing that LLLT (wavelength 660 nm, output power 20 mW, energy density 5 J/cm2) induced a reduction in TNF-α expression after 1 and 7 days and a reduction in TGF-β after 7 days [22]. TGF-β is related to the formation of scar tissue during muscle repair and regulates the production of ECM-degrading enzymes, such as collagenases and gelatinases, as well as the synthesis of enzymes that inhibit the degradation of ECM, such as tissue inhibitors of metalloproteinases and plasminogen activator inhibitor 1 [9]. The present findings are in agreement with these data, as a reduction in the amount of collagen and better organization were found after 7 days, associated with an increase in MMP-2 activity. Assis et al. [26] found that infrared laser (wavelength 808 nm, output power 30 mW, energy density 180 J/cm2) caused a reduction in TGF-β1 and type I collagen in mouse muscle 4 days following injury.
In the present study, the acute phase of muscle repair was also analyzed based on the histopathological aspects of the different experimental groups. The quantitative analysis revealed that LLLT led to a reduction in the number of total inflammatory cells and myonecrosis after 1 day. These results are in agreement with findings reported by Barbosa et al. [46], who administered red laser (wavelength 685 nm, energy density 4.2 J/cm2) on the gastrocnemius muscle of mice subjected to injury by the venous snake Bothrops jararacussu and found an 83.5 % reduction in myonecrosis after 24 h. Moreover, Cressoni et al. [19] who found that LLLT (wavelength 785 nm, energy density 2.7 J/cm2) led to a reduction in the number of polymorphonuclear and mononuclear leukocytes and accelerated tissue regeneration by stimulating the maturation of muscle cells and the proliferation of fibroblasts, thereby providing conditions for improving the organization and alignment of regenerating muscle fibers.
An increase in the number of blood vessels was found after 3 and 7 days in the injury + LLLT group. Angiogenesis is an important part of the muscle regeneration process, as it reestablishes circulation to the injury site, thereby limiting ischemic necrosis and facilitating regeneration [24]. After 7 days, an increase in the number of new muscle fibers with a greater degree of maturation was found in the injury + LLLT group. In a previous study conducted by our research group [24], no statistically significant difference was found regarding this aspect in the treated group after 7 days, but different laser irradiation parameters were used (wavelength 660 nm, output power 20 mW, total energy density 1.6 J).
In summary, the results of the present study demonstrate that LLLT has a positive effect on the inflammatory process, MMP-2 activity and collagen organization and distribution in the repair process of rat skeletal muscle.
References
Bischoff R, Heintz C (1994) Enhancement of skeletal muscle regeneration. Dev Dyn 201:41–54
Chargé SB, Rudnick MA (2004) Cellular and molecular regulation of muscle regeneration. Physiol Rev 84:209–238
Shi X, Garry DJ (2006) Muscle stem cells in development, regeneration, and disease. Genes Dev 20:1692–1708
Huard J, Li Y, Fu FH (2002) Muscle injuries and repair: current trends in research. J Bone Joint Surg Am 84:822–832
Tidball JG (2005) Inflammatory processes in muscle injury and repair. Am J Physiol Regul Integr Comp Physiol 288:345–353
Tidball JG, Villalta SA (2010) Regulatory interactions between muscle and the immune system during muscle regeneration. Am J Physiol Regul Integr Comp Physiol 298:1173–1187
Hawke TJ, Garry DJ (2001) Myogenic satellite cells: physiology to molecular biology. J Appl Physiol 91:534–551
Dogra C, Hall SL, Wedhas N, Linkhart TA, Kumar A (2007) Fibroblast growth factor inducible-14 (Fn14) is required for the expression of myogenic regulatory factors and differentiation of myoblasts into myotubes: evidence for TWEAK-independent functions of Fn14 during myogenesis. J Biol Chem 282:15000–15010
Mann CJ, Perdiguero E, Kharraz Y, Aguilar S, Pessina P, Serrano AL et al (2011) Review: aberrant repair and fibrosis development in skeletal muscle. Skeletal Muscle 1:21
Carmeli E, Moas M, Reznick AZ, Coleman R (2004) Matrix metalloproteinases and skeletal muscle: a brief review. Muscle Nerve 29:191–197
Kjaer M (2004) Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev 84:649–698
Tang M, Zhou F, Zhang W, Guo Z, Shang Y, Lu H et al (2011) The role of thrombospondin-1-mediated TGF-β1 on collagen type III synthesis induced by high glucose. Mol Cell Biochem 346:49–56
Chen X, Li Y (2009) Role of matrix metalloproteinases in skeletal muscle: migration, differentiation, regeneration and fibrosis. Cell Adhesion & Migration 3:337–341
Carmeli E, Haimovitch TG, Nemcovsky CE (2006) Expression of matrix metalloproteinase 2 and heat shock protein-72 in immobilized muscle in rats. J Musculoskelet Neuronal Interact 6:96–102
Barnes BR, Szelenyi ER, Warren GL, Urso ML (2009) Alterations in mRNA and protein levels of metalloproteinases-2, -9, and −14 and tissue inhibitor of metalloproteinase-2 responses to traumatic skeletal muscle injury. Am J Physiol Cell Physiol 297:1501–1508
Chang C, Werb Z (2001) The many faces of metalloproteases: cell growth, invasion, angiogenesis, and metastasis. Trends Cell Biol 11:37–43
Zemowaska M, Krzysztof HO, Swierzynska M, Streminska M, Ciemerych MA (2012) Decrease of MMP-9 activity improves soleus muscle regeneration. Tissue Eng Part A 18:1–11
Deveci D, Marshall JM, Egginton S (2002) Chronic hypoxia induces prolonged angiogenesis in skeletal muscles of rat. Exp Physiol 87:287–291
Cressoni MD, Dib Giusti HH, Casarotto RA, Anaruma CA (2008) The effects of a 785-nm AlGaInP laser on the regeneration of rat anterior tibialis muscle after surgically-induced injury. Photomed Laser Surg 26:461–466
Silva LH, Silva MT, Gutierrez RM, Conte TC, Toledo CA, Aoki MS, Liebano RE, Miyabara EH (2012) GaAs 904-nm laser irradiation improves myofiber mass recovery during regeneration of skeletal muscle previously damaged by crotoxin. Lasers Med Sci 27:993–1000
Nakano J, Kataoka H, Sakamoto J, Origuchi T, Okita M, Yoshimura T (2009) Low-level laser irradiation promotes the recovery of atrophied gastrocnemius skeletal muscle in rats. Exp Physiol 94:1005–1015
Mesquita-Ferrari RA, Martins MD, Silva JA Jr, da Silva TD, Piovesan RF, Pavesi VC et al (2011) Effects of low-level laser therapy on expression of TNF-α and TGF-β in skeletal muscle during the repair process. Lasers Med Sci 26:335–340
Baptista J, Martins M, Pavesi V, Bussadori S, Fernandes KPS, Mesquita-Ferrari RA (2010) Influence of laser photobiomodulation on collagen IV during skeletal muscle tissue remodeling following injury in rats. Photomed Laser Surg 29:12–17
Souza TO, Mesquita DA, Ferrari RA, Dos Santos PD, Jr CL, Bussadori SK, Fernandes KP, Martins MD (2011) Phototherapy with low-level laser affects the remodeling of types I and III collagen in skeletal muscle repair. Lasers Med Sci 26:803–814
Medrado AP, Pugliese LS, Reis SRA, Andrade ZA (2003) Influence of low level laser therapy on wound healing and its biological action upon myofibroblasts. Lasers Surg Med 32:239–244
Assis L, Moretti AL, Abrahão TB, De Souza HP, Hamblin MR, Parizotto NA (2012) Low-level laser therapy (808 nm) contributes to muscle regeneration and prevents fibrosis in rat tibialis anterior muscle after cryolesion. Lasers Med Sci 28(3):947–955
Miyabara EH, Aoki MS, Soares AG, Moriscot AS (2005) Expression of tropism-related genes in regenerating skeletal muscle of rats treated with cyclosporin-A. Cell Tissue Res 319:479–489
Fernandes KPS, Alves AN, Nunes FD, Souza NHC, Silva JA, Bussadori SK, Mesquita-Ferrari RA (2012) Effect of photobiomodulation on expression of IL-1β in skeletal muscle following acute injury. Lasers Med Sci
França CM, de Loura Santana C, Takahashi CB, Alves AN, De Souza Mernick AP, Fernandes KP, de Fátima Teixeira da Silva D, Bussadori SK, Mesquita-Ferrari RA (2012) Effect of laser therapy on skeletal muscle repair process in diabetic rats. Lasers Med Sci
Junqueira LC, Montes GS, Sanchez EM (1982) The influence of tissue section thickness on the study of collagen by the Picrosirius-polarization method. Histochemistry 74:153–156
Hadi AM, Mouchaers KT, Schalij I, Grunberg K, Meijer GA, Vonk-Noordegraaf A et al (2011) Rapid quantification of myocardial fibrosis: a new macro-based automated analysis. Cell Oncol (Dordr) 34:343–354
Cleutjens JP, Kandala JC, Guarda E, Guntaka RV, Weber KT (1995) Regulation of collagen degradation in the rat myocardium after infarction. J Mol Cell Cardiol 27:1281–1292
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Albertini R, Villaverde AB, Aimbire F, Bjordal J, Brugnera A, Mittmann J et al (2008) cytokine mrna expression is decreased in the subplantar muscle of rat paw subjected to carrageenan-induced inflammation after low-level laser therapy. Photomed Laser Surg 26:19–24
Bellayr IH, Mu X, Li Y (2009) Biochemical insights into the role of matrix metalloproteinases in regeneration: challenges and recent developments. Future Med Chem 1:1095–1111
Stetler-Stevenson WG (1999) Matrix metalloproteinases in angiogenesis: a moving target for therapeutic intervention. J Clin Invest 103:1237–1241
Miyazaki D, Nakamura A, Fukushima K, Yoshida K, Takeda S, Ikeda S (2011) Matrix metalloproteinase-2 ablation in dystrophin-deficient mdx muscles reduces angiogenesis resulting in impaired growth of regenerated muscle fibers. Hum Mol Genet 20:1787–1799
Yamamoto Y, Kono T, Kotani H, Kasai S, Mito M (1996) Effect of low-power laser irradiation on procollagen synthesis in human fibroblasts. J Clin Laser Med Surg 14:129–132
Pereira AN, Eduardo CP, Matson E, Marques MM (2002) Effect of low-power laser irradiation on cell growth and procollagen synthesis of cultured fibroblasts. Lasers Surg Med 31:263–267
Reddy GK, Stehno-Bittel L, Enwemeka CS (1998) Laser photostimulation of collagen production in healing rabbit Achilles tendons. Lasers Surg Med 22:281–287
Marques MM, Pereira AN, Fujihara NA, Nogueira FN, Eduardo CP (2004) Effect of low-power laser irradiation on protein synthesis and ultrastructure of human gingival fibroblasts. Lasers Surg Med 34:260–265
Chiquet M, Matthison M, Koch M, Tannheimer M, Chiquet-Ehrismann R (1996) Regulation of extracellular matrix synthesis by mechanical stress. Biochem Cell Biol 74:737–744
Han XY, Wang W, Komulainen J, Koskinen SO, Kovanen V, Vihko V et al (1999) Increased mRNAs for procollagens and key regulating enzymes in rat muscle following downhill running. Pflugers Arch 437:857–864
Myllyla R, Myllyla VV, Tolonen U, Kivirikko KI (1982) Changes in collagen metabolism in diseased muscle. I. Biochemical studies. Arch Neurol 39:752–755
Gillies AR, Lieber RL (2011) Structure and function of the skeletal muscle extracellular matrix. Muscle Nerve 44:318–331
Barbosa AM, Villaverde AB, Sousa LG, Munin E, Fernandez CM, Cogo JC et al (2009) Effect of low-level laser therapy in the myonecrosis induced by Bothrops jararacussu snake venom. Photomed Laser Surg 27:591–597
Acknowledgments
The authors would like to thank UNINOVE and FAPESP (2011/17638-2; 2011/04452-8) for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alves, A.N., Fernandes, K.P.S., Melo, C.A.V. et al. Modulating effect of low level-laser therapy on fibrosis in the repair process of the tibialis anterior muscle in rats. Lasers Med Sci 29, 813–821 (2014). https://doi.org/10.1007/s10103-013-1428-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-013-1428-9