Abstract
Promising effects of phototherapy on markers of exercise-induced muscle damage has been already demonstrated in constant load or isokinetic protocols. However, its effects on more functional situations, such as plyometric exercises, and when is the best moment to apply this treatment (pre- or post-exercise) remain unclear. Therefore, the purpose of this study was to investigate the effect of low-level laser therapy (LLLT) before or after plyometric exercise on quadriceps muscle damage markers. A randomized, double-blinded, placebo-controlled trial was conducted with 24 healthy men, 12 at pre-exercise treatment group and 12 at post-exercise treatment group. Placebo and LLLT (810 nm, 200 mW per diode, 6 J per diode, 240 J per leg) were randomly applied on right/left knee extensor muscles of each volunteer before/after a plyometric exercise protocol. Muscular echo intensity (ultrasonography images), soreness (visual analogue scale - VAS), and strength impairment (maximal voluntary contraction - MVC) were assessed at baseline, 24, 48, and 72 h post-exercise. Legs treated with LLLT before or after exercise presented significantly smaller increments of echo intensity (values up to 1 %) compared to placebo treatments (increased up to ∼7 %). No significant treatment effect was found for VAS and MVC, although a trend toward better results on LLLT legs have been found for VAS (mean values up to 30 % lesser than placebo leg). In conclusion, LLLT applied before or after plyometric exercise reduces the muscle echo intensity response and possibly attenuates the muscle soreness. However, these positive results were not observed on strength impairment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Exercise-induced muscle damage is a well-described phenomenon that occurs in response to an unaccustomed physical activity, mostly the ones involving eccentric contractions [1]. It can be divided into the following two phases: primary or mechanical damage and secondary or inflammatory damage [1, 2]. Primary phase involves damage to microscopic muscular structures, such as the Z-line, sarcoplasmatic membrane, sarcoplasmatic reticulum, T-tubules, myofibrils, and cytoskeletal system [1]. It triggers the secondary damage, caused by alteration in Ca2+ homeostasis, and induces inhibition of mitochondrial function, adenosine triphosphate (ATP) depletion, inflammatory response, and proteolytic enzyme activation, resulting in further damage to muscle tissue [1–3]. This process can last up to several days and leads to impairments of muscle function, limitation of athletic performance, and increased injury risk [1]. Therefore, delayed onset of muscle soreness (DOMS), strength impairment, inflammatory response, and presence of muscle proteins into the blood (especially creatine kinase - CK) are the most common markers used in studies on exercise-induced muscle damage [4]. Additionally, muscular echo intensity (or echogenicity), assessed through ultrasonography images, has been used as a reliable non-invasive method to assess the muscle damage level in humans [5–7].
Different therapeutic modalities have been studied on the attempt to reduce muscle damage impairments, including anti-inflammatory drugs, nutritional supplements, cryotherapy, massage, stretching, electrical stimulation, endurance and resistance exercise, among others [1, 2]. In this scenario, phototherapy (low-level laser therapy - LLLT); and light-emitting diode therapy - LEDT), a non-pharmacological agent widely used by physiotherapists to treat musculoskeletal injuries [8, 9], emerges as a possible intervention to counteract the undesirable effects of exercise-induced muscle damage. LLLT/LEDT act by interacting with cytochrome c oxidase, a mitochondrial enzyme of the respiratory chain, improving ATP production and delaying cellular acidosis and its negative effect on cell metabolism [10, 11], besides an anti-inflammatory action mediated by reduced reactive species of oxygen and oxidative stress [12].
Benefits of phototherapy on exercise-induced muscle damage have been already shown by some animal studies [13–15]. In humans, trials concerning LLLT/LEDT application before [16–18], during [19], or after [16, 20, 21] exercise demonstrated positive effects (or at least a trend to) on DOMS [16–18, 20, 21], strength impairment [17, 18, 21], CK levels [17–19], and lactate dehydrogenase (LDH) levels [18]. These trials have used constant load [16, 19–21] or isokinetic [17, 18] exercise protocols to induce muscle damage. Since its practical application may be limited as it does not reproduce sport activities, efforts have been made to evaluate the effects of phototherapy front to more functional tasks, such as cycling [22, 23], running [24], and even a volleyball match [25]. However, these investigations assessed muscle damage solely through CK levels and with a single time point few minutes [22–24] or 24 h [25] after exercise, which limits the understanding of phototherapy effects on muscle response to damage induced by functional exercises.
Considering currently available evidence, there is a lack on the literature about the effect of phototherapy on main markers of muscle damage induced by functional/sportive tasks and no consensus in relation to the best moment for phototherapy application, pre-exercise vs. post-exercise. Therefore, the purpose of this study was to investigate the effects of LLLT (810 nm) before or after plyometric exercise on quadriceps muscle echo intensity, soreness, and strength impairment up to 72 h after exercise in healthy subjects.
Methods
Trial design
This study was a randomized, double-blinded, placebo-controlled trial. The data collection was conducted at the Physiotherapy Laboratory of the Universidade Federal de Ciências da Saúde de Porto Alegre (Brazil).
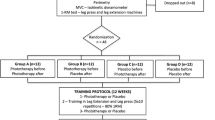
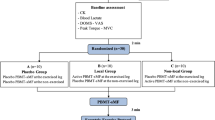
Initially, volunteers were divided in two groups: (1) pre-exercise treatment and (2) post-exercise treatment. Thereafter, a computer randomization program was used to determine which lower limb would be treated with LLLT in each volunteer. Consequently, the contralateral limb would receive placebo treatment. Care was taken to ensure a similar number of subjects treated with active therapy in preferred and non-preferred limbs. A researcher responsible for therapy application was the only one aware of the LLLT/placebo randomization. Volunteers and researchers responsible by the evaluations were blinded to the treatment allocation.
Volunteers have been to the laboratory in four consecutive days (Monday through Thursday), with an interval of 24 ± 1 h between them. At the first day, participants underwent primary baseline assessments of quadriceps muscle echo intensity, soreness, and strength. Thereafter, subjects were submitted to the LLLT/placebo application and the plyometric exercise, respecting the sequence order determined by group allocation (pre- or post-exercise treatment). Participants have been re-evaluated 24, 48, and 72 h after plyometric exercise (Fig. 1).
Participants
Healthy and physically active males aged between 18 and 30 years were invited to participate in the study. Volunteers who had been engaged in any type of systematized lower limb strength training program in the last 3 months as well as who presented musculoskeletal injuries in the lower limbs or contraindications to maximal exercise performance (as cardiovascular, respiratory, or neurological diseases) were not included in the study. Individuals who used analgesic or anti-inflammatory drugs or performed any type of vigorous physical exercise for the lower limbs during the study protocol were excluded.
Echo intensity
Echo intensity of rectus femoris muscle was assessed through ultrasonography with a GE Vivid I (General Electric, Fairfield, USA) equipment, along with a linear array probe (48 mm, 7.5 MHz) of the same manufacturer. All measurements were made by an experienced investigator using this technique. Before each assessment, individuals remained in supine position with knees and hips in neutral position and rested for 10 min [26]. The distance between the greater trochanter and the articular line of the knee was measured, and the mid-point was set as the reference for the evaluations. Four transversal images were captured of each lower limb on each day. Special attention was given to determine the specific site where the images were collected from. At the first day, a waterproof pen was used to mark the exact site where the subsequent assessments should be done. In addition, a bubble level was attached to the ultrasound probe to ensure a minimum inclination during data collection. Posteriorly, all images were analyzed with ImageJ software (National Institutes of Health, USA) by a blinded researcher to LLLT/placebo randomization. Briefly, a square with the area of 1 cm2 [7] was positioned at the mid-point of rectus femoris for the echogenicity calculation (gray scale histogram 0 = full black picture; 255 = full white picture). The echo intensity mean value between the four images from each day was used for analysis.
Muscle soreness
Muscle soreness was assessed through the visual analogue scale (VAS), a 100-mm horizontal line without marks or numbers, just indications of “no soreness” at left the beginning and “extreme soreness” at the end of the line [18]. In each evaluation, the same researcher instructed the subjects to go down on a step (16 cm) and mark a vertical line on the scale that best reflected their soreness during the quadriceps eccentric action. Muscle soreness was quantified by the distance between the initial point line (0 mm) and the point marked by the subject [17, 18, 20, 21].
Maximal voluntary contraction
Peak torque during maximal voluntary contraction (MVC) of knee extensor muscles from each lower limb was assessed with the isokinetic dynamometer Biodex System 4 Pro (Biodex Medical Systems, Shirley, USA). The volunteers were positioned as the manufacturer’s recommendations for evaluation of knee flexion-extension movements. A 5-min warm-up on a cycle ergometer followed by 20 concentric knee flexion-extension repetitions at 180°seg−1 were performed before each evaluation. Individuals performed three MVCs at 60° of knee flexion (0° = full knee extension) for 5 s, and the highest peak torque value obtained and sustained for 0.5 s was considered. If there was more than 10 % difference between tests, a fourth test was performed [27]. A 2-min rest was given between each MVC to minimize possible fatigue effects [27]. Volunteers were previously instructed to perform maximal force, and verbal encouragements were given by researchers during tests.
LLLT/placebo
LLLT and placebo were randomly applied by the same researcher with the equipment Chattanooga Intelect Advanced 2766 (Chattanooga, USA) on knee extensor muscles. Half of volunteers received LLLT at the preferred lower limb (the one used to kick a ball), while the other half received the active treatment at the non-preferred lower limb. Therapy was delivered about 2 min before or after the plyometric protocol, respecting the group division. Phototherapy was delivered with a cluster probe composed by five 810-nm laser diodes, each one with 200 mW (Chattanooga Corp., Chattanooga, USA). Eight sites of the quadriceps muscle were defined by palpation and treated: two at vastus lateralis, three at vastus medialis, and three at rectus femoris (Fig. 2). Each site was treated for 30 s, leading to a dose of 6 J per diode, 30 J per site, and 240 J per leg [27]. The probe was held stationary in skin contact at 90° angle with light skin pressure. Placebo and active therapies were applied on the same way but with the device turned off during placebo application. During LLLT/placebo application, subjects were kept lying and blindfolded. Headphones with music of their own choice were used to prevent any equipment beep could provide clues about the active or placebo application.
Plyometric protocol
The plyometric protocol suggested by Marginson et al. [28] was chosen to promote the exercise-induced muscle damage. It consists of 10 sets of 10 repetitions of the counter-movement jump with a 1-min rest period between sets. Before protocol commencement, volunteers received standardized instructions and performed familiarization jumps. They were asked to stand with feet shoulder-width apart and to keep hands on hips during jumps. They should bend knees and hips until approximately 90° of flexion, jump as high as possible, return to the knee bend position, and then to the same initial standing position. Verbal guidance and encouragement were given during the protocol, and participants were asked to repeat the jump when it was wrongly performed (i.e., when individuals did not achieve a knee flexion near to 90° before/after jump). All protocols were controlled by the same instructor.
Statistical analysis
Data distribution and homogeneity were checked through Shapiro-Wilk and Levene tests, respectively. Normalized data (percent change compared to baseline time point) of echo intensity and MVC were analyzed through a two-way repeated measures ANOVA [treatment (pre-LLLT, pre-placebo, post-LLLT, post-placebo) × time (baseline, post-24 h, post-48 h, post-72 h)], followed by LSD post hoc test. All subjects declared no pain at baseline evaluation; therefore, raw data of muscle soreness were analyzed with the same statistical approach. A significance level of 5 % (α < 0.05) was adopted for all procedures.
Results
Twenty-four participants completed the full study schedule, 12 in each group. No between-group difference (p > 0.05) was found to age, body mass, or height (Table 1). In the same way, similar values (p > 0.05) of quadriceps echo intensity, soreness, and MVC were found at baseline evaluation (Table 1).
There was no treatment-time interaction for any outcome (p > 0.05 for all), while time effect was found for echo intensity (p = 0.028), soreness (p < 0.001), and MVC (p < 0.001).
Significant treatment effect was found for echo intensity (p = 0.011; Fig. 3). Limbs treated with LLLT before or after exercise presented smaller echo intensity increments compared to placebo treatments. No difference was found between pre-LLLT and post-LLLT or between pre-placebo and post-placebo (p > 0.05 for both comparisons).
Results (mean ± SE) of muscular echo intensity change for LLLT and placebo legs throughout the study protocol. Letter a indicates the difference than baseline (p < 0.05), letter b the difference than post-24 h (p < 0.05), letter c the difference than post-48 h (p < 0.05), and letter d the difference than post-72 h (p < 0.05)
There was no significant treatment effect for muscle soreness (p > 0.05; Fig. 4). Pre-LLLT limbs reached soreness mean values 17, 14, and 30 % smaller compared to placebo limb at 24, 48, and 72 h after exercise, respectively. Very close mean values were observed between legs in post-exercise treatment group at 24-h assessment (LLLT 2 % smaller than placebo), while limbs treated with LLLT presented mean values 16 % fewer than placebo limbs at 48 and 72 h post-exercise.
Results (mean ± SE) of muscle soreness for LLLT and placebo legs throughout the study protocol. Letter a indicates the difference than baseline (p < 0.05), letter b the difference than post-24 h (p < 0.05), letter c the difference than post-48 h (p < 0.05), and letter d the difference than post-72 h (p < 0.05)
We found no significant treatment effect for MVC loss (p > 0.05; Fig. 5). LLLT prior to exercise leads to MVC impairments around 6–22 % less expressive than placebo treatment throughout the study protocol. In the post-exercise treatment group, the mean MVC loss of LLLT limb was 12 % higher than placebo limb 24 h after exercise, while assessments at 48 and 72 h showed 13–14 % less expressive losses in LLLT compared to placebo limb.
Results (mean ± SE) of maximal voluntary contraction (MVC) change for LLLT and placebo legs throughout the study protocol. Letter a indicates the difference than baseline (p < 0.05), letter b the difference than post-24 h (p < 0.05), letter c the difference than post-48 h (p < 0.05), and letter d the difference than post-72 h (p < 0.05)
Discussion
In this clinical trial, we investigated the effect of LLLT applied before or after plyometric exercise on markers of exercise-induced muscle damage. Our initial hypotheses were (1) in view of previous findings, both LLLT protocols (pre- or post-exercise) would be able to reduce muscle damage markers compared to placebo applications and (2) pre-exercise LLLT would achieve better results than post-exercise LLLT, because pre-exercise treatment could affect primary and secondary phases of muscle damage, while post-exercise treatment would affect only the secondary phase. These hypotheses were partially confirmed by our findings.
Evaluations of the echo intensity response to exercise have been widely studied recently, and an increased signal was previously described as a marker of muscle damage. Although the full mechanism behind the echo intensity change in response to muscle damage remains unclear, evidence suggest that it represents connective tissue damage and inflammation, as well as muscle swelling or increase in plasma enzyme levels, causing an increment on interstitial space between fibers [5–7]. Lower limb muscles have more discreet changes in echo intensity values due to muscle damage compared to upper extremity muscles [5], and placebo limb behavior (increased 3–7 %) was as expected for a quadriceps muscle affected by exercise-induced muscle damage [5, 6]. Since echo intensity values remained practically unchanged throughout the 72 h at limbs treated with LLLT before or after exercise, the therapy effectiveness on preventing inflammatory response seems to be demonstrated for the first time through echo intensity levels in ultrasonography images.
DOMS assessment is probably the most common tool used to measure exercise-induced muscle damage in humans [4], and VAS is a largely used and validated instrument [17, 18, 20, 21]. Positive results on VAS have been shown in some of the analyzed time points after exercise when phototherapy was applied before [17] and after [20, 21] damaging exercise. However, some authors [18, 29, 30] have reported data with high dispersion in soreness perception, making it difficult to obtain significant results from a statistical approach. One factor that may have contributed to this large data dispersion is that volunteers were not allowed to see the mark made on previous days in VAS. As they could not compare their current soreness levels with the previous ones, it may have caused some confusion and acted as a limiting factor. Nonetheless, mean values of soreness experienced in both groups suggest a beneficial effect of LLLT (see Fig. 4). In the pre-exercise treatment group, mean values indicated that subjects experienced up to 30 % less pain in LLLT limb compared to placebo limb throughout the subsequent days of plyometric exercise. Note that a 30 % difference in pain score is considered a clinically worthwhile response [31]. Since this consideration depends on individual perspectives, an even lesser analgesic effect may be very welcome in athletes and non-athletes experiencing DOMS.
Strength loss following an exercise bout is a traditional functional impairment due to muscle damage, and MVC is considered one of the most reliable measurements of strength impairment on damaged muscles [4]. Although our volunteers have suffered smaller strength losses compared to those submitted to isokinetic eccentric exercise of the knee extensor muscles [17, 18], the time effect observed on MVC analysis may be added to DOMS and echo intensity data in order to make clear that our plyometric protocol was able to promote expressive levels of muscle damage. Earlier studies which investigated the influence of phototherapy on muscle recovery have shown positive effects on knee extensors MVC from 24 to 96 h after eccentric exercise [17, 18, 21], which cannot be supported by findings of Felismino et al. [19] or by our results. We are not able to state if the type of exercise used for inducing muscle damage (plyometric vs. isokinetic vs. constant load), the study design (crossover vs. paired groups), or the different phototherapy parameters are responsible for these controversial results. However, it is interesting that echo intensity, soreness, and strength had specific responses to phototherapy in our study, suggesting that connective tissue damage and inflammation are not directly related to nociceptive sensation and functional status of the muscle. The reason for the different response of these three muscle damage markers to phototherapy should be further investigated.
There is no consensus on the literature about the optimal phototherapy protocol for reducing the effects of exercise-induced muscle damage; then, we have chosen to apply the parameters successfully used in our previous trials [18, 27] and supported by a meta-analysis study [32]. However, it is important to note that other parameters than energy may affect the tissue responses to phototherapy. Since the light wavelength is considered a key factor on biological tissue absorption, one could argue that better results could be found with a different wavelength than 810 nm. A previous animal study showed that phototherapy protects skeletal muscle tissue against damage with wavelengths of 660 and 905 nm but not of 830 nm [33], while human trials have shown both positive [16, 18, 23] and no significant effects [19, 34] with wavelengths near to 830 nm. Furthermore, discussion about the differences between LLLT and LEDT can be raised up here. On one hand, there is the idea that “light is light”; thus, there is no big difference between the light sources to prevent exercise-induced muscle damage if the total dose applied per muscle group is adequate [25]. On the other hand, it has been currently suggested that therapeutic advantage can be achieved if different wavelengths and/or light sources are applied at the same time [17, 33, 35]. Therefore, we encourage studies comparing the effects of different wavelengths and light sources on exercise-induced muscle damage in humans.
The moment of application is also a factor that might influence the therapy effectiveness. Time response for improving both muscle ATP content and resistance to fatigue by phototherapy applied over mice skeletal muscles were already demonstrated [36], and a recent clinical trial of the same research group demonstrated positive effects on CK levels with phototherapy applied 40–60 min before a volleyball match in professional players [25]. While some authors [25, 36] argued that the waiting time would allow to muscles a larger time to respond to the light and possibly would promote better results, studies [16–18] have shown good results on different muscle damage markers (i.e., strength, pain, CK, and LDH) with phototherapy delivered few minutes before exercise. Additional evidence from studies with positive results on muscle damage treated with phototherapy during [19] and after exercise [16, 21] contributed to our research question about the best moment to apply phototherapy aiming to counteract exercise-induced muscle damage.
To the best of our knowledge, only dos Reis et al. [16] have already compared the effects of phototherapy applied before and after an exercise bout. However, the exercise characteristics (knee flexion-extension repetitions until exhaustion) and the outcomes (lactate clearance and CK collected 5 min after exercise) suggest that this study [16] was more focused on the recovery from the fatigue process than muscle damage, as well as other studies with phototherapy application before cycling [22, 23] and running [24] tests. Therefore, the present study is the first one to compare the effects of pre- and post-exercise phototherapy on the classical markers of muscle damage throughout subsequent days of an exercise protocol. Findings of this study refuted our initial hypothesis that pre-exercise treatment would be the best choice, suggesting that adequate LLLT parameters used during application (e.g., energy, power, wavelength) seem to be more important than moment of application (immediately before or after exercise) for counteracting the exercise-induced muscle damage. However, more investigations on this topic are needed to optimize phototherapy treatments in the exercise field.
Different from previous studies involving phototherapy and exercise-induced muscle damage, we choose a crossover design with LLLT or placebo being applied in each leg of a same volunteer submitted to a single exercise session. Since preferred and non-preferred arms respond similarly to maximal eccentric exercise in regards to muscle damage markers [37], we suppose that a similar behavior may be attributed to the lower limbs submitted to plyometric exercise (executed bilaterally; e.g., the same body weight being unloaded on each leg). The main advantage of this crossover design is that it limits the influence of intervenient factors found in a two-group design or a two-session design, such as baseline status and motivation in exercise performing, as well as sleep [38], diet [39], and daily activities [1] after damaging protocol. In each group of our study (pre- and post-exercise treatment), both lower limbs (LLLT and placebo) of each volunteer experienced the same magnitude of these intervenient factors, minimizing possible biases. As disadvantage, this study design makes it impossible to assess biochemical markers of inflammation or muscle damage, as the commonly used CK.
In summary, the LLLT protocol used in this study had no effect on strength fall induced by muscle damage; then, caution is needed for recommending phototherapy to reduce this functional impairment. However, LLLT applied both before and after plyometric exercise was able to reduce the muscular echo intensity, indicating a reduced inflammatory response mediated by phototherapy. In addition, our findings suggest a slight effect (not statistically significant) on muscle soreness, which might seem too small for most individuals but can make a crucial difference for high-performance athletes.
References
Howatson G, van Someren KA (2008) The prevention and treatment of exercise-induced muscle damage. Sports Med 38(6):483–503
Cheung K, Hume P, Maxwell L (2003) Delayed onset muscle soreness: treatment strategies and performance factors. Sports Med 33(2):145–164
Fredsted A, Gissel H, Madsen K, Clausen T (2007) Causes of excitation-induced muscle cell damage in isometric contractions: mechanical stress or calcium overload? Am J Physiol Regul Integr Comp Physiol 292(6):R2249–R2258. doi:10.1152/ajpregu.00415.2006
Warren GL, Lowe DA, Armstrong RB (1999) Measurement tools used in the study of eccentric contraction-induced injury. Sports Med 27(1):43–59
Chen TC, Lin KY, Chen HL, Lin MJ, Nosaka K (2011) Comparison in eccentric exercise-induced muscle damage among four limb muscles. Eur J Appl Physiol 111(2):211–223. doi:10.1007/s00421-010-1648-7
Chen TC, Tseng WC, Huang GL, Chen HL, Tseng KW, Nosaka K (2013) Low-intensity eccentric contractions attenuate muscle damage induced by subsequent maximal eccentric exercise of the knee extensors in the elderly. Eur J Appl Physiol 113(4):1005–1015. doi:10.1007/s00421-012-2517-3
Radaelli R, Bottaro M, Wilhelm EN, Wagner DR, Pinto RS (2012) Time course of strength and echo intensity recovery after resistance exercise in women. J Strength Cond Res 26(9):2577–2584. doi:10.1519/JSC.0b013e31823dae96
Tumilty S, Munn J, McDonough S, Hurley DA, Basford JR, Baxter GD (2010) Low level laser treatment of tendinopathy: a systematic review with meta-analysis. Photomed Laser Surg 28(1):3–16. doi:10.1089/pho.2008.2470
Jang H, Lee H (2012) Meta-analysis of pain relief effects by laser irradiation on joint areas. Photomed Laser Surg 30(8):405–417. doi:10.1089/pho.2012.3240
Karu TI (2010) Multiple roles of cytochrome c oxidase in mammalian cells under action of red and IR-A radiation. IUBMB Life 62(8):607–610. doi:10.1002/iub.359
Hayworth CR, Rojas JC, Padilla E, Holmes GM, Sheridan EC, Gonzalez-Lima F (2010) In vivo low-level light therapy increases cytochrome oxidase in skeletal muscle. Photochem Photobiol 86(3):673–680. doi:10.1111/j.1751-1097.2010.00732.x
Lim W, Lee S, Kim I, Chung M, Kim M, Lim H, Park J, Kim O, Choi H (2007) The anti-inflammatory mechanism of 635 nm light-emitting-diode irradiation compared with existing COX inhibitors. Lasers Surg Med 39(7):614–621. doi:10.1002/lsm.20533
Liu XG, Zhou YJ, Liu TC, Yuan JQ (2009) Effects of low-level laser irradiation on rat skeletal muscle injury after eccentric exercise. Photomed Laser Surg 27(6):863–869. doi:10.1089/pho.2008.2443
Sussai DA, Carvalho Pde T, Dourado DM, Belchior AC, dos Reis FA, Pereira DM (2010) Low-level laser therapy attenuates creatine kinase levels and apoptosis during forced swimming in rats. Lasers Med Sci 25(1):115–120. doi:10.1007/s10103-009-0697-9
Camargo MZ, Siqueira CP, Preti MC, Nakamura FY, de Lima FM, Dias IF, Toginho Filho Dde O, Ramos Sde P (2012) Effects of light emitting diode (LED) therapy and cold water immersion therapy on exercise-induced muscle damage in rats. Lasers Med Sci 27(5):1051–1058. doi:10.1007/s10103-011-1039-2
Dos Reis FA, da Silva BA, Laraia EM, de Melo RM, Silva PH, Leal-Junior EC, de Carvalho Pde T (2014) Effects of pre- or post-exercise low-level laser therapy (830 nm) on skeletal muscle fatigue and biochemical markers of recovery in humans: double-blind placebo-controlled trial. Photomed Laser Surg 32(2):106–112. doi:10.1089/pho.2013.3617
Antonialli FC, De Marchi T, Tomazoni SS, Vanin AA, dos Santos Grandinetti V, de Paiva PR, Pinto HD, Miranda EF, de Tarso Camillo de Carvalho P, Leal-Junior EC (2014) Phototherapy in skeletal muscle performance and recovery after exercise: effect of combination of super-pulsed laser and light-emitting diodes. Lasers Med Sci 29(6):1967–1976. doi:10.1007/s10103-014-1611-7
Baroni BM, Leal Junior EC, De Marchi T, Lopes AL, Salvador M, Vaz MA (2010) Low level laser therapy before eccentric exercise reduces muscle damage markers in humans. Eur J Appl Physiol 110(4):789–796. doi:10.1007/s00421-010-1562-z
Felismino AS, Costa EC, Aoki MS, Ferraresi C, de Araujo Moura Lemos TM, de Brito Vieira WH (2014) Effect of low-level laser therapy (808 nm) on markers of muscle damage: a randomized double-blind placebo-controlled trial. Lasers Med Sci 29(3):933–938. doi:10.1007/s10103-013-1430-2
Douris P, Southard V, Ferrigi R, Grauer J, Katz D, Nascimento C, Podbielski P (2006) Effect of phototherapy on delayed onset muscle soreness. Photomed Laser Surg 24(3):377–382. doi:10.1089/pho.2006.24.377
Borges LS, Cerqueira MS, dos Santos Rocha JA, Conrado LA, Machado M, Pereira R, Pinto Neto O (2014) Light-emitting diode phototherapy improves muscle recovery after a damaging exercise. Lasers Med Sci 29(3):1139–1144. doi:10.1007/s10103-013-1486-z
Leal Junior EC, Lopes-Martins RA, Baroni BM, De Marchi T, Rossi RP, Grosselli D, Generosi RA, de Godoi V, Basso M, Mancalossi JL, Bjordal JM (2009) Comparison between single-diode low-level laser therapy (LLLT) and LED multi-diode (cluster) therapy (LEDT) applications before high-intensity exercise. Photomed Laser Surg 27(4):617–623. doi:10.1089/pho.2008.2350
Leal Junior EC, Lopes-Martins RA, Baroni BM, De Marchi T, Taufer D, Manfro DS, Rech M, Danna V, Grosselli D, Generosi RA, Marcos RL, Ramos L, Bjordal JM (2009) Effect of 830 nm low-level laser therapy applied before high-intensity exercises on skeletal muscle recovery in athletes. Lasers Med Sci 24(6):857–863. doi:10.1007/s10103-008-0633-4
De Marchi T, Leal Junior EC, Bortoli C, Tomazoni SS, Lopes-Martins RA, Salvador M (2012) Low-level laser therapy (LLLT) in human progressive-intensity running: effects on exercise performance, skeletal muscle status, and oxidative stress. Lasers Med Sci 27(1):231–236. doi:10.1007/s10103-011-0955-5
Ferraresi C, Dos Santos RV, Marques G, Zangrande M, Leonaldo R, Hamblin MR, Bagnato VS, Parizotto NA (2015) Light-emitting diode therapy (LEDT) before matches prevents increase in creatine kinase with a light dose response in volleyball players. Lasers Med Sci 30(4):1281–1287. doi:10.1007/s10103-015-1728-3
Baroni BM, Rodrigues R, Franke RA, Geremia JM, Rassier DE, Vaz MA (2013) Time course of neuromuscular adaptations to knee extensor eccentric training. Int J Sports Med 34(10):904–911. doi:10.1055/s-0032-1333263
Baroni BM, Rodrigues R, Freire BB, Franke Rde A, Geremia JM, Vaz MA (2015) Effect of low-level laser therapy on muscle adaptation to knee extensor eccentric training. Eur J Appl Physiol 115(3):639–647. doi:10.1007/s00421-014-3055-y
Marginson V, Rowlands AV, Gleeson NP, Eston RG (2005) Comparison of the symptoms of exercise-induced muscle damage after an initial and repeated bout of plyometric exercise in men and boys. J Appl Physiol 99(3):1174–1181. doi:10.1152/japplphysiol.01193.2004
French DN, Thompson KG, Garland SW, Barnes CA, Portas MD, Hood PE, Wilkes G (2008) The effects of contrast bathing and compression therapy on muscular performance. Med Sci Sports Exerc 40(7):1297–1306. doi:10.1249/MSS.0b013e31816b10d5
White JP, Wilson JM, Austin KG, Greer BK, St. John N, Panton LB (2008) Effect of carbohydrate-protein supplement timing on acute exercise-induced muscle damage. J Int Soc Sports Nutr 5:5. doi:10.1186/1550-2783-5-5
Yelland MJ, Schluter PJ (2006) Defining worthwhile and desired responses to treatment of chronic low back pain. Pain Med 7(1):38–45. doi:10.1111/j.1526-4637.2006.00087.x
Leal-Junior EC, Vanin AA, Miranda EF, de Carvalho PT, Dal Corso S, Bjordal JM (2015) Effect of phototherapy (low-level laser therapy and light-emitting diode therapy) on exercise performance and markers of exercise recovery: a systematic review with meta-analysis. Lasers Med Sci 30(2):925–939. doi:10.1007/s10103-013-1465-4
Santos LA, Marcos RL, Tomazoni SS, Vanin AA, Antonialli FC, Grandinetti Vdos S, Albuquerque-Pontes GM, de Paiva PR, Lopes-Martins RA, de Carvalho PT, Bjordal JM, Leal-Junior EC (2014) Effects of pre-irradiation of low-level laser therapy with different doses and wavelengths in skeletal muscle performance, fatigue, and skeletal muscle damage induced by tetanic contractions in rats. Lasers Med Sci 29(5):1617–1626. doi:10.1007/s10103-014-1560-1
Higashi RH, Toma RL, Tucci HT, Pedroni CR, Ferreira PD, Baldini G, Aveiro MC, Borghi-Silva A, de Oliveira AS, Renno AC (2013) Effects of low-level laser therapy on biceps braquialis muscle fatigue in young women. Photomed Laser Surg 31(12):586–594. doi:10.1089/pho.2012.3388
Rossato M, Dellagrana RA, Lanferdini FJ, Sakugawa RL, Lazzari CD, Baroni BM, Diefenthaeler F (2016) Effect of pre-exercise phototherapy applied with different cluster probe sizes on elbow flexor muscle fatigue. Lasers Med Sci 31(6):1237–1244. doi:10.1007/s10103-016-1973-0
Ferraresi C, de Sousa MV, Huang YY, Bagnato VS, Parizotto NA, Hamblin MR (2015) Time response of increases in ATP and muscle resistance to fatigue after low-level laser (light) therapy (LLLT) in mice. Lasers Med Sci 30(4):1259–1267. doi:10.1007/s10103-015-1723-8
Newton MJ, Sacco P, Chapman D, Nosaka K (2013) Do dominant and non-dominant arms respond similarly to maximal eccentric exercise of the elbow flexors? J Sci Med Sport 16(2):166–171. doi:10.1016/j.jsams.2012.06.001
Dattilo M, Antunes HK, Medeiros A, Monico Neto M, Souza HS, Tufik S, de Mello MT (2011) Sleep and muscle recovery: endocrinological and molecular basis for a new and promising hypothesis. Med Hypotheses 77(2):220–222. doi:10.1016/j.mehy.2011.04.017
Sousa M, Teixeira VH, Soares J (2014) Dietary strategies to recover from exercise-induced muscle damage. Int J Food Sci Nutr 65(2):151–163. doi:10.3109/09637486.2013.849662
Acknowledgments
The authors would like to thank to CNPq and UFCSPA by the research scholarships.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare they have no competing interests and the project had no founding sources. The study was approved by the institutional ethics in research committee (protocol number 924944) and was prospectively registered on ClinicalTrials.gov (ID NCT02493556). All volunteers have signed the written informed consent.
Additional information
An erratum to this article is available at http://dx.doi.org/10.1007/s10103-016-2113-6.
Rights and permissions
About this article
Cite this article
Fritsch, C.G., Dornelles, M.P., Severo-Silveira, L. et al. Effects of low-level laser therapy applied before or after plyometric exercise on muscle damage markers: randomized, double-blind, placebo-controlled trial. Lasers Med Sci 31, 1935–1942 (2016). https://doi.org/10.1007/s10103-016-2072-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-016-2072-y