Abstract
The aim of this work was to evaluate the effects of low-level laser therapy (LLLT) on exercise performance, oxidative stress, and muscle status in humans. A randomized double-blind placebo-controlled crossover trial was performed with 22 untrained male volunteers. LLLT (810 nm, 200 mW, 30 J in each site, 30 s of irradiation in each site) using a multi-diode cluster (with five spots - 6 J from each spot) at 12 sites of each lower limb (six in quadriceps, four in hamstrings, and two in gastrocnemius) was performed 5 min before a standardized progressive-intensity running protocol on a motor-drive treadmill until exhaustion. We analyzed exercise performance (VO2 max, time to exhaustion, aerobic threshold and anaerobic threshold), levels of oxidative damage to lipids and proteins, the activities of the antioxidant enzymes superoxide dismutase (SOD) and catalase (CAT), and the markers of muscle damage creatine kinase (CK) and lactate dehydrogenase (LDH). Compared to placebo, active LLLT significantly increased exercise performance (VO2 max p = 0.01; time to exhaustion, p = 0.04) without changing the aerobic and anaerobic thresholds. LLLT also decreased post-exercise lipid (p = 0.0001) and protein (p = 0.0230) damages, as well as the activities of SOD (p = 0.0034), CK (p = 0.0001) and LDH (p = 0.0001) enzymes. LLLT application was not able to modulate CAT activity. The use of LLLT before progressive-intensity running exercise increases exercise performance, decreases exercise-induced oxidative stress and muscle damage, suggesting that the modulation of the redox system by LLLT could be related to the delay in skeletal muscle fatigue observed after the use of LLLT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The metabolism during contractile activity produces reactive oxygen species (ROS) [1], which can lead to muscle oxidative stress [2]. This can be one factor associated with a reduction in contractile function and muscle fatigue develops [3]. To counteract these effects, organisms present antioxidant defenses, such as the enzymes superoxide dismutase (SOD) and catalase (CAT), responsible for the dismutation of the superoxide (O2•-) radical and hydrogen peroxide (H2O2), respectively [2].
Skeletal muscle fatigue is characterized by impairment of muscle ability to generate and maintain force production during muscle activity. In submaximal activities, skeletal muscle fatigue is denoted as a failure to continue activity at the initial intensity [4]. The development of muscle fatigue is a complex and multifaceted process involving several physiological and biomechanical elements [5], including muscle fiber type, the intensity and duration of the activity [6], and oxidative stress [3].
The use of low-level laser therapy (LLLT) and light-emitting diode therapy before exercise has shown positive results in delaying skeletal muscle fatigue [7] and improving skeletal muscle recovery in athletes [8, 9]. These studies were done with a single muscle group and with short-duration exercises at high intensities. However, in sport activities, several muscle groups are involved in the exercise. Therefore, it is important to know the effects of LLLT in more complex and long-duration exercise activities.
In this perspective, the aim of this work was to study LLLT effects on human exercise performance, oxidative stress, and muscle damage in a progressive-intensity running exercise.
Methods
Ethical aspects
The study was approved by the Ethics Committee of the University de Caxias do Sul. In accordance with the Declaration of Helsinki, all subjects were advised about the procedure and they signed an informed consent prior to participation in the study.
Subjects
Twenty-two volunteers were selected for this study. The number of the participants was calculated using a statistical power of 80% and a significance level of p <0.05 (or 5%). The individuals were recruited among healthy untrained male volunteers, with ages between 20 and 25 years, from the University of Caxias do Sul, Brazil. Exclusion criteria included any previous musculoskeletal injury to the hip, knee, or ankle region in the previous 3 months and the use of any kind of nutritional supplements or pharmacological agents.
Randomization and blinding procedures
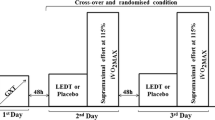
The study was designed as a randomized double-blinded placebo-controlled crossover trial. Randomization was performed by a simple drawing of lots (A or B), which determined whether active LLLT (A) or placebo LLLT (B) would be given at the first exercise session. During the second session, participants were crossed over to receive treatment A or B, i.e., the one which had not been given at the first session. The code from the drawing of lots was delivered to a technician who preset the control unit accordingly to either active LLLT or placebo LLLT mode. The technician was instructed to not communicate the type of treatment neither to the participants nor to the therapist applying the LLLT source to the lower limbs. Thus, the allocation of treatments was concealed to the participants and the therapist. Blinding was further maintained by the use of opaque goggles by participants and the therapist during LLLT procedures.
Low-level laser therapy
LLLT was performed exactly 5 min before the progressive running protocol. The therapy was applied by using a multi-diode cluster (with five diode spots; THOR® Photomedicine, London, UK) at 12 sites of each lower limb (six in quadriceps: two centrally - musculus rectus femoris and musculus vastus intermedius, two laterally - musculus vastus lateralis, and two medially - musculus vastus medialis; four in hamstrings; and two in gastrocnemius), as shown in Fig. 1. LLLT characteristics and application mode are shown in Table 1. The laser device was calibrated before and after data acquisition, the equipment showed the same power output in both calibrations. The optical power was measured using a Newport multifunction optical meter model 1835 C. The stability of the laser during the laser irradiation was measured collecting light with a partial reflect (4%). The dose was chosen based on previous studies of our research group. In these studies we observed an effective decrease in muscle damage induced by short-duration and high-intensity exercise involving a single muscle group [16, 19].
Exercise protocol
Subjects performed a standardized progressive running protocol on a motor-driven treadmill with a fixed inclination of 1%. The initial velocity was 3 km.h-1 during the first 3 min (warm-up phase). After the warm-up phase, the velocity was increased 1 km.h-1 at each minute until it reached 16 km.h-1. Volunteers performed the exercise protocol until exhaustion. The exercise protocol could be finished at any moment, if the volunteers asked (by hand sign). After the exercise protocol, volunteers performed a recovery phase with a velocity of 6 km.h-1 for 3 min. During the exercise protocol, the rates of oxygen uptake (VO2), dioxide carbon production (CO2) (measured using a VO2000 gas analyzer, Inbrasport®, Brazil), total time until exhaustion, and heart rate (measured using a digital electrocardiograph from Micromed®, Brazil) were monitored. Aerobic and anaerobic thresholds were also measured through the methodology proposed by Wasserman et al. [10].
Blood samples and biochemical assays
Blood samples were collected by a qualified nurse blinded to group allocation and were obtained from an antecubital vein before exercise and exactly 5 min after the end of the progressive exercise protocol. Blood was centrifuged at 2,700 × g for 10 min at 4°C. Serum was immediately pipetted into Eppendorf tubes and stored at −80°C until analysis. Lipid damages were measured spectrophotometrically (Shimadzu spectrophotometer Model UV-1700, Shimadzu®, Japan) by determining thiobarbituric acid reactive substances (TBARS) as previously described by Wills [11]. Results were expressed as nmol per ml. The oxidative damage to proteins was assessed by determining carbonyl groups based on the reaction with 2,4-dinitrophenylhydrazine (DNPH), as previously described by Levine et al. [12]. Results were expressed as DNPH nmol per mg of proteins. SOD activity was determined measuring the inhibition of the rate of auto-catalytic adrenochrome formation at 480 nm (Shimadzu spectrophotometer Model UV-1700, Shimadzu®, Japan), in a reaction medium containing 1 mM adrenaline (pH 2.0) and 50 mM glycine (pH 10.2), (both from E. Merck) as described by Bannister and Calabrese [13]. This reaction was conducted at 30°C for 3 min and the results were expressed as units per gram of protein. One SOD unit was defined as the amount of enzyme that inhibits the rate of adrenochrome formation by 50% per gram of protein. CAT activity was measured according to the method described by Aebi [14]. The assay principle is based on determining the rate of hydrogen peroxide (E. Merck) decomposition at 240 nm (Shimadzu spectrophotometer Model UV-1700, Shimadzu®, Japan). This reaction was conducted at 30°C for 1 min and the results were expressed as CAT units per milligram of protein. One unit of CAT decomposed one μmol of hydrogen peroxide per mg of protein per minute at pH 7.4. Total protein levels were evaluated using the Total Proteins kit from Labtest® (Protein Kit, Labtest Diagnostica S.A., Brazil). CK and LDH activity were measured by using a commercial kit (CK - Labtest® - Brazil, LDH - Bioclin® - Brazil). CK catalyzes the dephosphorylation of creatine phosphate to produce adenosine triphosphate, which reacts with glucose in the presence of hexokinase forming glucose-6-phosphate. Glucose-6-phosphate by glucose-6-phosphate dehydrogenase is oxidized to phosphogluconate and reduces NADP+ to NADPH. The rate of increase in absorbance at 340 nm is proportional to CK activity in the sample. The LDH catalyzes the reduction of pyruvate with NADH, resulting in lactate and NAD+. The catalytic concentration is determined from the rate of decomposition of NADH, measured by the decrease in absorptive at 340 nm. Results were expressed as units per liter-1.
Statistical analysis
Data from exercise protocol, oxidative stress and muscle damage markers were expressed as mean and standard deviation (± SD) and tested statistically by a two-sided paired t-test through the software SPSS 18.0 for Windows. The significance level was set at p < 0.05.
Results
Volunteers in this study were 22.02 ± 3.02 years old, body weight was 74.22 ± 11.54 kg with a height of 176.30 ± 7.75 cm. Results of the progressive-intensity running exercise with and without LLLT application are shown in Table 2. Total time to reach exhaustion during exercise protocol, as well as oxygen consumption - VO2 max (both in absolute than in relative values) were significantly increased (p< 0.05) by pre-exercise irradiation with active LLLT. On the other hand, aerobic and anaerobic thresholds did not change with LLLT.
The progressive-intensity running exercise induced an increase in lipid (TBARS) and protein oxidative damages, as well as a decrease in SOD activity. The application of laser in the active LLLT group prevents these changes, avoiding the damages to the biomolecules and the decrease in the antioxidant enzyme SOD. Neither the progressive-intensity running exercise nor the active LLLT changed post-exercise CAT activity (Table 3). Baseline values for TBARS, protein oxidative damages, SOD and CAT was similar without significant difference (p > 0.05) in both treatments tested.
Pre-exercise activity of CK (active LLLT 151.74 ± 45.15 U/l-1, placebo LLLT 150.10 ± 48.60 U/l-1) and LDH (active LLLT 281.89 ± 44.36 U/l-1, placebo LLLT 274.93 ± 37.62 U/l-1) were similar in two sections of exercise (with and without LLLT). Progressive-intensity running exercise increases CK (active LLLT 178.26 ± 82.36, placebo LLLT 290.42 ± 127.11) and LDH (active LLLT 276.80 ± 32.86, placebo LLLT 332.72 ± 63.07) activities. Active LLLT reduces the increase in the activities of these enzymes, as shown in Figs. 2 and 3.
Discussion
Several animal and human trials have shown positive effects of LLLT on inflammatory disorders both in acute and in chronic phases [15]. However, skeletal muscle fatigue and post-exercise recovery are new areas of research in LLLT and few studies have been performed on this subject. To our knowledge, this is the first study to examine the effects of LLLT applied previously to complex exercise involving several muscle groups. Studies have shown positive effects of red and infrared LLLT in reducing markers of fatigue and muscle damage [7, 8, 9, 16, 19]. However, we decided to use LLLT (810 nm), because infrared LLLT has greater skin penetration than red LLLT [17].
We observed that pre-exercise irradiation in healthy untrained subjects submitted to LLLT significantly increased VO2 max both in absolute and in relative values, as well as the time to reach exhaustion (Table 2). In spite of a low increase of 14 s in exercise length, this enhancement seems to be clinically relevant, considering that in the last 10 years the world record 5,000-meter running (of approx. 13 min) improved only 12 s [18]. It illustrates how difficult it is to improve exercise performance at this level of effort. On the other hand, we did not observe any significant changes in aerobic and anaerobic thresholds (Table 2), which means that although LLLT leads to performance improvement, it does not change the metabolic pathway of energy production.
Pre-exercise irradiation with LLLT also decreased the post-exercise improvement in the activities of CK and LDH (Figs. 2 and 3), which means that LLLT can protect skeletal muscle against exercise-induced damage in progressive-intensity long-duration exercises, which could, at least in part, explain the performance improvement observed in our study. Our findings corroborate previous studies that used pre-exercise LLLT irradiation in single muscle groups to decrease exercise-induced muscle damage [16, 19].
When volunteers did not receive pre-exercise active LLLT, an increase in oxidative damage, both in lipids and in proteins (Table 3) was observed, corroborating other studies [20, 21]. Active LLLT can prevent the exercise-induced increase in oxidative damage both in lipids and in proteins and prevent the decrease in SOD antioxidant activity (Table 3). Previous studies have already shown that LLLT can modulate SOD activity [22, 23] or stimulate its synthesis in the cell [24]. This redox modulation can explain, at least in part, the decrease in oxidative damage observed in our study. On the other hand, no statistical difference in CAT activity was observed after the progressive-intensity running exercise. Some studies show that acute exercise did not cause any changes in the concentrations of CAT [25, 26], corroborating our findings. Other studies about this biochemical parameter should be evaluated to confirm this data.
The mechanisms through which ROS play a role in the development of fatigue are not fully understood [4], however, it is known that oxidative stress leads to an impairment of the contractile muscle function resulting in muscle fatigue [3]. Therefore, our findings suggest that the reduction of oxidative stress is related to LLLT delaying skeletal muscle fatigue and protecting against exercise-induced damage. Others mechanisms for LLLT effects could include the increase in microcirculation, potential anti-inflammatory activity, and improvement of mitochondrial function [27, 28].
Conclusions
As far as we know, this is the first investigation about the effects of LLLT on a complex exercise. It is possible to conclude that pre-exercise LLLT application decreases oxidative stress leading to a delay in the development the skeletal muscle fatigue, improvement of skeletal muscle performance, and prevention of muscle damage. Optimal parameters of application and dose-response patterns still need to be identified in further studies.
References
Almar M, Villa JG (2002) Urinary levels of 8-hydroxydeoxyguanosine as a marker of oxidative damage in road cycling. Free Radic Res 36:247–253
Halliwell B, Gutteridge JC (2000) Free Radicals in Biology and Medicine. New York, Oxford
Reid MB, Haack KE, Franchek KM, Valberg PA, Kobzik L, West MS (1992) Reactive oxygen in skeletal muscle. I. Intracellular oxidant kinetics and fatigue in vitro. J Appl Physiol 73:1797–1804
Allen DG, Lamb GD, Westerblad H (2008) Skeletal muscle fatigue: cellular mechanisms. Physiol Rev 88:287–332
Ivey FM, Roth SM, Ferrell RE, Tracy BL, Lemmer JT, Hurlbut DE, Martel GF, Siegel EL, Fozard JL, Jeffrey Metter E, Fleg JL, Hurley BF (2000) Effects of age, gender, and myostatin genotype on the hypertrophic response to heavy resistance strength training. J Gerontol A Biol Sci Med Sci 55:641–648
Lamb GD, Stephenson DG, Bangsbo J, Juel C (2006) Point: Counterpoint: Lactic acid accumulation is an advantage/disadvantage during muscle activity. J Appl Physiol 100:1410–1414
Leal Junior EC, Lopes-Martins RA, Dalan F, Ferrari M, Sbabo FM, Generosi RA, Baroni BM, Penna SC, Iversen VV, Bjordal JM (2008) Effect of 655-nm low-level laser therapy on exercise-induced skeletal muscle fatigue in humans. Photomed Laser Surg 26:419–424
Leal Junior EC, Lopes-Martins RA, Baroni BM, De Marchi T, Rossi RP, Grosselli D, Generosi RA, de Godoi V, Basso M, Mancalossi JL, Bjordal JM (2009) Comparison between single-diode low-level laser therapy (LLLT) and LED multi-diode (cluster) therapy (LEDT) applications before high-intensity exercise. Photomed Laser Surg 27:617–623
Leal Junior EC, Lopes-Martins RA, Baroni BM, De Marchi T, Taufer D, Manfro DS, Rech M, Danna V, Grosselli D, Generosi RA, Marcos RL, Ramos L, Bjordal JM (2009) Effect of 830 nm low-level laser therapy applied before high-intensity exercises on skeletal muscle recovery in athletes. Lasers Med Sci 24:857–863
Wasserman K, Hansen JE, Sue DY, Whipp BJ (1987) Principles of Exercise Testing and Interpretation. Lea & Febiger, Philadelphia
Wills ED (1996) Mechanism of lipid peroxide formation in animal tissues. Biochem J 99:667–676
Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, Ahn BW, Shaltiel S, Stadtman ER (1990) Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol 186:464–478
Bannister JV, Calabrese L (1987) Assays for superoxide dismutase. Methods Biochem Anal 32:279–312
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Huang YY, Chen AC, Carroll JD, Hamblin MR (2009) Biphasic dose response in low-level light therapy. Dose Response 7:358–383
Leal Junior EC, Lopes-Martins RA, Frigo L, De Marchi T, Rossi RP, de Godoi V, Tomazoni SS, da Silva DP, Basso M, Lotti Filho P, Corsetti FV, Iversen VV, Bjordal JM (2010) Effects of low-level laser therapy (LLLT) in the development of exercise-induced skeletal muscle fatigue and changes in biochemical markers related to post-exercise recovery. J Orthop Sports Phys Ther 40:524–532
Enwemeka CS (2009) Intricacies of dose in laser phototherapy for tissue repair and pain relief. Photomed Laser Surg 27:387–393
International Association of Athletics Federations – IAAF (2010) Available at: http://www.iaaf.org/statistics/toplists/index.html. Accessed: August 20
Leal Junior EC, Lopes-Martins RA, Rossi RP, De Marchi T, Baroni BM, de Godoi V, Marcos RL, Ramos L, Bjordal JM (2009) Effect of cluster multi-diode light emitting diode therapy (LEDT) on exercise-induced skeletal muscle fatigue and skeletal muscle recovery in humans. Lasers Surg Med 41:572–577
Alessio HM (1993) Exercise-induce oxidative stress. Med Sci Sports Exerc 25:218–224
Reid MB (2008) Free Radicals and muscle fatigue: Of ROS, canaries, and the IOC. Free Radic Biol Med 44:169–179
Fillipin LI, Mauriz JL, Vedovelli K, Moreira AJ, Zettler CG, Lech O, Marroni NP, González-Gallego J (2005) Low-level laser therapy (LLLT) prevents oxidative stress and reduces fibroses en rat traumatized Achilles tendon. Lasers Surg Med 37:293–300
Liu XG, Zhou YJ, Liu TC, Yuan JQ (2009) Effects of low-level laser irradiation on rat skeletal muscle injury after eccentric exercise. Photomed Laser Surg 27:863–869
Lubart R, Eichler M, Lavi R, Friedman H, Shainberg A (2005) Low-energy laser irradiation promotes cellular redox activity. Photomed Laser Surg 23:3–9
Ji LL (1995) Exercise and oxidative stress: role of the cellular antioxidant system. Exerc Sport Sci Rev 23:135–166
Powers SK, Jackson MJ (2008) Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev 88:1243–1276
Tullberg M, Alstergren PJ, Ernberg MM (2003) Effects of low-power laser exposure on masseter muscle pain and microcirculation. Pain 105:89–96
Xu X, Zhao X, Liu TC, Pan H (2008) Low-intensity laser irradiation improves the mitochondrial dysfunction of C2C12 induced by electrical stimulation. Photomed Laser Surg 26:197–202
Acknowledgments
The authors would like to thank the volunteers who participated in the study, the staff of the Laboratories of Oxidative Stress and Antioxidants and the Sports Medicine Institute, especially Juliano Augusto Ziembowicz and Luciana Maria Machado. We also thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES-Brazil) and Caxias do Sul University for their support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
De Marchi, T., Leal Junior, E.C.P., Bortoli, C. et al. Low-level laser therapy (LLLT) in human progressive-intensity running: effects on exercise performance, skeletal muscle status, and oxidative stress. Lasers Med Sci 27, 231–236 (2012). https://doi.org/10.1007/s10103-011-0955-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-011-0955-5