Abstract
The purpose of the present study was to determine the effect of low level laser therapy (LLLT) treatment before knee extensor eccentric exercise on indirect markers of muscle damage. Thirty-six healthy men were randomized in LLLT group (n = 18) and placebo group (n = 18). After LLLT or placebo treatment, subjects performed 75 maximal knee extensors eccentric contractions (five sets of 15 repetitions; velocity = 60° seg−1; range of motion = 60°). Muscle soreness (visual analogue scale—VAS), lactate dehydrogenase (LDH) and creatine kinase (CK) levels were measured prior to exercise, and 24 and 48 h after exercise. Muscle function (maximal voluntary contraction—MVC) was measured before exercise, immediately after, and 24 and 48 h post-exercise. Groups had no difference on kineanthropometric characteristics and on eccentric exercise performance. They also presented similar baseline values of VAS (0.00 mm for LLLT and placebo groups), LDH (LLLT = 186 IU/l; placebo = 183 IU/l), CK (LLLT = 145 IU/l; placebo = 155 IU/l) and MVC (LLLT = 293 Nm; placebo = 284 Nm). VAS data did not show group by time interaction (P = 0.066). In the other outcomes, LLLT group presented (1) smaller increase on LDH values 48 h post-exercise (LLLT = 366 IU/l; placebo = 484 IU/l; P = 0.017); (2) smaller increase on CK values 24 h (LLLT = 272 IU/l; placebo = 498 IU/l; P = 0.020) and 48 h (LLLT = 436 IU/l; placebo = 1328 IU/l; P < 0.001) post-exercise; (3) smaller decrease on MVC immediately after exercise (LLLT = 189 Nm; placebo = 154 Nm; P = 0.011), and 24 h (LLLT = 249 Nm; placebo = 205 Nm; P = 0.004) and 48 h (LLLT = 267 Nm; placebo = 216 Nm; P = 0.001) post-exercise compared with the placebo group. In conclusion, LLLT treatment before eccentric exercise was effective in terms of attenuating the increase of muscle proteins in the blood serum and the decrease in muscle force.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Theodore Hough, in 1902, suggested that certain types of exercise could cause microdamage on muscle cells structure, establishing the concept of exercise-induced muscle damage (Hough 1902). Currently, damage has been suggested to happen when subjects perform an unaccustomed physical activity. Eccentric actions induce higher levels of muscle damage (Morgan and Allen 1999; Proske and Morgan 2001; Byrne et al. 2004; Howatson and van Someren 2008), mainly when performed on the descending limb of the force–length relationship, as proposed by the popping-sarcomere theory (Morgan 1990). The most vulnerable structure to damage induced by exercise seems to be the Z-line, with damage also occurring on the sarcoplasmic membrane, sarcoplasmic reticulum, T-tubules, myofibrils and cytoskeletal system (in particular, titin and desmin) (Morgan and Allen 1999; Fridén and Lieber 2001).
This microscopic structural damage promotes functional impairment on the whole muscle, resulting in a decrease of force production capacity, change in force production optimal length and increase of passive tension (Morgan and Allen 1999; Proske and Morgan 2001). Delayed onset muscle soreness (DOMS), increase of muscle proteins in the blood, swelling and inflammatory response are also associated with damage (Morgan and Allen 1999; Proske and Morgan 2001; Clarkson and Hubal 2002; Cheung et al. 2003). Altogether, these changes lead to limitation of athletic performance (Byrne et al. 2004) and increased risk of injury (Cheung et al. 2003).
Direct verification of muscle damage in humans is possible using biopsy or magnetic resonance images. While biopsy may underestimate or overestimate the real damage, since the results of a small tissue fraction are extrapolated to the whole muscle, evaluation of magnetic resonance images still generates controversy related to the methods of image analysis in determining muscle damage (Clarkson and Hubal 2002). Therefore, most of the research in the area has used indirect markers of muscle damage. DOMS estimation is probably the most used tool for measuring muscle damage in humans, followed by the determination of muscle proteins in the blood (especially creatine kinase—CK) and the assessment of muscle force, which is considered as one of the best ways for measuring the damage magnitude (Warren et al. 1999).
Parallel to understanding the mechanisms of exercise-induced muscle damage, a growing interest in determining effective strategies to reduce their undesirable effects has been observed. Careful reviews (Cheung et al. 2003; Howatson and van Someren 2008), evaluating preventive and therapeutic modalities against muscle damage effects, showed the efficacy of strategies involving exercise, nutritional supplementation and pharmacological therapy. However, the lack of scientific evidence of some methods widely used in the field (e.g. cryotherapy, massage, stretching and electrical stimulation) emphasizes the importance of studying innovative non-invasive therapies in this area.
Therapeutic action of low level laser therapy (LLLT) has been the focus of research since the 1960s. The mechanism of action of this therapy on tissues has been attributed to the ability of light energy to be absorbed by cells, stimulating or inhibiting intracellular processes according to the parameters used (Huang et al. 2009). Numerous studies have reported the effects of phototherapy on cutaneous wound healing (Al-Watban et al. 2007); regeneration of tendinous (Oliveira et al. 2009), muscle (Cressoni et al. 2008) and nervous tissues (Rochkind et al. 2009); and analgesic (Chow et al. 2009) and anti-inflammatory action (Yamaura et al. 2009).
Recent studies also showed the effectiveness of phototherapy in reducing muscle damage in animal experiments (Liu et al. 2009; Sussai et al. 2010; Leal Junior et al. 2010), corroborating previous findings observed in humans on DOMS (Douris et al. 2006) and blood markers (Leal Junior et al. 2009). These promising results perhaps can be explained by the anti-inflammatory effects of phototherapy (Yamaura et al. 2009), such as the ability of this therapy to reduce reactive oxygen species release (Rizzi et al. 2006), increase antioxidant capacity (Avni et al. 2005) and improve mitochondrial function (Xu et al. 2008).
Therefore, given the need for effective techniques against the undesirable effects of exercise-induced muscle damage and the promising results involving phototherapy, the purpose of this study was to determine the effects of LLLT (810 nm) before knee extensor eccentric exercise on DOMS, muscle proteins in blood and muscle function up to 48 h after exercise.
Methods
Subjects
Thirty-six male subjects aged between 19 and 35 years agreed to participate in the study through a written informed consent. All were healthy and physically active students, enrolled in recreational sports, but not competing at any specific level. They had no recent experience with isokinetic eccentric exercise, were not enrolled in strength training programs of the lower body and declared to be non-users of nutritional supplements. Volunteers agreed to not perform physical exercise or use any other therapeutic modalities and avoided the consumption of alcoholic beverages between data collection sessions.
Experimental design
This study was approved by the university Ethics in Research Committee and was designed as a randomized double-blind placebo-controlled trial to verify the effects of LLLT before eccentric exercise on indirect markers of muscle damage. Subjects were randomized in LLLT group (n = 18) and placebo group (n = 18) by a technician responsible for LLLT or placebo application. Researchers were blinded to the subjects’ allocation to the groups, and were not present in the room during treatment application. Similarly, volunteers were blinded to LLLT or placebo applications, since both treatments did not result in any thermal stimulus for the subjects.
Procedures
Each volunteer completed three visits to the laboratory with an interval of 24 ± 1 h between visits. In the first session, volunteers were subjected to (1) kineanthropometric evaluation; (2) quadriceps muscle soreness evaluation through a visual analogue scale (VAS); (3) blood sampling for subsequent measurement of serum levels of lactate dehydrogenase (LDH) and creatine kinase (CK); (4) muscle function evaluation through maximal voluntary contraction (MVC) of knee extensors before eccentric exercise; (5) LLLT or placebo treatment; (6) eccentric exercise protocol; and (7) muscle function evaluation through knee extensors MVC immediately after eccentric exercise.
In the following sessions (24 and 48 h post-eccentric exercise), subjects returned to the laboratory for (1) blood sampling (LDH and CK); (2) quadriceps soreness evaluation (VAS); and (3) muscle function evaluation (MVC).
Kineanthropometry
All body composition evaluations were performed by the same technician (level II of the International Society for the Advancement of Kineanthropometry—ISAK) using procedures established by ISAK (Marfell-Jones et al. 2006). Height, weight, length, diameter, perimeter and skinfold measurements allowed fractioning of total body mass into five components (fat, muscle, residual, bone and skin mass), which were used for group comparison as an additional way to ensure the group homogeneity.
Muscle soreness–VAS
A visual analogue scale (VAS) was used to assess the volunteers’ muscle soreness. In each evaluation, the same researcher instructed subjects in a standardized manner to perform a sub-maximal voluntary isometric knee extensor contraction, marking a vertical line at the scale point that best reflected their muscle soreness. The 100-mm horizontal line of the VAS did not have any marks or numbers, having just indications of “no soreness” at beginning and “extreme soreness” at end of the line. The soreness quantification was determined by the distance between the initial point line (0 mm) and the point marked by the subject (French et al. 2008; White et al. 2008; Wilson et al. 2009).
Blood markers—LDH and CK
A licensed professional collected approximately 10 ml of blood from a suitable antecubital forearm vein before exercise, and 24 and 48 h after exercise. Blood was carefully transferred to evacuated blood collection tubes and centrifuged at 3,000 rpm for 20 min. Serum samples were removed and stored at −80°C until subsequent analysis. Enzymatic activity of lactate dehydrogenase (LDH) and creatine kinase (CK) were determined in duplicate using commercially available kinetic UV assay kits (Labtest Diagnóstica, Lagoa Santa–MG, Brazil) according to the manufacturer’s guidelines. The mean values obtained between the two samples were used for statistical analysis.
Muscle function–MVC
Subjects were properly positioned with the dominant lower limb on the Isokinetic Dynamometer Biodex System 3 Pro (Biodex Medical System, Shirley–NY, USA), following the manufacturer’s recommendations for evaluation of knee flexion–extension movements. Before each evaluation a warm-up (10 concentric knee flexion–extension repetitions at 180° seg−1 and maximal range of motion) was performed. Muscle function was assessed through the highest torque value obtained among three 5-s maximal voluntary contractions (MVC) at 60° of knee flexion (0° = full knee extension). Two-minute rest between each MVC was observed to minimize possible fatigue effects. Volunteers were previously instructed to perform maximal force and verbal encouragement was given by researchers in each MVC.
Treatment–LLLT or placebo
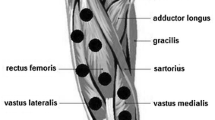
Both treatments were applied using the equipment THOR DD2 Control Unit (THOR®–London, UK), with the Infra-Red Laser Cluster Probe of the same manufacturer, consisting of five 810 nm diodes, each one with 0.029 cm2 area and 200 mW output power. Placebo treatment was performed exactly the same way as LLLT treatment, but with the device turned off. Treatments were performed before eccentric exercise with the probe held stationary in skin contact at a 90° angle with slight pressure. Figure 1 illustrates the six application points, defined by palpation of muscle bellies: two points on the distal region of vastus medialis; two points on the distal region of vastus lateralis; and two points on the central region of rectus femoris. LLLT or placebo treatment was applied for 30 s in each point. In this way, 30 J were applied per point (6 J each diode), resulting in a total of 180 J for the whole quadriceps muscle.
Eccentric exercise protocol
In the first session, a 2-min rest was observed between the last MVC and the eccentric exercise familiarization. Thus, five sub-maximal knee extensor eccentric contractions were used to familiarize subjects with the movement, angular velocity and range of motion of the subsequent eccentric protocol used to induce muscle damage. Before each eccentric contraction, the volunteer’s limb was passively extended to 30° of knee flexion. Each subject was encouraged to promote a knee extensor contraction as soon as the dynamometer arm reached this position. In response to the subject’s extensor torque, the dynamometer droved the segment to 90° of knee flexion (range of motion = 60°) at an angular velocity of 60° seg−1. One minute after familiarization, LLLT or placebo treatment were administered as previously described.
After treatment, a 2-min rest period was observed before starting the eccentric exercise protocol. Muscle damage was induced through 75 maximal knee extensor eccentric contractions, which were divided in five sets of 15 repetitions with 30 s’ rest between them. The exercise was performed at an angular velocity of 60° seg−1 (both passive and eccentric phases) and with a 60° range of motion (30°–90° of knee flexion). Thus, each eccentric contraction lasted 1 s, followed by 1-s rest during which the limb was passively extended, with each set lasting 30 s. Immediately after eccentric exercise, the dynamometer automatically positioned the lower limb at a knee joint angle of 60°, and about 10 s after exercise was finished, a new 5-s knee extensor MVC was performed. The MVC peak torque value was considered the volunteer’s maximal force production capacity immediately after exercise.
Statistical analyses
Kineanthropometric data of LLLT and placebo groups were compared through a One-Way ANOVA. The same statistical procedure was used to compare the eccentric exercise performance through the knee extensors peak torque per set and the knee extensors work per set.
A Two-Way ANOVA (group × time) was used to compare each of muscle soreness (VAS), enzymatic activity of lactate dehydrogenase (LDH), enzymatic activity of creatine kinase (CK) and muscle function (MVC). The same statistical procedure was adopted to compare the percentage change of LDH, CK and MVC. Data sphericity was tested through Mauchly’s Test, and Greenhouse-Geisser correction factor was used when necessary. When interaction was observed, a repeated measures ANOVA was used within each group to determine changes between variables along the time, followed by a Bonferroni post-hoc test. In addition, a One-Way ANOVA was used within each time period (pre-exercise, immediately after exercise, 24 and 48 h after exercise) to compare the groups.
All statistical analyses were performed with an alpha level of P < 0.05. Results are presented in the text and tables as mean ± standard deviation and in the figures as mean ± standard error.
Results
Significant differences were not observed between groups for kineanthropometric variables (P > 0.05; Table 1). There was no difference (P > 0.05) between groups on peak torque of knee extensors per set or knee extensor work per set (i.e. eccentric exercise performance). LLLT and placebo groups showed the following eccentric peak torque values: set 1 (303.40 ± 47.02 Nm; 298.14 ± 55.65 Nm); set 2 (275.91 ± 51.26 Nm; 274.97 ± 55.52 Nm); set 3 (262.28 ± 59.63 Nm; 265.74 ± 56.09 Nm); set 4 (262.38 ± 61.67 Nm; 252.63 ± 42.18 Nm); and set 5 (249.45 ± 60.25 Nm; 245.73 ± 41.84 Nm). LLLT and placebo groups presented the following eccentric knee extensor work values: set 1 (3009.75 ± 438.77 J; 3002.92 ± 734.94 J); set 2 (2619.01 ± 534.74 J; 2713.37 ± 768.15 J); set 3 (2555.24 ± 682.59 J; 2532.51 ± 746.94 J); set 4 (2414.64 ± 699.33 J; 2354.39 ± 535.88 J); and set 5 (2313.24 ± 744.49 J; 2142.40 ± 580.59 J).
Only data from the VAS did not show group by time interaction (P = 0.066). VAS values were similar between groups (P = 0.071) and increased with time in both groups (Table 2). Groups were also similar (P > 0.05) on baseline values for all the other muscle damage markers (Table 2). However, LLLT group presented (1) smaller increase of absolute (Table 2; P = 0.017) and percentage (Fig. 2; P = 0.013) LDH values 48 h after exercise; (2) smaller increase of absolute (Table 2; P = 0.020) and percentage (Fig. 2; P = 0.023) CK values 24 h after exercise; (3) smaller increase of absolute (Table 2; P < 0.001) and percentage (Fig. 2; P = 0.001) CK values 48 h after exercise; (4) smaller fall of absolute (Table 2; P = 0.011) and percentage (Fig. 3; P = 0.031) MVC values immediately after exercise; (5) smaller fall of absolute (Table 2; P = 0.004) and percentage (Fig. 3; P = 0.002) MVC values 24 h after exercise; and (6) smaller fall of absolute (Table 2; P = 0.001) and percentage (Fig. 3; P = 0.001) MVC values 48 h after exercise compared with the placebo treatment group.
Discussion
The aim of the present study was to verify the effect of LLLT before knee extensor eccentric exercise on the most commonly used indirect markers of muscle damage in humans: muscle soreness, blood circulating proteins and impairment of muscle function. VAS data did not show group by time interaction, indicating that treatments promoted similar effects on muscle soreness. However, results indicated that subjects treated with LLLT before eccentric exercise show attenuation of some indirect markers of muscle damage: LLLT group showed a smaller increment of LDH levels 48 h after exercise, a smaller increment of CK levels 24 and 48 h after exercise, as well as a less marked decrease in force immediately after exercise and a better recovery of muscle function 24 and 48 h post-exercise.
Muscle soreness subjective perception is the most used measurement tool in studies involving exercise-induced muscle damage in humans (Warren et al. 1999). Although VAS is a validated instrument largely used in muscle damage studies (Cheung et al. 2003), several authors reported data with high dispersion in soreness perception (Brown et al. 1997; French et al. 2008; White et al. 2008). This variability makes it difficult to obtain significant results from a statistical point of view, as observed in this trial. Previous studies showed mixed results about the effects of LLLT on DOMS. While Douris et al. (2006) applied phototherapy after arm eccentric exercise each day for five consecutive days and found a reduction of DOMS, Craig et al. (1999) did not observed beneficial effects of this therapy. In the present study, even though the statistical approach indicated no protective effect of LLLT on DOMS, a trend in favour of this therapy can be observed in the VAS mean values.
Since intensity of exercise (Chen et al. 2007), number of repetitions (Brown et al. 1997), angular velocity (Chapman et al. 2006), range of motion (Váczi et al. 2009), and the use of upper or lower limbs (Jamurtas et al. 2005) directly influence damage magnitude, comparison with studies that used different exercise protocols is difficult. In addition, due to the large discrepancy of CK peak values observed in response to eccentric exercise (236–25.244 IU/l) (Nosaka and Clarkson 1996), some researchers have chosen to classify subjects into low and high responders (Totsuka et al. 2002), while some studies minimize this discrepancy through logarithmical transformations (Brown et al. 1997; French et al. 2008). One possibility to decrease variability would be to perform a crossover experimental design, which was not done in the present study due to the frequent reports in the literature about the protective effect promoted by repeated bouts of eccentric exercise (Brown et al. 1997; Nosaka et al. 2001). Therefore, subjects were selected with similar physical characteristics and randomized into two groups that, according to Table 1, presented similar kineanthropometric characteristics. In addition, a higher sample size per group than most studies found in the literature was used in order to minimize this heterogeneity (Brown et al. 1997; Nosaka et al. 2001; Jamurtas et al. 2005; Chapman et al. 2006; Chen et al. 2007; French et al. 2008; White et al. 2008; Wilson et al. 2009).
The smaller increase in blood markers after eccentric exercise in LLLT group agrees with recent reports (Liu et al. 2009; Leal Junior et al. 2009; Sussai et al. 2010; Leal Junior et al. 2010). Liu et al. (2009) showed histological and biochemical evidence that phototherapy reduces muscle damage in the gastrocnemius muscle of rats subjected to downhill running. Sussai et al. (2010) demonstrated that this therapy attenuates CK levels and apoptosis during forced swimming in rats. Leal Junior et al. (2010), in a study of the effects of LLLT on muscle fatigue, also found smaller CK activity after electrically stimulated contractions in the tibialis anterior muscle of rats treated with LLLT. Moreover, in humans, Leal Junior et al. (2009) observed lower signs of damage (CK) and inflammatory response (C-reactive protein) immediately after exercise when athletes were treated with phototherapy previously to exercise using free weights.
Other human studies used LLLT after eccentric exercise for the treatment of muscle damage (Craig et al. 1999; Douris et al. 2006). However, we chose to apply a single LLLT treatment immediately before eccentric exercise as a preventative measure against muscle damage. Thus, we tried to verify possible effects of this therapy on isometric MVC immediately after exercise, when the fall in force production is due to both muscle damage and fatigue (Proske and Morgan 2001). A previous study from our group, focused on phototherapy effects on exercise-induced muscle fatigue, showed attenuation in the fall of quadriceps MVC values immediately after isokinetic concentric exercise when volunteers were treated with this therapy pre-exercise (Baroni et al. 2010). The main findings of the present study confirm these previous results, reinforcing the effectiveness of phototherapy on decreasing muscle fatigue independent of the contraction type.
While impairments of force production capacity due to fatigue are completely reversed at relatively short time periods (a few hours), damaged muscles need several days for total functional recovery (Hough 1902; Clarkson and Hubal 2002). Thus, MVC values from 24 to 48 h after exercise show the LLLT effect specifically on exercise-induced muscle damage, since maximal isometric torque evaluation is one of the most reliable ways to assess this phenomenon (Warren et al. 1999). In this way, reported results showed that impairments to muscle function until 2 days after eccentric exercise were considerably attenuated by LLLT treatment, a finding with an interesting practical applicability to sports medicine. However, recovery of force levels was measured only up to 48 h after eccentric exercise, and maximal isometric torques remained lower than baseline values at this time. It would be interesting in future studies to assess recovery for a longer period of time in order to determine the time needed by LLLT and placebo groups to completely restore their muscle function.
The exact mechanism of action by which LLLT acts on the reduction of muscle damage remains to be established. The present study was not designed at identifying these mechanisms, and the evaluated outcomes do not allow for direct determination of these mechanisms. However, some inferences may be made from etiologic knowledge of damage, which has been divided into two stages: the primary damage, caused mainly by mechanical stress (Howatson and van Someren 2008); and the secondary damage, caused by a cascade of metabolic events triggered from Ca2+ homeostasis disruption (Morgan and Allen 1999; Howatson and van Someren 2008), and that promotes the inflammatory response (Clarkson and Hubal 2002) and the deleterious action of reactive oxygen species (Nikolaidis et al. 2008), among others. Thus, when phototherapy is applied after exercise (e.g. Craig et al. 1999; Douris et al. 2006), this therapy acts just on the secondary damage stage, whilst the treatment prior to exercise influences the intracellular processes since the primary damage stage.
Evidence about the anti-inflammatory effects of phototherapy have been shown in the literature (Yamaura et al. 2009), such as the effect of LLLT in reducing the release of reactive oxygen species (Rizzi et al. 2006) and in increasing antioxidant capacity (Avni et al. 2005). In addition, phototherapy improves the mitochondrial function, as observed by experiments involving muscle cell cultures (Xu et al. 2008) and rat injured muscles (Silveira et al. 2009), suggesting that LLLT may induce an increase of ATP synthesis in humans. Altogether, these evidences help perhaps to explain the decrease in muscle damage markers observed in the LLLT group. Nevertheless, further research on the phototherapy mechanisms at the cellular level is necessary before these explanations can be linked to the observed results.
Conclusion
LLLT treatment before knee extensor eccentric exercise was effective in terms of attenuating the deleterious effects of muscle damage on muscle function, and reduced the increment of LDH and CK serum levels. To the best of our knowledge, this seems to be the first study to demonstrate the positive effects of this therapy on muscle force recovery after eccentric exercise, which is an interesting aspect with practical applicability to sports training and performance. Although more studies involving phototherapy and muscle damage need to be performed, LLLT is presented here as a possible non-pharmacologic and non-invasive therapy against undesirable effects of exercise-induced muscle damage.
References
Al-Watban FA, Zhang XY, Andres BL (2007) Low-level laser therapy enhances wound healing in diabetic rats: a comparison of different lasers. Photomed Laser Surg 25:72–77
Avni D, Levkovitz S, Maltz L, Oron U (2005) Protection of skeletal muscles from ischemic injury: low-level laser therapy increases antioxidant activity. Photomed Laser Surg 23:273–277
Baroni BM, Leal Junior ECP, Geremia JM, Diefenthaeler F, Vaz MA (2010) Effect of light emmiting diodes therapy (LEDT) on knee extensor muscle fatigue. Photomed Laser Surg (in press)
Brown SJ, Child RB, Day SH, Donnelly AE (1997) Exercise-induced skeletal muscle damage and adaptation following repeated bouts of eccentric muscle contractions. J Sports Sci 15:215–222
Byrne C, Twist C, Eston R (2004) Neuromuscular function after exercise-induced muscle damage—theoretical and applied implications. Sports Med 34:49–69
Chapman D, Nexton M, Sacco P, Nosaka K (2006) Greater muscle damage induced by fast versus slow velocity eccentric exercise. Int J Sports Med 27:591–598
Chen TC, Nosaka K, Sacco P (2007) Intensity of eccentric exercise, shift of optimum angle, and the magnitude of repeated-bout effect. J Appl Physiol 102:992–999
Cheung K, Hume P, Maxwell L (2003) Delayed onset muscle soreness—treatment strategies and performance factors. Sports Med 33:145–164
Chow RT, Johnson MI, Lopes-Martins RA, Bjordal JM (2009) Efficacy of low-level laser therapy in the management of neck pain: a systematic review and meta-analysis of randomised placebo or active-treatment controlled trials. Lancet 374:1897–1908
Clarkson PM, Hubal MJ (2002) Exercise-induced muscle damage in humans. Am J Phys Med Rehabil 81:S52–S69
Craig JA, Barron J, Walsh DM, Baxter GD (1999) Lack of effect of combined low-intensity laser therapy/phototherapy (CLILT) on delayed onset muscle soreness in humans. Lasers Surg Med 24:223–230
Cressoni MD, Dib Giusti HH, Casarotto RA, Anaruma CA (2008) The effects of a 785-nm AlGaInP laser on the regeneration of rat anterior tibialis muscle after surgically-induced injury. Photomed Laser Surg (Ahead of print). doi:10.1089/pho.2007.2150
Douris P, Southard V, Ferrigi R, Grauer J, Katz D, Nascimento C, Podbielski P (2006) Effect of phototherapy on delayed onset muscle soreness. Photomed Laser Surg 24:377–382
French DN, Thompson KG, Garland SW, Barnes CA, Portas MD, Hood PE, Wilkes G (2008) The effects of contrast bathing and compression therapy on muscular performance. Med Sci Sports Exerc 40:1297–1306
Fridén J, Lieber RL (2001) Eccentric exercise-induced injuries to contractile and cytoskeletal muscle fibre components. Acta Physiol Scand 171:321–326
Hough T (1902) Ergographic studies in muscular soreness. Am J Physiol 7:76–92
Howatson G, van Someren KA (2008) The prevention and treatment of exercise-induced muscle damage. Sports Med 38:483–450
Huang YY, Chen AC, Carroll JD, Hamblin MR (2009) Biphasic dose response in low level light therapy. Dose Response 7:358–383
Jamurtas AZ, Theocharis V, Tofas T, Tsiokanos A, Yfanti C, Paschalis V, Koutedakis Y, Nosaka K (2005) Comparison between leg and arm eccentric exercises of the same relative intensity on indices of muscle damage. Eur J Appl Physiol 95:179–185
Leal Junior EC, Lopes-Martins RB, Rossi RP, De Marchi T, Baroni BM, Godoi V, Marcos RL, Ramos L, Bjordal JM (2009) Effect of cluster multi-diode light emitting diode therapy (LEDT) on exercise-induced skeletal muscle fatigue and skeletal muscle recovery in humans. Lasers Surg Med 41:572–577
Leal Junior EC, Lopes-Martins RA, de Almeida P, Ramos L, Iversen VV, Bjordal JM (2010) Effect of low-level laser therapy (GaAs 904 nm) in skeletal muscle fatigue and biochemical markers of muscle damage in rats. Eur J Appl Physiol 108:1083–1088
Liu XG, Zhou YJ, Liu TC, Yuan JQ (2009) Effects of low-level laser irradiation on rat skeletal muscle injury after eccentric exercise. Photomed Laser Surg 27:863–869
Marfell-Jones M, Olds T, Stewart A, Carter L (2006) International standards for anthropometric assessment. ISAK, Potchefstroom (South Africa)
Morgan DL (1990) New insights into the behavior of muscle during active lengthening. Biophys J 57:209–221
Morgan DL, Allen DG (1999) Early events in stretch-induced muscle damage. J Appl Physiol 87:2007–2015
Nikolaidis MG, Jamurtas AZ, Paschalis V, Fatouros IG, Koutedakis Y, Kouretas D (2008) The effect of muscle-damaging exercise on blood and skeletal muscle oxidative stress: magnitude and time-course considerations. Sports Med 38:579–606
Nosaka K, Clarkson PM (1996) Variability in serum creatine kinase response after eccentric exercise of the elbow flexors. Int J Sports Med 17:120–127
Nosaka K, Sakamoto K, Newton M, Sacco P (2001) How long does the protective effect on eccentric exercise-induced muscle damage last? Med Sci Sports Exerc 33:1490–1495
Oliveira FS, Pinfildi CE, Parizoto NA, Liebano RE, Bossini PS, Garcia EB, Ferreira LM (2009) Effect of low level laser therapy (830 nm) with different therapy regimes on the process of tissue repair in partial lesion calcaneous tendon. Lasers Surg Med 41:271–276
Proske U, Morgan DL (2001) Muscle damage from eccentric exercise: mechanism, mechanical signs, adaptation and clinical applications. J Physiol 537:333–345
Rizzi CF, Mauriz JL, Freitas Corrêa DS, Moreira AJ, Zettler CG, Filippin LI, Marroni NP, González-Gallego J (2006) Effects of low-level laser therapy (LLLT) on the nuclear factor (NF)-kappaB signaling pathway in traumatized muscle. Lasers Surg Med 38:704–713
Rochkind S, Geuna S, Shainberg A (2009) Chapter 25: phototherapy in peripheral nerve injury: effects on muscle preservation and nerve regeneration. Int Rev Neurobiol 87:445–464
Silveira PC, Silva LA, Fraga DB, Freitas TP, Streck EL, Pinho R (2009) Evaluation of mitochondrial respiratory chain activity in muscle healing by low-level laser therapy. J Photochem Photobiol B 95:89–92
Sussai DA, Carvalho PTC, Dourado DM, Belchior ACG, Reis FA, Pereira DM (2010) Low-level laser therapy attenuates creatine kinase levels and apoptosis during forced swimming in rats. Lasers Med Sci 25:115–120
Totsuka M, Nakaji S, Suzuki K, Sugawara K, Sato K (2002) Break point of serum creatine kinase release after endurance exercise. J Appl Physiol 93:1280–1286
Váczi M, Costa A, Rácz L, Tihanyi J (2009) Effects of consecutive eccentric training at different range of motion on muscle damage and recovery. Acta Physiol Hung 96:459–468
Warren GL, Lowe DA, Armstrong RB (1999) Measurement tools used in the study of eccentric contraction-induced injury. Sports Med 27:43–59
White JP, Wilson JM, Austin KG, Greer BK, St. John N, Panton LB (2008) Effect of carbohydrate-protein supplement timing on acute exercise-induced muscle damage. J Int Soc Sports Nutr 5:5
Wilson JM, Kim JS, Lee SR, Rathmacher JA, Dalmau B, Kingsley JD, Koch H, Manninen AH, Saadat R, Panton LB (2009) Acute and timing effects of beta-hydroxy-beta-methylbutyrate (HMB) on indirect markers of skeletal muscle damage. Nutr Metab (Lond) 6:6
Xu X, Zhao X, Liu TC, Pan H (2008) Low-intensity laser irradiation improves the mitochondrial dysfunction of C2C12 induced by electrical stimulation. Photomed Laser Surg 26:197–202
Yamaura M, Yao M, Yaroslavsky I, Cohen R, Smotrich M, Kochevar IE (2009) Low level light effects on inflammatory cytokine production by rheumatoid arthritis synoviocytes. Lasers Surg Med 41:282–290
Acknowledgments
The authors would like to thank Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES-Brazil) and Conselho Nacional de Pesquisa (CNPq-Brazil) for financial support and our colleagues Rodrigo Rodrigues and Giovani dos Santos Cunha for technical assistance during data collection.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Arnold de Haan.
Rights and permissions
About this article
Cite this article
Baroni, B.M., Leal Junior, E.C.P., De Marchi, T. et al. Low level laser therapy before eccentric exercise reduces muscle damage markers in humans. Eur J Appl Physiol 110, 789–796 (2010). https://doi.org/10.1007/s00421-010-1562-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-010-1562-z