Abstract
The use of plastic is increasing every year and is a major environmental problem. A possible solution to reduce plastic pollution is to convert it into synthetic fuel, which can be achieved by a pyrolysis process. The catalyst used can influence the quality of the pyrolysis products. In the present work, we tested the influence of ZAP USY zeolite on the quality and quantity of pyrolysis oils produced from high-density polyethylene, low-density polyethylene, polypropylene, and polystyrene plastic from municipal waste. The pyrolysis process was carried out in a laboratory-scale single batch fixed bed pyrolysis reactor at the temperature of about 400 °C ± 30 °C. The mass ratio of plastic and catalyst was 10:1. The obtained results show that the use of a catalyst reduces the pyrolysis oil production and solid residues’ formation. The use of a catalyst also decreases the calorific value, density, surface tension, and kinematic viscosity of the obtained oils slightly. The use of a catalyst increases the plastic degradation rate, which is evident from the result of the carbon number distribution. The pyrolysis oils obtained by catalytic pyrolysis contain a higher amount of shorter chain molecules than oils obtained by thermal pyrolysis of waste plastic. The obtained results of the analyzed pyrolysis oils indicate that they have a high potential for possible use in internal combustion engines as an additive to conventional fuels, or as their substitute.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plastic is a very useful material that has played a significant role in our daily lives. In the last few decades, several materials have been replaced by various plastics. The increasing world population, the constant need for economic growth, low production cost and short production time are just some of the reasons for the rapid increase in the use of plastic (Lee et al. 2015). In 2018, more than 359 million tons of plastic were produced worldwide. Most of the plastic was produced in Asia. Europe accounts for about 17% of global plastic production, which was about 62 million tons in 2018 (Europe and EPRO 2019). Polypropylene (PP), high-density polyethylene (HDPE), and low-density polyethylene (LDPE) represent about half of all plastics produced in Europe. In 2018, 29.1 million tons of plastic waste was collected in Europe, of which 32.5% was recycled, 42.6% was used in energy recovery facilities and 25% was landfilled (Europe and EPRO 2019). Landfilling of plastic presents major environmental problems, as plastic is usually not biodegradable (Benavides et al. 2017). Recycling of plastic also has its limitations, as some additives need to be added during the process to increase the final quality of the recycled plastic, which limits the endless recycling process of plastic (Thompson et al. 2009). According to Zhou et al. (2021), merely 2% of recycled plastic is the same quality as the plastic waste which enters the recycling process. On the other hand, plastic waste can be used for the production of synthetic fuels. The use of synthetic fuels can reduce dependence on fossil fuel consumption, which accounts for more than 90% of total energy consumption in the EU Transport sector (EEA 2019).
Synthetic fuels are usually produced from plastic waste by pyrolysis. Pyrolysis is a process in which long chains of plastic polymer molecules are degraded thermally into less-complex, short chain molecules. This occurs at elevated temperatures and in the absence of oxygen (Anuar Sharuddin et al. 2016). A pyrolysis process that does not use a catalyst is also known as thermal pyrolysis. The pyrolysis gas, oil and solid products are the three main products obtained during the pyrolysis process. The pyrolysis process is influenced by the reaction conditions and the type of pyrolysis reactor, both of which affect the quantity and quality of the final product (Anuar Sharuddin et al. 2016). If optimal conditions prevail during the pyrolysis process, more than 90% of plastic waste HDPE, LDPE, PP and polystyrene (PS) can be converted into pyrolysis oil (PO) (Kunwar et al. 2016). Polyvinyl chloride (PVC) and polyethylene terephthalate (PET) plastic types have lower conversion rates to pyrolysis oils and generally yield higher ratios of solid residues and pyrolysis gas. Sogancioglu et al. (2017) performed pyrolysis of HDPE, LDPE, PP, PET and PS plastics in a laboratory-scale single batch reactor with a fixed bed. The highest amount of PO, almost 89%, was obtained with HDPE plastic, followed by PP plastic, LDPE plastic and PS plastic at 300 °C. Slightly lower conversion rates of PO were obtained in the work of Santaweesuk and Janyalertadun (2017). They used a rotary kiln reactor and were able to recover 80% of the pyrolysis oil from PP plastic, 73% from LDPE and 70% from HDPE plastic. Very high conversion rates of PP, PS, HDPE and LDPE plastics were also obtained by Phanisankar et al. (2020). Less than 50% of HDPE plastic was converted to pyrolysis oil at 620 °C using a microwave-assisted pyrolysis system in the work of Zhou et al. (2021).
The quality and quantity of pyrolysis oils can be influenced further by the use of catalysts. The process is commonly referred to as catalytic pyrolysis. The most commonly used catalysts in a pyrolysis process are red mud, fly ash, FCC, ZSM-5, HZSM-5, Y-zeolites, Fe2O3, Al2O3, Ca(OH)2, and natural zeolites (Kumaran and Sharma 2020). The main differences between catalysts are acidity, BET surface area, pore size and crystalline structure. The use of catalysts usually reduces the energy input of the process and increases the formation of the lighter fraction of pyrolysis oils (Kumaran and Sharma 2020). In the work of Zhang et al. (2019), several different active carbons were tested for the production of jet fuel by pyrolysis of LDPE plastic. They concluded that the acidity of the activated carbon used is the critical factor of the catalyst in the production of jet fuel from LDPE plastic by catalytic pyrolysis. LDPE plastic was also used in the work of Singh et al. (2020), where fly ash and zeolite were used as catalysts. Both catalysts reduced the formation of liquid products and increased the overall gaseous yield. Su et al. (2019) tested eutectic salt as a catalyst in the pyrolysis of waste packaging polyethylene (WPE). The use of eutectic salt in the catalytic pyrolysis of WPE reduces the cracking temperature compared to thermal pyrolysis. It also reduces the heavy oil component content in the oil product.
Zeolite catalysts differ in the ratio of SiO2 and Al2O3, which affects their acidity. Higher acidity (a low SiO2/AI2O3 ratio), usually leads to lower pyrolysis oil production and higher pyrolysis gas production Artetxe et al. (2013); Miandad et al. (2016), tested fresh, spent and regenerated ZSM-5 zeolite in the catalytic pyrolysis of mixed plastic waste to investigate the loss of zeolite activity and its influence on the obtained products. The properties of the fresh, spent and regenerated zeolite differed in BET surface area, micro-pore volume (MPV) and micro-pore area (MPA). The BET surface area was 412 m2/g for fresh zeolite and 291.6 m2/g for spent zeolite. The MPV and MPA for fresh zeolite were 0.1 cm3/g and 346.1 m2/g, and 1.0E − 03 cm3/g and 3.9 m2/g for spent zeolite, respectively. These differences in zeolite properties further influence the quality of the pyrolysis products obtained. The aromatic content of the pyrolysis oils and the fraction of C5–C9 carbon number decreased when spent zeolite was used as the catalyst in the pyrolysis process. Despite the differences in properties, the use of all zeolites increased the amount of gas and decreased the production of pyrolysis oil. Their use also affected the increase in aromatics in the PO and produced higher amounts of short chain chemicals (C5–C9). This phenomenon was also noted in the work of Serrano et al. (2005) and is explained by the presence of strong acid sites in the zeolite where aromatization reactions are favored. The catalyst they used (Serrano et al. 2005) again differed in its physicochemical properties. The Al-MCM-41 catalyst has the highest BET surface area of 1441 m2/g, the highest average pore diameter of 22.8 nm, an MPV of 1.023 cm3/g and an SiO2/AlO3 ratio of 40, while the HZSM-5 zeolite has the lowest BET of 390 m2/g, the average pore diameter of 5.5 nm, an MPV of 0.18 cm3/g and an SiO2/AlO3 ratio of 34. The difference between the catalyst properties affects the profile of the degradation species, which have a lower proportion of aromatic compounds and a higher complex mixture of long chain linear, branched and cyclic hydrocarbon species when Al-MCM-41 was used as the catalyst in the pyrolysis of LDPE plastic. The effect of the surface area of BET on the yield of pyrolysis product was tested in the work of Rehan et al. (2017), where they tested natural zeolite with a BET surface area of 4.3 m3/g and synthetic zeolite with a BET surface area of 780 m2/g. They concluded that the higher BET surface area of zeolite results in a lower pyrolysis oil yield and higher pyrolysis gas production. They also concluded that zeolites with different BET surface areas produce pyrolysis oils that have slightly different calorific value and chemical composition. The influence of zeolite properties on the pyrolysis process and PO oil quality was also tested in the work of Marcilla et al. (2009). They tested the HUSY zeolite with a higher BET surface area of 614 m2/g, weak acidic sites and pore volume of 0.29 cm3/g, along with HSZM-5 zeolite, which has a smaller BET of 341 m2/g, smaller pore size volume of 0.16 cm3/g and both weak and strong acidic sites. The use of HZSM-5 zeolite as a catalyst in the pyrolysis process of HDPE and LDPE plastics resulted in a higher yield of gases and a lower yield of PO compared to HUYS zeolite. The mechanism for such a result is related to the differences in the acid sites and pore sizes of the zeolite. The larger pore size of the HUSY zeolite, compared to HZSM-5, allows the formation of bulky coke precursors that lead to rapid deactivation of the role of the HUSY zeolite as a catalyst. The differences in zeolite properties affected the properties of the pyrolysis products. The use of USY zeolite with a BET surface area of 550 m2/g, MPV of 0.1 cm3/g and SiO2/Al2O3 ratio of 15 in the catalytic pyrolysis of polyethylene and polypropylene was tested in the works of Kassargy et al. (2017; 2018). They concluded that the use of USY zeolite increases the production of gas products and the influence on the chemical composition of the obtained pyrolysis oils.
The zeolites are one of most popular catalyst for pyrolysis of plastic waste. (Fadillah et al. 2021) As can be seen from the above-mentioned literature review, the influence of catalysts in the pyrolysis process is not negligible. The main objective of the study was the production of useful pyrolysis oils from waste HDPE, LDPE, PP and PS plastics types, which are presenting the majority of produced plastic waste. With the aim of decreasing solid residue, and obtaining better quality and composition of the pyrolysis oils produced, pyrolysis was also carried out using ZAP USY zeolite with a low SiO2/Al2O3 ratio. The low SiO2/Al2O3 ratio indicates on higher acidity of used zeolite, which was also presented in work of Fadillah et al. (2021) and Kassargy et al. (2018). The acidity of catalyst decreases the activation energy (Silva et al. 2017) and accelerates the decomposition reaction which increases the potential of secondary reaction. So the other objectives of the manuscript was to test if used zeolite, with SiO2/Al2O3 ratio (high acidity), can minimize the amount of solid residue, decreases activation energy, increases the formation of short chain hydrocarbons by over-cracking as proven in work of Fadillah et al. (2021) and influences the composition of the pyrolysis oils. The pyrolysis process was performed in a single batch, fixed bed pyrolysis reactor, with and without usage of ZAP USY zeolite as a catalyst. The obtained pyrolysis oils were analyzed further by different test methods and compared with diesel and gasoline fuels produced according to the EN 590 and EN 228 Standards. To the authors’ knowledge, no USY zeolite with this specific low SiO2/Al2O3 ratio has been used to study its influence on HDPE, LDEP, PP and PS plastic degradation during the pyrolysis process. Since the study focuses on usage of several types of collected waste plastic, the results can give deeper insight into possible usage of large amounts of collected plastic waste for obtaining pyrolysis oils, and their potential as an additive to conventional fuels, or as their substitute.
The thermal and catalytic pyrolysis processes were performed in the laboratories of the Faculty of Mechanical Engineering at the University of Maribor in 2020. The characterization of the pyrolysis oils, with the exception ATR FT-IR spectroscopy, was carried out in the laboratories of CICT of the Universidad de Jaén.
Materials and methods
Plastic
The plastics used in the present study were collected from municipal waste. The collected plastic was sorted according to each type of plastic and washed before being cut into smaller pieces of approximately 1 × 2 cm in size. The HDPE, LDPE, PP and PS types of plastics were used in the presented study.
Zeolite
The catalyst used in the pyrolysis experiment was zeolite ZAP USY, produced by SILKEM d.o.o., with a BET surface area of 540 m2/g, MPV of 0.09 cm3/g and SiO2/Al2O3 ratio of 13. The main properties of the used zeolite and commonly used zeolite are presented in Table 1. During catalytic pyrolysis, the mass ratio of plastic/catalyst was 10/1.
Pyrolysis process
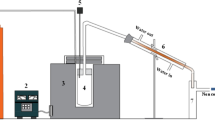
In the present work, pyrolysis was carried out in a laboratory-scale single batch pyrolysis reactor with a fixed bed. The schematic representation of the experimental setup is shown in Fig. 1.
The reactor was made of stainless steel and was heated with a 3300 W gas stove. The reactor was thermally insulated with a jacket of glass wool. A thermocouple was inserted into the reactor to monitor the temperature during the process. In the final position, the thermocouple was in direct contact with the plastic sample under investigation. The application for monitoring the pyrolysis process was created in the program LabVIEW. A National Instruments DAQ carrier WLS-9163 and an NI-9219 module were used for temperature data acquisition. Type K thermocouples were used.
At the beginning of each experiment, the plastic was cut into smaller pieces of approximately 2 cm2. 100 g of the plastic was weighed and placed in the reactor. If a catalyst was used, 10 g of zeolite was weighed and mixed with the plastic before it was added to the reactor. The reactor was sealed and connected to the condenser using copper tubes. The temperature inside the reactor was raised from ambient to 400 °C ± 30 °C. The vapors of the plastic (pyrolysis products) flowed through the condenser, which was cooled with tap water flowing in the opposite direction to the plastic vapors. The condensate (pyrolysis oil) was collected in an oil recovery tank, while the non-condensable gasses were released to the atmosphere without further analysis. The pyrolysis process was stopped (heat source turned off) when the time interval between oil droplets in three consecutive droplets increased above 10 s. The solid product remained in the reactor and was removed after the pyrolysis processes were completed. The pyrolysis oils obtained were analyzed further.
Material analysis and characterization
The density of the pyrolysis oils was determined using a portable density meter, Mettler Toledo Density2Go, in a temperature range from 15 to 40 °C. The same temperature range was also used for determining the surface tension of the pyrolysis oils, which was measured using a Krüss EasyDyne K20 tensiometer. The calorific value of the tested oils was determined using a 6050 compensated jacket calorimeter designed by the Parr Company. A TruSpec Micro analyzer from LECO was used to determine the carbon (C), hydrogen (H), nitrogen (N), and sulfur (S) composition of the PO. The carbon number distribution of pyrolysis oils was determined using a Thermo Quadrupole GC–MS Model DSQ II-Trace Ultra GC analyzer. ATR FT-IR spectra were recorded on a Perkin Elmer Spectrum GX spectrometer. The ATR accessory (supplied by Specac Ltd, UK) included a diamond crystal. A total of 16 scans were recorded for each sample, with a resolution of 4 cm−1 at room temperature. The spectra of the pyrolysis oils were recorded in the wave number range between 4000 and 650 cm−1.
Results and discussion
The results of the pyrolysis process, the properties of the obtained pyrolysis oil, its comparison with conventional fuels and characterization by GS-MS and FT-IR spectroscopy are presented in the following chapter.
Ratios of the pyrolysis products
The results of the pyrolysis products obtained experimentally are shown in Table 2. The values in the table present the percentage of each pyrolysis product for all tested plastic types, with and without a catalyst. The results of catalytic pyrolysis are indicated by the abbreviation z.
Pyrolysis oil and solid residue yields were determined by weighing the mass of each product obtained and dividing by the total mass of plastic used. Since the gas phase was not collected, its mass was calculated from the difference between the total mass of plastic used and the mass of oil and solid residue obtained. We assume that the catalyst used did not evaporate and remained in the solid residue. Therefore, its mass was subtracted from the total mass of the residue remaining in the reaction chamber.
As can be seen from the obtained results, the catalyst does not have a uniform effect on the production of pyrolysis products. When comparing the results for high-density polyethylene, 76.9% of the raw material was converted into pyrolysis oil without the catalyst (HDPE), and only 62.7% with the catalyst (HDPE_z). The amount of pyrolysis gas obtained from HDPE plastic increased from 8.8% to almost 32.8% when the catalyst was used.
The same trend was observed with the use of low-density polyethylene and polypropylene plastic. The use of a catalyst decreased the amount of PO from 75.1 to 65.5% when LDPE plastic was used, and from 80.6 to 59.9% when using PP plastic. The amount of pyrolysis gas was increased by up to 31.8% when using a catalyst and low-density polyethylene (LDPE_z), and by up to 34.7% when using a catalyst and polypropylene plastic (PP_z).
The opposite trend was observed when polystyrene plastic was used. In this case, thermal pyrolysis of PS plastic converted only 58.6% of the raw plastic to PO, and 81.5% when the plastic and catalyst were mixed (PS_z). The amount of pyrolysis gas produced decreased from 18.8 (PS) to 8.3% when the catalyst was used (PS_z).
The presented results also show that the use of the catalyst reduces the formation of the solid residue. This indicates that the use of USY zeolite as a catalyst enhances and promotes the decomposition reactions of plastics into oil and gas products, which have a higher potential for further use due to the higher heating value than the solid residue (Saeaung et al. 2021), but can be less favorable to the Petrochemical industry, which favors the production of naphtha and other feedstocks (Zhou et al. 2021).
The last line in Table 2 presents the results of time reduction when the catalyst was used during the pyrolysis process. The results show a significant reduction in time for catalytic pyrolysis compared to thermal pyrolysis. The highest reduction in reaction time, 34%, was obtained when the catalyst and plastic PP were mixed, followed by LDPE plastic, with a time reduction of 31%, HDPE plastic with a time reduction of 26% and PS plastic with a time reduction in a reaction time of 12%. From this, we can conclude that use of ZAP USY zeolite as a catalyst in the pyrolysis process reduces the required activation energy for all tested plastic types. The use of a catalyst also increases the conversion ratio of plastic volatiles to pyrolysis oil and gas, which resulted in less solid residue.
Pyrolysis oil characterization
In order to use pyrolysis oils as fuel, they must meet the physical properties prescribed in the EN 590 Standard for diesel fuel and EN 228 for gasoline fuel. The following chapter presents the properties of the obtained pyrolysis oils. Table 3 presents the characteristics of the obtained PO and required values for European Standards.
Pyrolysis oil density
Fuel density has a significant effect on the fuel injection process in internal combustion engines, since the speed of sound of the fuel is a function of the square root of the fuel density. This is particularly important in compression ignition engines with mechanically controlled injection systems. If the start injection is delayed, which can happen at lower fuel density, the start of combustion can be delayed, which can, further, affect slower combustion rate, shorter ignition delay, higher pressure and higher temperature in the combustion chamber (Torres-Jimenez et al. 2011; Shareef and Mohanty 2020). If the fuel density increases, the injection timing can be advanced, resulting in faster combustion, higher ignition delay, and lower pressure and temperature in the combustion chamber (Shareef and Mohanty 2020; Shameer and Ramesh 2018).
These changes further affect engine performance and emissions’ formation. Fuel density further affects the injected fuel mass. When the mass of injected fuel is reduced, which can happen when fuel density is lower, the nominal engine power is reduced, and vice versa.
The results of the obtained PO densities at different ambient temperatures are presented in Fig. 2.
The density of the pyrolysis HDPE oil at 15 °C was 788.9 kg/m3. The pyrolysis oils from LDPE and PP plastic waste have densities of 787.4 kg/m3 and 789 kg/m3 at 15 °C. The highest density of PO was obtained with PS plastic waste and was 924.3 kg/m3 at 15 °C. The presented results show that the PO obtained from the plastic wastes of HDPE, LDPE and PP provided lower densities than those specified in the EN 590 Standard for diesel fuel, Table 3 and Fig. 2. Comparing the results with the density specified in the Gasoline Fuel Standard EN 228, the obtained pyrolysis oils had slightly higher densities at 15 °C, Table 3 and Fig. 2. The pyrolysis oil produced from PS plastic had higher densities compared to the values given in the Standard for both diesel and gasoline fuels. The obtained PO densities are in agreement with the results obtained by other authors where the same types of plastic were used for production of pyrolysis oils (Kumaran et al. 2020; Singh et al. 2020; Kassargy et al. 2017).
As the test temperature was increased, the density of the pyrolysis oil decreased, which follows normal fuel behavior. By using the catalyst, the PO density decreased at all temperatures, which is in agreement with the results of Singh et al. (2020) and Kassargy et al. (2017). The decrease in PO density, in the case of catalytic pyrolysis, was more or less the same for all pyrolysis oils obtained, by 1% for PP and PS plastics, 2% for HDPE plastic and 3% for LDPE plastic. Since the density of pyrolysis oils is slightly lower, the injection delay can be expected in engines with mechanically controlled injection systems. Due to the lower density of pyrolysis oil, a slightly lower mass of fuels will be injected if the injection duration will not be increased.
Pyrolysis oil surface tension
Surface tension has a major effect on fuel spray distribution. When the surface tension is increased, it can increase the internal forces in the fuel, which further increases the length of the spray and decreases its cone angle. Higher surface tension of the fuel increases the mean Sauter diameter of the spray droplets, which slows down their break-up and evaporation (Liu et al. 2019).
The results obtained for all pyrolysis oils at different temperatures are presented in Fig. 3.
The experimental values of surface tension are shown in Fig. 3. These results are in agreement with, or slightly lower than, the surface tension values for diesel fuel obtained in the work of Ren et al. (2007) and Chhetri and Watts (2013). The values they obtained for diesel fuel were 28.6 mN/m at 28 °C (Ren et al. 2007), 25.84 mN/m at 20 °C in (Chhetri and Watts 2013), and 23.8 mN/m at 20 °C in (Liu et al. 2019). The values for gasoline were slightly lower. Liu et al. (2019) determined a surface tension of 22 mN/m at 20 °C for gasoline.
The results of PO surface tension are highly dependent on the temperature of the measurement. As the temperature of the oil increases, its surface tension decreases. The catalyst decreased the surface tension of the obtained pyrolysis oils from HDPE, LDPE and PP plastic waste, while it increased the surface tension of PO from PS plastic waste. The reduction in surface tension of pyrolysis oil from HDPE was in the range of 9 to 12% when the catalyst was used, depending on the test temperature. The LDPE plastic experienced a surface tension reduction of 10 to 14% at different temperatures. The decrease in surface tension of PP pyrolysis oils ranged from 1 to 5% when the catalyst was used. At 30 °C, the catalyst caused the reverse trend and increased the surface tension of PP pyrolysis oil. The increase in surface tension of pyrolysis oils was obtained when the catalyst was used at all test temperatures for PS pyrolysis oils. The increase was in the range of 8 to 10%, depending on the test temperature.
According to the good agreement between the surface tension of the oils obtained in thermal pyrolysis and conventional fuels, no major influence on spray development and break-up is expected if pyrolysis oil were used in internal combustion engines. Slightly lower spray lengths and wider spray angles can be expected with the use of pyrolysis oils obtained during the catalytic pyrolysis due to their lower surface tension.
Pyrolysis oil calorific value
The calorific value (CV) of fuels determines how much energy is released when the fuel is burned. Higher values lead to higher heat release, and vice versa. Figure 4 shows the results of the obtained PO gross calorific values.
The results in Fig. 4 show that all pyrolysis oils have a high calorific value, comparable to the calorific value of diesel (42.8–43.4 MJ/kg) and gasoline (44.4 MJ/kg) (Kassargy et al. 2017; Lešnik et al. 2016). When comparing the results for thermal pyrolysis, the highest calorific value of pyrolysis oil, 46 MJ/kg, was obtained for the LDPE plastic waste, followed by HDPE pyrolysis oil with a CV of 46 MJ/kg, PP pyrolysis oil with a CV of 44.4 MJ/kg and PS with 40.9 MJ/kg. The catalytic pyrolysis oils had slightly lower calorific values. The highest CV in catalytic pyrolysis was obtained when using HDPE plastic, 44.4 MJ/kg, followed by PP plastic with a CV of 43.5 MJ/kg and LDPE plastic with a CV of 43.2 MJ/kg. The lowest calorific value was again obtained by PS plastic, which resulted in a pyrolysis oil with a CV of 40.1 MJ/kg.
The obtained results agree well with the results of other authors, where a catalyst and the same plastic types were used. Kumaran et al. (2020) produced PO from several different types of plastic waste using dolomite and zeolite as catalysts. The calorific value of the delivered oils was in the range of 41.7–44.2 MJ/kg. Only LDPE plastic was used in the work of Sing et al. (2020). The obtained pyrolysis oil had a calorific value of 40.37 MJ/kg, which is slightly less than in our study. The use of PP and PE plastics in catalytic pyrolysis using USY zeolite, with properties similar to our zeolite, resulted in a CV of 45.8 MJ/kg for PP and 45.1 MJ/kg for PE plastic in the work of Kassargy et al. (2017).
Pyrolysis oil viscosity
Fuel viscosity is also a very important parameter. According to Kim et al. (2019), higher fuel viscosity affects a lower injection rate, longer ignition delay, lower needle lift speed and slower increase in the cross-sectional area of the fuel flow. This can further affect the nominal engine rated power and emission formation. Changes in fuel viscosity also affect fuel spray injection parameters. Higher fuel viscosity results in longer spray type penetration depth and a narrower spray cone angle (Achebe et al. 2020). The results of kinematic viscosity for the obtained oils are presented in Fig. 5.
The presented results of the kinematic viscosity of the pyrolysis oils show that the use of the catalyst decreased the viscosity of the majority of obtained pyrolysis oils. This can be attributed to changes in the composition of the PO obtained during catalytic pyrolysis which promote the formation of short chain molecules, Figs. 6 and 7. The same mechanism was also presented in the work of Miandad et al. (2016).
The lowest decrease in kinematic viscosity upon catalyst pyrolysis was obtained when PS plastic was used and ranged from 4.9 to 8.4%, depending on the temperature. The highest decrease in kinematic viscosity, 37.8%, was obtained with catalyst pyrolysis of LDPE plastic at a 35 °C test temperature.
The results show that, as the test temperature increased, the kinematic viscosity of the pyrolysis oil decreased, which follows normal fuel behavior.
The Standard for diesel fuel, EN 590, specifies the required kinematic viscosity of the fuel, which should be in the range of 2 to 4.5 mm2/s at 40 °C (Kassargy et al. 2018). According to them, thermal pyrolysis of HDPE, LDPE and PP plastic oils fulfilled the required viscosity for diesel fuel, while, in all other cases, the obtained viscosity of PO was lower than required by the Standard. Lower viscosity can increase the fuel injection rate and shorten ignition delay. The obtained kinematic viscosities of PO were slightly lower than in the work of Sing et al. (2020), where LDPE plastic was used. Slightly higher kinematic viscosities, compared to our values, were obtained in Santaweesuk et al. (2017), where LDPE, HDPE and PP plastic types were used, while Kurniawati et al. (2021) obtained the same value of kin. viscosity when using HDPE plastic in the thermal pyrolysis process.
Pyrolysis oil elementary composition
To understand the quality of pyrolysis oil better, information is needed about the chemical composition of the oil. The chemical composition of the fuel can affect its calorific value, emission formation during combustion, etc. Since the content of nitrogen and sulfur in all PO oils is almost negligible (less than 0.1% for N and less than 0.5% for S), the focus was on the amount of C and H.
The results of pyrolysis oil’s elementary composition are presented in Table 4.
From the presented results of the PO elemental composition, it can be concluded that the use of the catalyst leads to a reduction in the carbon and hydrogen content in the pyrolysis oils.
The C/H ratio of all HDPE, LDPE and PP pyrolysis oils was similar to the ratio found in diesel and gasoline fuels, 6.2–6.3, while both pyrolysis oils produced from PS had a C/H ratio greater than 10.
The results also show that carbon and hydrogen make up the most of the composition of pyrolysis oils. Since all plastics contain a considerable amount of additives, such as plasticizers, flame retardants, stabilizers, fillers, reinforcements (Hahladakis et al. 2018), we assume that they account for up to 100% of the difference in the composition of the obtained pyrolysis oils.
Pyrolysis oil carbon number distribution
The results of carbon number distribution are shown in Fig. 6.
As can be seen from the carbon number distribution results, the oils obtained from thermal pyrolysis of HDPE, LDPE and PP plastic wastes contained hydrocarbons with longer chains and higher molecular weights compared to oils obtained from catalytic pyrolysis of the same types of plastics. The high-density polyethylene contained hydrocarbons with a wide range of carbon numbers (6–36), with the highest area percentage of C19 (15.9%), C16 (9.4%), and C15 (8.4%) molecules. The catalytic pyrolysis of HDPE plastic had a narrower range of carbon number distribution (5–10) with a lower proportion of higher hydrocarbons. The highest area percentage of HDPE_z pyrolysis oil was obtained at C8 (28.4%), followed by C7 (16.7%) and C9 (15.7%). More or less the same trend was obtained for LDPE_z pyrolysis oil, with the highest area percentage of C8 (22.7%), C7 (15.6%) and C9 (15.1%). Non-catalytic pyrolysis of low-density polyethylene again yielded oil with a wider carbon number distribution and with a higher area percentage at C8 (13.9%), followed by C19 (11.5%) and C7 (9.6%). The higher area percentage for PP plastic was obtained for C9 (17.3%), C18 (14%) and C13 (12.6%) for non-catalytic pyrolysis, and for C9 (26.5%), C8 (23.3%) and C7 (12.7%) for catalytic pyrolysis of waste polypropylene plastic. The oils obtained with both pyrolyses of PS waste plastic contained a similar carbon number distribution, with maximum area percentage at C8 (41.8% for PS and 41.6% for PS_z), followed by C9 (20.1% for PS and 17.1% for PS_z) and C16 (8.1% for PS and 8.8% for PS_z).
According to Marcilla et al. (2009), the typical carbon number distribution for gasoline is in the range of C4 to C12 and from C12 to C24. Comparing these values with the results of pyrolysis oils, it can be seen that non-catalytic pyrolysis oils from HDPE, LDPE and PP plastic waste produce oils that have a similar C number distribution to diesel fuel. The oils obtained from the catalytic pyrolysis of polypropylene, high and low-density polyethylene, have a carbon number distribution similar to gasoline.
In work of Kassargy et al. (2017), the USY zeolite, with slightly higher SiO2/Al2O3 ratio (lower acidic) compared to ours, produced a similar carbon number distribution of pyrolysis oils obtained from PE and PP plastic. Comparing the results, one can see that the slightly lower acidic of their zeolite resulted in production of longer chain molecules with a C number higher than 10. This shows that the lower SiO2/Al2O3 ratio of zeolite used in the presented work promoted the cracking of plastic and produced short chain molecules.
In order to investigate the influence of the catalyst on the tested plastic degradation rate, the amount of area fraction up to carbon number C10 is shown in Fig. 7.
The results presented in Fig. 7 show that the use of the catalyst increased the rate of degradation of the plastics, resulting in a higher proportion of short chain molecules up to the C10 carbon number. This is indicated by the higher area fraction for all the plastics in the case of catalytic pyrolysis. The highest increase in degradation rate was seen for HDPE plastic, followed by LDPE and PP plastics. The higher degradation rate and higher proportion of short chain molecules influenced the lower fuel density, surface tension and viscosity of the catalytic pyrolysis oils presented in Figs. 2, 3 and 5.
ATR FT-IR spectroscopy
The results of the FT-IR spectra for oils obtained during thermal pyrolysis are shown in Fig. 8, and the results for catalytic pyrolysis are shown in Fig. 9. The spectrum of conventional diesel fuel without additives was added for better comparison.
The spectrum of conventional diesel fuel (D2), pyrolysis oils of HDPE, LDPE and PP plastics with and without the use of the catalyst showed the typical signals of aliphatic hydrocarbons in the range of 2960 cm−1 to 2850 cm−1, at 1460 cm−1 and at 1375 cm−1, respectively, due to the presence of –CH3 and –CH2 groups.
According to the literature data (Su et al. 2019), the olefins in the FT-IR spectra show the following typical signals; a signal at 3078 cm−1 attributed to the antisymmetric stretching of –CH2; two signals, one at 990 cm−1 and the other at about 910 cm−1, are typical of a monosubstituted double bond (R- CH=CH2), a signal at 720 cm−1 representing a cis-disubstituted double bond, and the peak at 965 cm−1. Typical olefin peaks around 3079 cm−1, 1643 cm−1, 991 cm−1 and 910 cm−1 were observed only in the spectra of thermally produced pyrolysis oils, Fig. 8. We can conclude that ZAP USY zeolite had some effect in limiting the formation of olefins.
The =C–H stretch in aromatics is observed at 3100–3000 cm−1 and is at a slightly higher frequency than the –C–H stretch in alkanes. Aromatic hydrocarbons show signals in the 1600–1585 cm−1 and 1500–1400 cm−1 ranges, which are due to carbon–carbon stretching vibrations in the aromatic ring. These signals were detected in pyrolysis oils in the PS FT-IR spectrum, the red spectrum in Figs. 8 and 9.
From the ATR FT-IR spectra, we found that the catalytic pyrolysis oils of HDPE, LDP and PP plastics produced mainly paraffins (alkanes), while the thermal pyrolysis oils of these plastics also contained a small amount of olefins (alkenes). We believe that the presence of double bonds in alkenes influences higher density, higher surface tension and higher kinematic viscosity of HDPE, LDPE and PP pyrolysis oils.
Catalytic and thermal pyrolysis oils obtained from PS plastics consist mainly of aromatic hydrocarbons.
Conclusion
The presented work discusses the influence of ZAP USY zeolite on the yield and quality of pyrolysis oils obtained from HDPE, LDPE, PP and PS plastic waste using a laboratory-scale fixed bed single batch pyrolysis reactor. The obtained results indicate that the use of ZAP USY zeolite decreased the required activation energy of all plastic waste, which resulted in decreased amounts of solid residue and increased gas production. The used zeolite also increased the degradation rate of plastics and promoted their decomposition, which resulted in the production of hydrocarbons with shorter chain length during catalytic pyrolysis. The FT-IR spectroscopy analysis showed that catalytic pyrolysis produced oils which consist mainly of alkanes, while thermal pyrolysis also produced a small amount of alkenes. This further influences the obtained pyrolysis oils’ composition and quality. Nevertheless, all the obtained pyrolysis oils had similar properties to gasoline and diesel fuel, which supports their possible use in internal combustion engines as an additive to conventional fuels. In addition, the usage of pyrolysis oils obtained from plastic waste can help to reduce the environmental impact by reducing the amount of plastic waste sent to landfill.
References
Achebe CH, Ogunedo BMO, Chukwuneke JL, Anosike NB (2020) Analysis of diesel engine injector nozzle spray characteristics fueled with residual fuel oil. Heliyon 6:e04637. https://doi.org/10.1016/j.heliyon.2020.e04637
Anuar Sharuddin SD, Abnisa F, Wan Daud WMA, Aroua MK (2016) A review on pyrolysis of plastic wastes. Energy Convers Manag 115:308–326. https://doi.org/10.1016/j.enconman.2016.02.037
Artetxe M, Lopez G, Amutio M et al (2013) Cracking of high density polyethylene pyrolysis waxes on HZSM-5 catalysts of different acidity. Ind Eng Chem Res 52:10637–10645. https://doi.org/10.1021/ie4014869
Benavides PT, Sun P, Han J et al (2017) Life-cycle analysis of fuels from post-use non-recycled plastics. Fuel 203:11–22. https://doi.org/10.1016/j.fuel.2017.04.070
Chhetri AB, Watts KC (2013) Surface tensions of petro-diesel, canola, jatropha and soapnut biodiesel fuels at elevated temperatures and pressures. Fuel 104:704–710. https://doi.org/10.1016/j.fuel.2012.05.006
EEA (2019) Transport : increasing oil consumption and greenhouse gas emissions hamper EU progress towards environment and climate objectives. Agência Eur Do Ambient. https://doi.org/10.2800/375771
Europe P, EPRO (2019) Plastics - the facts 2019
Fadillah G, Fatimah I, Sahroni I et al (2021) Recent progress in low-cost catalysts for pyrolysis of plastic waste to fuels. Catalysts. https://doi.org/10.3390/catal11070837
Hahladakis JN, Velis CA, Weber R et al (2018) An overview of chemical additives present in plastics: migration, release, fate and environmental impact during their use, disposal and recycling. J Hazard Mater 344:179–199. https://doi.org/10.1016/j.jhazmat.2017.10.014
Kassargy C, Awad S, Burnens G et al (2017) Experimental study of catalytic pyrolysis of polyethylene and polypropylene over USY zeolite and separation to gasoline and diesel-like fuels. J Anal Appl Pyrolysis 127:31–37. https://doi.org/10.1016/j.jaap.2017.09.005
Kassargy C, Awad S, Burnens G et al (2018) Gasoline and diesel-like fuel production by continuous catalytic pyrolysis of waste polyethylene and polypropylene mixtures over USY zeolite. Fuel 224:764–773. https://doi.org/10.1016/j.fuel.2018.03.113
Kim J, Lee J, Kim K (2019) Numerical study on the effects of fuel viscosity and density on the injection rate performance of a solenoid diesel injector based on AMESim. Fuel 256:115912. https://doi.org/10.1016/j.fuel.2019.115912
Kumaran KT (2020) Sharma I (2020) catalytic pyrolysis of plastic waste: a review. Adv Sci Eng Technol Int Conf ASET 2:822–838. https://doi.org/10.1109/ASET48392.2020.9118286
Kunwar B, Cheng HN, Chandrashekaran SR, Sharma BK (2016) Plastics to fuel: a review. Renew Sustain Energy Rev 54:421–428. https://doi.org/10.1016/j.rser.2015.10.015
Kurniawati D, Putra N, Abdullah N et al (2021) An experimental analysis of diesel fuel produced from HDPE (high-density polyethylene) waste using thermal and catalytic pyrolysis with passive heat pipe cooling system. Therm Sci Eng Prog 23:100917. https://doi.org/10.1016/j.tsep.2021.100917
Lee S, Yoshida K, Yoshikawa K (2015) Application of waste plastic pyrolysis oil in a direct injection diesel engine: for a small scale non-grid electrification. Energy Environ Res 5:18–32. https://doi.org/10.5539/eer.v5n1p18
Lešnik L, Biluš I (2016) The effect of rapeseed oil biodiesel fuel on combustion, performance, and the emission formation process within a heavy-duty di diesel engine. Energy Convers Manag 109:140–152. https://doi.org/10.1016/j.enconman.2015.12.001
Liu J, Feng L, Wang H et al (2019) Spray characteristics of gasoline/PODE and diesel/PODE blends in a constant volume chamber. Appl Therm Eng 159:113850. https://doi.org/10.1016/j.applthermaleng.2019.113850
Marcilla A, Beltrán MI, Navarro R (2009) Thermal and catalytic pyrolysis of polyethylene over HZSM5 and HUSY zeolites in a batch reactor under dynamic conditions. Appl Catal B Environ 86:78–86. https://doi.org/10.1016/j.apcatb.2008.07.026
Miandad R, Barakat MA, Aburiazaiza AS et al (2016) Catalytic pyrolysis of plastic waste: a review. Process Saf Environ Prot 102:822–838. https://doi.org/10.1016/j.psep.2016.06.022
Phanisankar BSS, Vasudeva Rao N, Manikanta JE (2020) Conversion of waste plastic to fuel products. Mater Today Proc. https://doi.org/10.1016/j.matpr.2020.02.880
Rehan M, Miandad R, Barakat MA et al (2017) Effect of zeolite catalysts on pyrolysis liquid oil. Int Biodeterior Biodegrad 119:162–175. https://doi.org/10.1016/j.ibiod.2016.11.015
Ren Y, Wang F, Huang Z (2007) Densities, surface tensions, and viscosities of diesel-oxygenate mixtures at the temperature 301.15 K. Energy Fuels 21:1628–1630. https://doi.org/10.1021/ef070065o
Saeaung K, Phusunti N, Phetwarotai W et al (2021) Catalytic pyrolysis of petroleum-based and biodegradable plastic waste to obtain high-value chemicals. Waste Manag 127:101–111. https://doi.org/10.1016/j.wasman.2021.04.024
Santaweesuk C, Janyalertadun A (2017) The production of fuel oil by conventional slow pyrolysis using plastic waste from a municipal landfill. Int J Environ Sci Dev 8:168–173. https://doi.org/10.18178/ijesd.2017.8.3.941
Serrano DP, Aguado J, Escola JM et al (2005) An investigation into the catalytic cracking of LDPE using Py-GC/MS. J Anal Appl Pyrolysis 74:370–378. https://doi.org/10.1016/j.jaap.2004.11.026
Shameer PM, Ramesh K (2018) Assessment on the consequences of injection timing and injection pressure on combustion characteristics of sustainable biodiesel fuelled engine. Renew Sustain Energy Rev 81:45–61
Shareef SM, Mohanty DK (2020) Experimental investigations of dairy scum biodiesel in a diesel engine with variable injection timing for performance, emission and combustion. Fuel 280:118647. https://doi.org/10.1016/j.fuel.2020.118647
Silva AOS, Souza MJB, Pedrosa AMG et al (2017) Development of HZSM-12 zeolite for catalytic degradation of high-density polyethylene. Microporous Mesoporous Mater 244:1–6. https://doi.org/10.1016/j.micromeso.2017.02.049
Singh TS, Verma TN, Singh HN (2020) A lab scale waste to energy conversion study for pyrolysis of plastic with and without catalyst: engine emissions testing study. Fuel. https://doi.org/10.1016/j.fuel.2020.118176
Sogancioglu M, Ahmetli G, Yel E (2017) A Comparative study on waste plastics pyrolysis liquid products quantity and energy recovery potential. Energy Procedia 118:221–226. https://doi.org/10.1016/j.egypro.2017.07.020
Su J, Fang C, Yang M et al (2019) Catalytic pyrolysis of waste packaging polyethylene using AlCl3-NaCl eutectic salt as catalyst. J Anal Appl Pyrolysis 139:274–281. https://doi.org/10.1016/j.jaap.2019.02.015
Thompson RC, Moore CJ, Saal FSV, Swan SH (2009) Plastics, the environment and human health: current consensus and future trends. Philos Trans R Soc B Biol Sci 364:2153–2166. https://doi.org/10.1098/rstb.2009.0053
Torres-Jimenez E, Jerman MS, Gregorc A et al (2011) Physical and chemical properties of ethanol-diesel fuel blends. Fuel 90:795–802
Zhang Y, Duan D, Lei H et al (2019) Jet fuel production from waste plastics via catalytic pyrolysis with activated carbons. Appl Energy. https://doi.org/10.1016/j.apenergy.2019.113337
Zhou N, Dai L, Lyu Y et al (2021) Catalytic pyrolysis of plastic wastes in a continuous microwave assisted pyrolysis system for fuel production. Chem Eng J 418:129412. https://doi.org/10.1016/j.cej.2021.129412
Acknowledgements
The authors wish to thank the Slovenian Research Agency (ARRS) for the financial support in the framework of the Research Program P2-0196 in Power, Process and Environmental Engineering and P2-0118 Textile Chemistry. The authors would like to thank the SILKEM d.o.o. Company for supplying the zeolite used. Many thanks also to Dr. Aljoša Košak and Dr. Marijana Lakić from the Institute for Environmental Protection and Sensors (IOS) for their support in determining the zeolite BET surface area. The authors would also like to thank the Universidad de Jaén (UJA, MINECO, Junta de Andalucía, FEDER) for their technical and human support provided by CICT, and to the program of the University of Jaén “Acción 1. Apoyo a las estructuras de investigación de la Universidad de Jaén para incrementar su competitividad atendiendo a sus singularidades.”
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Editorial responsibility: Samareh Mirkia.
Rights and permissions
About this article
Cite this article
Palomar-Torres, A., Torres-Jimenez, E., Kegl, B. et al. Catalytic pyrolysis of plastic wastes for liquid oils’ production using ZAP USY zeolite as a catalyst. Int. J. Environ. Sci. Technol. 20, 17–30 (2023). https://doi.org/10.1007/s13762-022-04023-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-022-04023-z