Abstract
In this paper, the influence on the system performance and greenhouse gas (GHG) emissions of different biomass pretreatment methods before gasification and Fischer–Tropsch (FT) crude production was evaluated. Entrained flow gasification has the benefit of producing a practically tar-free synthesis gas with nearly complete carbon conversion. This gasifier type requires a relatively dry fuel, with small particle size, at high pressure. The size can be acquired by milling, which is energy intensive and feeding is challenging. Torrefaction of biomass facilitates milling; it thus requires less electricity, however, the torrefaction process requires heat. Pyrolysis decomposes the biomass into gaseous, liquid, and solid parts, respectively. This further makes feeding easier, but comes with a greater heat demand than torrefaction. The impact of the different pretreatment methods on the overall energy system has been evaluated using process integration methodology. The results show that the excess heat from an FT process with a biomass input of 300 MWHHV can replace the bark boiler in a large chemical pulp and paper mill, producing 350,000 tonnes of bleached paperboard annually. With the preconditions given for this study, thermal pretreatment of biomass may be beneficial in terms of wood-to-FT crude efficiency, with efficiencies up to 68 %, assuming 40 % electrical efficiency. Pretreatment using pyrolysis performed the best in regards to GHG emissions, if CO2 from acid gas removal was vented, while milling, with an annual reduction of around 700,000 tonnes of CO2,eq, had the best results if the CO2 was captured and sequestrated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Integration of different biorefinery concepts with a pulp and paper (PP) mill has been discussed as a way to increase profitability of the existing PP mill and to reduce overall CO2 emissions. Different courses of action towards sustainable and profitable biorefineries have previously been discussed by, for example, Lundberg et al. (2014) dealing with extraction of lignin and hemicelluloses for production of value-added products and Andersson et al. (2014) who present a concepts based on thermochemical conversion of biomass. Jönsson and Berntsson (2012) consider the possibility to implement carbon capture and storage (CCS) in the European pulp and paper industry as a way of reducing the industry’s impact on global climate change. Geographical and infrastructural factors were found to make it difficult for CCS to be a viable option for northern European PP mills, while the potential is greater for mills in central Europe situated closer to large “capture clusters.” A capture cluster is a region where several CO2 emission sources are located near each other, and thereby benefit from economy of scale for common CCS infrastructure. One potential biorefinery route is gasification of biomass and upgrading into a gas consisting mainly of hydrogen and carbon monoxide (synthesis gas or syngas). This makes possible downstream production of virtually any hydrocarbon, e.g. transportation fuels or chemicals. In many cases, commercial processes, developed for fossil feedstock, are available for syngas processing as well as the final synthesis of the end product. Joelsson and Gustavsson(2012) studied integration possibilities of two different gasification processes with a pulp mill: gasification of black liquor with dimethyl ether (DME) production and biomass gasification with Fischer–Tropsch (FT) production. The processes were compared in terms of system efficiency and CO2 emissions. In that study, they conclude that integration of biofuel production with a PP mill will always be more efficient than a corresponding stand-alone plant. Other studies, however, have shown that a stand-alone plant can perform better in terms of CO2 emissions and economy, under certain conditions (Ljungstedt et al. 2013; Isaksson et al. 2012). The study by Isaksson et al. (2012) assessed biomass gasification and production of three different end-products integrated with a mechanical PP mill, in contrast to previously mentioned studies where the focus was on chemical pulp mills. Heat integration with a mechanical mill introduces different possibilities compared to a chemical pulp because of the lack of a chemical recovery cycle, including the recovery boiler, while instead heaving a substantially higher demand for electricity for the mechanical refiners. Similar approaches of integrating gasification-based biorefineries in existing industry have been investigated in other studies, for example by Brau et al. (2013) where wood-based hydrogen was produced for an oil refinery.
Different biomass gasification technologies have been suggested for these types of processes, for example, indirect dual bed gasification and direct gasification in a fluidized bed or in an entrained flow (EF) reactor, each having their respective advantages and disadvantages. The most commonly used gasifier type today is the EF gasifier, using coal as feedstock, which produces a raw gas practically free from hydrocarbons and tars, thus making subsequent crackers and reformers unnecessary. This type puts rigorous demands on feeding, which is particularly challenging if the raw material is fibrous biomass. The short residence time in the reactor requires a fuel which is relatively dry and has a small particle size, to be pressurized from ambient conditions up to typically 20–80 bar. Milling of biomass is, however, energy intensive, this means that there are reasons to have as large particle size as possible. When using coal as a fuel in EF reactors, the size requirement is in the range of 50–100 µm in order to reach high carbon conversion (>99 %). However, since biomass is more reactive than coal, the size requirement may be less stringent. Methods of dealing with the energy demand connected to sizing of biomass can include a torrefaction or pyrolysis step prior to gasification. Both of these technologies have the capability of reducing the amount of electricity required for milling and pressurization, but on the expense of introducing a heating demand.
This paper presents a study of the integration of FT crude production from gasified biomass with an existing chemical PP mill, focusing on assessing the impact of biomass pretreatment on heat integration. Three different pretreatment methods before EF gasification were evaluated in terms of energy efficiency and greenhouse gas (GHG) emissions: milling only, torrefaction followed by milling, and pyrolysis (milling of residual coke). No previous study is known to the authors that present the impact of biomass pretreatment in a gasification-based forest biorefinery, on heat integration potential, system performance, and GHG emissions.
Methodology
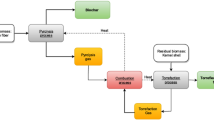
The mass and energy balances of the processes in question must be attained prior to evaluating their performance in terms of efficiency and GHG emissions. The commercial process simulation software Aspen Plus (Aspen Tech 2010) has been used to retrieve these balances for the different process units, shown in Fig. 1. Simulation conditions and assumptions are described in “The Fischer–Tropsch process” section for each unit. Excess heat from the FT process is assumed to replace steam production from the existing bark boiler at the PP mill. Identification of the potential for heat integration between the PP mill and the FT process, as well as the possibility for integration of a steam cycle between the two processes, has been performed using Pinch technology and split grand composite curves (GCCs), also referred to as background/foreground analysis. The concepts of pinch analysis were first formulated by Linnhoff and Flower (1978). The driving force for heat recovery within the processes, represented by the global ΔT min, was set to 8 °C, which corresponds to the current steam consumption for the PP mill, and the biomass input for FT production was fixed at 300 MWHHV (HHV = higher heating value). That size may be considered large enough to benefit from economy of scale while still ensuring durable supply of raw material. The minimum size for biomass gasification has been estimated by McKeough and Kurkela (2008) to be about 200 MWLHV (LHV = lower heating value).
Performance indicators
Two different energy performance indicators were used to evaluate the different cases, FT energy yield, Eq. (1), and wood fuel-to-FT crude efficiency, Eq. (2). The energy yield is defined as the FT energy output divided by the biomass input (HHV basis). The second indicator is defined as the FT energy output divided by the increased use of biomass and increased/decreased use of electricity compared to a stand-alone PP mill. Net increase, or decrease, in electricity demand (\(\Delta \dot{P}\)) is valued as the corresponding amount of biomass needed to produce the electricity in a biomass-based power plant, given a specified wood fuel-to-electricity efficiency, η el . That way, this performance indicator is a measure of the total biomass resource efficiency. The wood fuel-to-electricity efficiency was varied from 25 % [current status for small-scale biomass-based steam cycle power plants (McHale and Associates Inc 2010)] to 55 % [possible to achieve 44 % on a HHV basis in a biomass-integrated gasification combined cycle with current technology (Sydkraft 2001)] to represent different production alternatives. HHV was chosen in favour of LHV since it includes all the latent and sensible energetic changes of a chemical reaction. HHV may therefore be considered more thermodynamically correct than LHV (Bossel 2003). However, the LHV for biomass and FT crude are also provided in order to enable comparisons with other studies: [HHVbiomass (50 % moisture) = 10.07 MJ kg−1 (wet), LHVbiomass (50 % moisture) = 8.14 MJ kg−1 (wet), HHVFT crude = 46.0 MJ kg−1, LHVFT crude = 42.8 MJ kg−1].
The net GHG emissions from the studied systems are calculated according to Eq. (3) and include feedstock handling and distribution, as well as effects of import or export of electricity and replacement of fossil fuels. The imported biomass is assumed to be chipped forest residues, accounting for total GHG emissions of 7.9 kg CO2,eq MWh−1 for production and distribution (Gode et al. 2011). The emissions are presented as CO2 equivalents.
\(\dot{E}_{\text{x}}\) denotes the energy flow of biomass, electricity (generated and consumed) or FT crude, and c x denotes the associated GHG emissions (kg CO2,eq MWh−1). \(m_{{{\text{CO}}_{2} ,{\text{CCS}}}}\) is CO2 that is captured from the process and stored (kg CO2 h−1) and t is the annual operating time (h year−1), which was set to 8,000 h year−1. For cases with capture and storage of CO2, additional electricity is required for compression of CO2. A positive ΔGHG corresponds to a decrease in global GHG emissions with the presented definition. Since FT crude must be upgraded at a refinery, the decrease in GHG emissions cannot be accounted for as the equivalent energy of diesel or petrol. Instead, we assume that the reduction of CO2 emissions is equal to the amount of “green” CO2 released during complete combustion of the FT crude (i.e. the “green” carbon replaces fossil carbon), as well as the GHG emissions prevented in association with the extraction of crude oil [23 kg CO2,eq MWh −1fuel (Gerdes and Skone 2009)]. Upgrading to transportation fuels in a conventional refinery and distribution, both of feedstock and of motor fuel, is assumed to generate approximately the same amount of emissions regardless of feedstock. GHG emissions associated with imported or exported electricity from/to the grid are evaluated for a span of possible electricity production technologies.
Process description
The FT process is assumed to be integrated with the PP mill through its steam and hot water networks and thereby replaces heat production from the existing bark boiler. Any steam surplus is assumed to be expanded in a condensing turbine to generate electricity.
Existing pulp and paper mill
The existing plant, with which the FT process was integrated, is a chemical PP mill, producing approximately 350,000 tonnes of bleached paperboard each year. The current steam production system consists of a recovery boiler and a bark boiler, both connected to the same back-pressure steam turbine for electricity generation. Steam data for the recovery boiler are 515 °C and 110 bar and were assumed to be the same for recovered steam from the FT process. When demand exists, about 8.3 MW of heat can be delivered to the local district heating network with the current setup.
The Fischer–Tropsch process
The process setup consists of a number of steps, visualized in Fig. 1. Assumptions behind the modelling of these steps are described in the following sections. For a thorough discussion about the different technical obstacles and challenges related to various steps within the biomass-to-FT process, see e.g. Noureldin et al. (2014).
Drying
The drying of incoming biomass is essential in order to ensure steady operation of the gasifier and a high quality syngas. A moisture content of 10 % was set as the target and drying was assumed to take place in a low temperature air dryer, heated with excess hot water from the PP mill. After drying, the wood was either sent to milling, torrefaction or pyrolysis.
Torrefaction
Torrefaction is a thermal pretreatment method which significantly improves several characteristics of biomass. Torrefied material has a higher energy density, is hydrophobic, brittle and has a more homogeneous composition. The benefit of using torrefaction as a pretreatment method before gasification is that the material is easier to mill, since the fibrous structure of the biomass has been destroyed. The biomass takes on the characteristics of coal and this method may therefore be appropriately connected to EF gasification. Another benefit from torrefaction is increased homogeneity of the feedstock and a more even size distribution, which is important for steady downstream operation. Torrefaction is carried out without the presence of oxygen at atmospheric pressure and at temperatures between 220 and 300 °C. For this study, information about the fraction of condensable species as well as the composition of permanent gases was gathered from an article by Tapasvi et al. (2012). The composition of the condensable phase was estimated for larch by Prins (2005) and was here assumed to represent an approximate composition for a coniferous wood species. The process was allowed to proceed until the energy requirement of the process was equal to the energy contained in the released volatiles, which were used to provide heat for the process.
Pyrolysis
Biomass pyrolysis is a process carried out in an inert atmosphere around 500 °C where biomass decomposes into a gaseous, a liquid and a solid part. The composition of the pyrolysis products has been predicted by applying equations suggested by Neves et al. (2011), who performed a literature review on pyrolysis characteristics which were structured and analysed. From these data empirical relationships were developed, which have been used in this paper to calculate mass and energy balances for the pyrolysis step. The model calculates the heat requirement for pyrolysis, the amount of ash, and the amount and composition of char and gases (condensable and permanent). Part of the char content was used to provide heat for the pyrolysis process, while part was milled and mixed with the liquid fraction into a pumpable slurry (Henrich and Weirich 2004).
Feeding
Milled, untreated wood was pressurized in lock-hoppers, using CO2 from downstream acid gas removal, and was fed to the gasifier with a screw feeder. Consumption of electricity for milling the biomass to 1 mm accounted for 14 kWel MW −1thermal (Svoboda et al. 2009), while electricity for feeding devices (compression of inert gas) has been estimated by van der Drift et al. (2004) to 21 kWel MW −1thermal .
After torrefaction, the material was milled down to a size of 400 µm with a specific electricity consumption of 5 kWel MW −1thermal . The particle size is smaller, compared to untreated wood, since the reactivity of the biomass decreases during the torrefaction process. The torrefied wood was pressurized in lock-hoppers requiring 25 kWel MW −1thermal and was fed to the gasifier pneumatically.
As described in the previous section, remaining char from pyrolysis was milled (assuming the same specific electricity consumption as for torrefied wood) and mixed with the pyrolysis oil, thus creating a slurry. Electricity consumption for slurry compression was neglected. The permanent gases were assumed to be fed into the hot raw gas stream after the gasifier.
Entrained flow gasification
EF gasification is a well proven method for coal but is still undergoing development for biomass use. The fuel particles are entrained along the flow of oxidant in a flame, which means that the fuel usually has relatively short residence time and that the process is carried out at a high temperature. Thanks to the high temperature, the raw gas is nearly tar-free and contains very little hydrocarbons. The gasifier was modelled to operate at 30 bar with the assumption that the reactions reach equilibrium, justified by experimental results produced by, for example, Qin et al. (2012). Oxygen (99.5 mol% purity) from a cryogenic air separation unit, with a specific electricity consumption of 250 kWh tonne O −12 (van Dijk et al. 1995), was added to reach a temperature of 1,350 °C. The high electricity consumption for oxygen production greatly influences the overall performance. However, more energy efficient solutions, for example membranes, are not feasible on such a large scale (Tranier et al. 2011). The hot raw gas may contain molten particles which can foul downstream heat exchanger surfaces. To avoid this, the gas was cooled in a gas quench, i.e. by recycling cooler gas, down to 900 °C. The gas was then further cooled through steam generation down to 400 °C.
Gas cleaning and upgrade
The raw gas from the gasifier was prepared for FT synthesis in three steps: filtration, adjustment of the H2:CO ratio, and separation of acid gases. Hot gas filtration operates at temperatures up to 900 °C, at pressures up to 8 MPa and in both reducing and oxidizing atmospheres. At these high temperatures only ceramic or metal filters are used. Hot gas filters are often constructed as candle filters where the filter cake builds up on the outside of the tube, while the gas flows out through the open end. The tubes are cleaned in groups by back-pulsing gas from a storage tank. Back-pulsing is in this study done with syngas, and not e.g. nitrogen, so the syngas is not diluted with inert gas (Heidenreich 2012).
The H2:CO ratio was adjusted to around 2 in a partial shift reactor in order to meet specifications for the FT synthesis. In the reactor, an excess of steam was mixed with the gas flow to form hydrogen and carbon dioxide through complete conversion of carbon monoxide. The desired H2:CO ratio was achieved by bypassing the shift reactor with part of the gas flow.
In order to prepare the gas for downstream processing, a methanol absorption unit (similar to Rectisol) was used to remove acid gas, which can poison the FT catalyst. Cooled gas entered the absorber where CO2 and H2S are absorbed in chilled methanol. The purified gas exited at the top of the column, while the absorbent is regenerated before it is recycled (Linde 2013). The electricity and steam demand of the Rectisol-like unit were estimated in accordance with a report by Liu et al. (2011) (Electricity (other than for refrigeration) = 1,900 kJ kmol (CO2 + H2S)−1; Refrigeration (MWel) = 3 × MWthermal from cooling input syngas 12 °C; 5 bar steam = 6.97 kg kmol (H2S + CO2)−1). Even if CO2 removal is done primarily for operational reasons, this is an opportunity to sequester the stream and store it to reduce the emissions of GHG from the plant. Compression of CO2 when CCS was applied has been estimated by Hannula and Kurkela (2012) to account for 0.36 MJ of electricity per kilogram of CO2. Only pure CO2 from the acid gas removal was taken into account when CCS was considered, not e.g. CO2 in flue gases from heat production for torrefaction or pyrolysis, which would require a different set of CO2 separation equipment.
FT-synthesis
The FT process is a way to derive synthetic hydrocarbons from a mixture of hydrogen and carbon monoxide. The process has, both historically and recently, been used to produce liquid transportation fuels from gasified coal, but there is growing interest in using it with renewable feedstock as well. For assumptions about the conversion of syngas into hydrocarbons, the reader is referred to Isaksson et al. (2012) where the synthesis step is described in detail. Only the production of straight paraffins and short olefins has been considered, in accordance with results reported by Fox and Tam (1995). For modern catalysts, a CO conversion of 90 % per pass may be assumed. The FT reactor can be operated with recycling (RC) to increase the overall conversion or, as a simpler solution, as a once-through (OT) process. Off-gases are assumed to be combusted in a boiler with 87 % efficiency, based on HHV. The FT crude is assumed to be sent to a conventional oil refinery for final separation and upgrading. As reported by Hancsók et al. (2014), integration of the final upgrading steps is possible to do in an average structure crude oil refinery. Additionally, the high cetane number of the biomass-based products makes is possible to blend with lower quality fuels in order to enhance profitability.
Results
The different process setups, shown in Fig. 1, have been evaluated concerning production of FT crude and electricity as well as through the wood fuel-to-FT crude efficiency indicator (Eq. 2) and GHG emission consequences. The assumptions behind these performance indicators were presented in “Performance indicators” section.
Energy analysis
As described earlier, the heat integration analysis was carried out using split GCCs. Figure 2 shows the milling case, with 300 MWHHV biomass input and once-through FT synthesis, as an example of this approach. The left figure shows the potential of integrating the FT process (foreground, dashed line) with the PP mill (background, black line). One can see that the “nose” of the PP mill and the heat pocket of the FT process create a good match between the processes. Integration of a steam cycle for utilization of the temperature gap between the gasification process and the PP mill is illustrated in the right figure. That figure shows a joint GCC for the two processes (FT process and PP mill) with an integrated steam cycle (dashed line). Remaining hot utility demand of the PP mill represents the steam production from the existing recovery boiler. Combustion of off-gases from the FT synthesis is depicted as the horizontal line at 900 °C.
Table 1 shows the main comparative results of the heat integration study, such as FT crude output and the electricity balance. The electricity generation (excluding back pressure generation from the recovery boiler, which is constant for all cases) and the main contributors to consumption of electricity are displayed separately, as well as net demand/surplus, in Fig. 3. Recycling of unconverted syngas increases the yield of FT with roughly eight to ten percentage points, while producing substantially less off-gas for steam production and electricity generation, compared to once-through cases. In other words, the FT process could be made smaller, in terms of biomass input, if once-through synthesis is applied instead of recycling if the sizing constraint of the FT process is to fully replace steam production from the bark boiler. The difference in FT crude production between the case with the largest yield (torrefaction + FT with recycling) and the lowest (pyrolysis + once-through FT) is greater than 20 %, because of loss of carbon for heat generation for pyrolysis and in the off-gas boiler in the latter case. This is also shown through the lower cold gas efficiency (CGE) for pyrolysis, compared to the other two pretreatment alternatives. A biomass input of 300 MWHHV is enough for all six FT processes to fully meet the heat demand of the PP mill, which is currently produced by a bark boiler on site, apart from the unaffected recovery boiler steam production, and to leave an excess for a condensing turbine. For some of the cases, it could be argued that the contribution from a condensing turbine would be too small to justify an investment. In such cases, it might be better to adjust the size of the FT process to allow for a larger condensing turbine, or to avoid it altogether.
Electricity is mainly consumed in processes which are not dependent on whether the FT process is run once-through or with recycling of off-gases, except consumption for additional acid gas removal and oxygen generation for tar cracking in the latter case. Consumption is therefore rather similar for both options. The production side, however, differs depending on whether the off-gases are combusted for steam generation or if they are recycled back to the reactor inlet, according to Fig. 3. Preparation of the wood, as well as pressurization and feeding, constitutes quite large shares of total consumption and has a significant impact on the overall performance. To arrive at the excess/deficit of electricity for the biorefinery in Table 1 (not just the net excess/deficit for the FT process, as shown in Fig. 3), the PP mill usage of 7.4 MW has been subtracted from the total electricity production in Fig. 3.
When looking at wood fuel-to-FT crude efficiencies (Eq. 2) in Fig. 4, one can see that this indicator benefits from higher electrical efficiencies since most cases have a deficit of electricity compared to the stand-alone PP mill. Only the pyrolysis once-through case has an excess of electricity (see Table 1) and therefore has a negative slope in the diagram; i.e. if marginal grid electricity is produced with low efficiency, it is better to produce the electricity in the FT process. At lower wood fuel-to-electricity efficiency, once-through cases perform well because of the higher internal electricity production from off-gases, but as the electrical efficiency of the marginal electricity producer improves, the wood fuel-to-FT crude efficiencies for cases with recycling of off-gases have a steeper increase. Milling and torrefaction show rather similar behaviour, where the higher yield of the torrefaction route and lower electricity consumption makes torrefaction preferable. Clearly, the way off-site electricity is produced, influences the results from this indicator to a great extent. Future biomass to electricity production units (for example, a biomass gasification combined cycle) are not expected to operate below 35 %, suggesting that high conversion into FT crude, i.e. recycling of off-gases, is favourable from a systems point of view. By comparing Figs. 4 and 5, one can see that the introduction of CCS lowers overall efficiency by roughly four to five percentage points, due to the added electricity demand for CO2 compression. Note that the differences in wood fuel-to-FT crude efficiency between CCS and non-CCS cases are not constant over the investigated wood fuel–electricity efficiency range.
The influence of CCS on results shown in Fig. 4
Greenhouse gas emission consequences
As described previously, the GHG emission consequences were assessed for a range of different technologies for marginal grid electricity production. The results from the GHG emissions balances are seen in Fig. 6 where the annual potential for GHG reduction is plotted against specific emissions from the marginal grid electricity production technology. Since the input of biomass to the gasifier is the same for all cases, and the product output is roughly the same for once-through and recycling cases respectively, the annual reduction of GHG emissions for those two groups is about the same if there are no emissions associated with grid electricity usage. The GHG emissions are then a function of emissions associated with biomass harvesting and transport and replacement of fossil crude oil with FT crude. The difference between the cases increases as offsite GHG emissions for electricity production grow. In the case of pretreatment technologies, pyrolysis always performs the best, followed by torrefaction and milling, which are almost identical. For comparison, a condensing coal power plant with CCS emits about 259 kgCO2 MWh −1el and a natural gas combined cycle without CCS about 376 kgCO2 MWh −1el (Axelsson and Harvey 2010). Since the electricity production sector has been found to have the largest potential for GHG emissions reduction (European Commission 2011), the recycling of off-gases could be argued to be the best solution for the future, from a systems perspective.
When CCS is applied to the FT process, the reduction potential of GHG emissions is obviously larger, as seen in Fig. 7, where the order of the evaluated technologies is also different compared to when CCS was not included in the analysis (Fig. 6). Now the milling case is the best performing route, since CCS was not assumed to be applied to flue gas streams from heat generation from coke and volatiles for pyrolysis and torrefaction, respectively. That means that more carbon is retained in the syngas stream for the milling cases, compared to both torrefaction and pyrolysis, which is available for capture in the acid gas removal unit and subsequent sequestration. When comparing Figs. 6 and 7, the introduction of CCS does not just shift the curves relative to the y-axis, but it also affects the slope. This is due to the introduced electricity demand for CO2 compression.
As a comparison, co-firing the same amount of biomass (215 MWHHV increase compared to stand-alone PP mill) in a condensing coal power plant would decrease annual CO2 emissions by 580,000 tonnes year−1. Such a comparison is relevant if biomass is considered a limited resource, and if other methods of producing renewable electricity do not meet the demand. However, if electricity from wind, solar and other renewable sources can replace coal power, carbon from biomass could instead be used for transportation fuels.
To gain a complete understanding of the differences between these various pretreatment methods, an economical evaluation would have to be carried out. At the moment, however, financial data especially for torrefaction are highly uncertain since the technology is not commercially proven. Furthermore pyrolysis may be considered an emerging technology, at least for this large a scale and for the type of application discussed in this article. The uncertainty related to determining an economic value for an intermediate product like FT crude also makes it difficult to estimate e.g. the EBITDA (earnings before interest, taxes, depreciation and amortization) for the different configurations. Such a comparison has therefore not been included in this study. Further integration with an expanded district heating system, compared to current demand, has not been evaluated in this study but is not expected to affect the results since access to excess heat is similar for all cases.
Conclusions and discussion
The choice of pretreatment method for gasification of biomass in an EF reactor and production of FT crude highly influences the electricity balance of the biorefinery. This, in turn, it increases the importance of assumptions about surrounding energy systems during evaluation of these technologies. Due to variations in electricity demand, the wood fuel-to-FT crude efficiency is highly dependent on the wood fuel-to-electricity efficiency for offsite electricity production. The route with pyrolysis, and an FT process with recycle, was shown to have the highest wood-to-FT crude efficiency, for a high wood fuel-to-electricity efficiency. The FT crude yield was slightly lower compared to when the wood fuel is simply milled. Still, on the other hand, the method requires less electricity for milling and pressurization. Recycling was beneficial for the system performance for the milling and torrefaction cases, even though the differences are very small at low off-site electrical efficiencies. One should bear in mind, though, that the recycling alternative comes with a significantly higher investment cost, and might therefore not be economically viable, although it is more energy efficient. The GHG emissions consequences follow the same trend as the wood fuel-to-FT crude efficiency, i.e. pyrolysis is shown to be the technology with the largest potential for global GHG reduction, followed by torrefaction and milling, under the assumption that CCS is not applied. As could be seen, the cases appeared in a different order when the CO2 from the acid gas removal unit was captured. If FT crude production from gasified biomass is to be considered a viable alternative to co-firing in a power plant, in terms of GHG emissions, CCS is a necessity.
References
Andersson J, Lundgren J, Marklund M (2014) Methanol production via pressurized entrained flow biomass gasification e Techno-economic comparison of integrated vs. stand-alone production. Biomass Bioenergy 64:256–268. doi:10.1016/j.biombioe.2014.03.063
Aspen Tech (2010) Aspen Plus v7.2. Houston
Axelsson E, Harvey S (2010) Scenarios for assessing profitability and carbon balances of energy investments in industry. The Alliance for Global Sustainability, Gothenburg
Bossel U (2003) Well-to-wheel studies, heating values, and the energy conservation principle. European Fuel Cell Forum, Oberrohrdorf
Brau JF, Morandin M, Berntsson T (2013) Hydrogen for oil refining via biomass indirect steam gasification: energy and environmental targets. Clean Technol Environ 15(3):501–512. doi:10.1007/s10098-013-0591-9
European Commission (2011) A roadmap for moving to a competitive low carbon economy in 2050. European Commission, Brussels, Belgium
Fox JM, Tam SS (1995) Correlation of slurry reactor Fischer–Tropsch yield data. Top Catal 2:285–300. doi:10.1007/BF01491973
Gerdes K, Skone T (2009) Consideration of crude oil source in evaluating transportation fuel GHG emissions. Department of Energy, National Energy Technology Laboratory, USA
Gode J, Martinsson F, Hagberg L, Öman A, Höglund J, Palm D (2011) Miljöfaktaboken 2011. Värmeforsk service AB, Stockholm, Sweden
Hancsók J, Eller Z, Pölczmann G, Varga Z, Holló A, Varga G (2014) Sustainable production of bioparaffins in a crude oil refinery. Clean Technol Environ. doi:10.1007/s10098-014-0743-6
Hannula I, Kurkela E (2012) Liquid transportation fuels via large-scale fluidised-bed gasification of lignocellulosic biomass. VTT Technology 91, Espoo, Finland
Heidenreich S (2012) Hot gas filtration: a review. Fuel 104:83–94. doi:10.1016/j.fuel.2012.07.059
Henrich E, Weirich F (2004) Pressurized entrained flow gasifiers for biomass. Environ Eng Sci 21(1):53–64. doi:10.1089/109287504322746758
Isaksson J, Pettersson K, Mahmoudkhani M, Åsblad A, Berntsson T (2012) Integration of biomass gasification with a Scandinavian mechanical pulp and paper mill—consequences for mass and energy balances and global CO2 emissions. Energy 44(1):420–428. doi:10.1016/j.energy.2012.06.013
Joelsson JM, Gustavsson L (2012) Reductions in greenhouse gas emissions and oil use by DME (di-methyl ether) and FT (Fischer–Tropsch) diesel production in chemical pulp mills. Energy 39:363–374. doi:10.1016/j.energy.2012.01.001
Jönsson J, Berntsson T (2012) Analysing the potential for implementation of CCS within the European pulp and paper industry. Energy 44:641–648. doi:10.1016/j.energy.2012.05.028
Linde (2013) Rectisol Wash. www.linde-engineering.com/en/process_plants/hydrogen_ and_ synthesis_gas_plants/gas_processing_plants/rectisol_wash/index.html. Accessed 18 Feb 2014
Linnhoff B, Flower JR (1978) Synthesis of heat exchanger networks: I. Systematic generation of energy optimal networks. AIChE J 24:633–642. doi:10.1002/aic.690240411
Liu G, Larson ED, Williams RH, Kreutz TG, Guo X (2011) Online Supporting Material for: making Fischer–Tropsch fuels and electricity from coal and biomass: performance and cost analysis. Energy Fuels 25:415–437. doi:10.1021/ef101184e
Ljungstedt H, Pettersson K, Harvey S (2013) Evaluation of opportunities for heat integration of biomass-based Fischer–Tropsch crude production at Scandinavian kraft pulp and paper mill sites. Energy 62:349–361. doi:10.1016/j.energy.2013.09.048
Lundberg V, Bood J, Nilsson L, Axelsson E, Berntsson T, Svensson E (2014) Converting a kraft pulp mill into a multi-product biorefinery: techno-economic analysis of a case mill. Clean Technol Environ. doi:10.1007/s10098-014-0741-8
McHale & Associates Inc (2010) Biomass technology review. Biomass Power Association, Portland
McKeough P, Kurkela E (2008) Process evaluations and design studies in the UCG project 2004–2007. VTT, Espoo
Neves D, Thunman H, Matos A, Tarelho L, Gómez-Barea A (2011) Characterization and prediction of biomass pyrolysis products. Prog Energ Combust 37(5):611–630. doi:10.1016/j.pecs.2011.01.001
Noureldin MMB, Bao B, Elbashir NO, El-Halwagi MM (2014) Benchmarking, insights, and potential for improvement of Fischer–Tropsch-based biomass-to-liquid technology. Clean Technol Environ 16(1):37–44. doi:10.1007/s10098-013-0589-3
Prins MJ (2005) Thermodynamic analysis of biomass gasification and torrefaction. Technische Universiteit Eindhoven, Eindhoven
Qin K, Arendt Jensen P, Lin W, Degn Jensen A (2012) Biomass gasification behavior in an entrained flow reactor: gas product distribution and soot formation. Energ Fuel 26:5992–6002. doi:10.1021/ef300960x
Svoboda K, Pohořelý M, Hartman M, Martinec J (2009) Pretreatment and feeding of biomass for pressurized entrained flow gasification. Fuel Process Technol 90(5):629–635. doi:10.1016/j.fuproc.2008.12.005
Sydkraft (2001) Värnamo demonstration plant: a demonstration plant for biofuel-fired combined heat and power generation based on pressurized gasification. Sydkraft, Trelleborg, Sweden
Tapasvi D, Khalil R, Skreiberg Ø, Tran K-Q, Grønli M (2012) Torrefaction of Norwegian Birch and Spruce: an experimental study using macro-TGA. Energy Fuel 26:5232–5240. doi:10.1021/ef300993q
Tranier J-P, Dubettier R, Darde A, Perrin N (2011) Air Separation, flue gas compression and purification units for oxy-coal combustion systems. Energy Procedia 4:966–971. doi:10.1016/j.egypro.2011.01.143
van der Drift A, Boerrigter H, Coda B, Cieplik MK, Hemmes K (2004) Entrained flow gasification of biomass—ash behaviour, feeding issues, and system analyses. ECN, Petten
van Dijk KM, van Dijk R, van Eekhout VJL, van Hulst H, Schipper W, Stam JH (1995) Methanol from natural gas—conceptual design & comparison of processes. Delft University of Technology, Delft
Acknowledgments
Financial support from Chalmers Energy Initiative and Holmen AB is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Isaksson, J., Åsblad, A. & Berntsson, T. Pretreatment methods for gasification of biomass and Fischer–Tropsch crude production integrated with a pulp and paper mill. Clean Techn Environ Policy 16, 1393–1402 (2014). https://doi.org/10.1007/s10098-014-0815-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10098-014-0815-7