Abstract
Large amounts of solid wastes are produced in manufacturing of leather and leather products. Nearly 80–85% of solid wastes is generated in leather production whereas 20–30% of leather is ended up as wastes from leather goods and footwear industries which poses significant concern regarding environmental pollution. An attempt was made to produce environmentally friendly bio-composite materials with higher mechanical characteristics by utilizing solid wastes released from leather industries. In this study, leather fibers (LF) from shaving dust and leather cutting scrap were used with plant fibers such as banana (Musa acuminata), pineapple (Ananas comosus), betel nut (Areca catechu), and moringa (Moringa oleifera) for making composite sheets reinforced with natural rubber latex (NRL). New composite materials were characterized by using thermogravimetric (TGA), Fourier transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM), and X-ray diffraction (XRD) techniques which confirmed the presence of desired physical and chemical properties in them. FTIR analysis showed characteristics absorption resonances at 3287, 2919, and 2855 cm−1 which were not apparent in starting single fibers. TGA data indicated that banana fiber (BF) composite is thermally more stable than others. The tensile strengths of pineapple fiber (PF) composite and BF sheet were 3.89 and 3.59 Mpa, respectively, which were higher than those observed in control sheet (CS). New composite sheets possess significant properties which make them suitable to be used as valuable raw materials for manufacturing of various footwear and leather goods. This interesting approach will reduce environmental pollution and ensure the sustainability of the respective ecosystem.
Graphical abstract

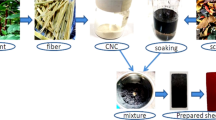
A—different fibrous sources (A1—leather scraps and A2–A4 are plant fibrous sources), B—raw fibers (B1 is leather fiber and B2-B4 are plants fibers), C—small-sized raw fibers, D—alkali treatment, E—crushing machine, F—different fibers (ready), G—hydraulic press machine, and H—prepared composite sheet
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The leather industries and leather products enterprises are important and promising export-oriented sector in many developing countries like Bangladesh. It generates huge employment and contributes significantly to national economy by earning foreign currency. However, leather industries discharge a large quantities of solid wastes during the manufacturing of finished leathers and leathers products, which cause severe environmental pollution in surrounding environment (Teklay et al. 2017a, b). Generally, leather industries generate about 800 kg of solid waste for producing only 200 kg of finished leather from one ton of salted hides or skins in the respective manufacturing processes (Ludvik et al. 1996, Thanikaivelan et al. 2005; Yorgancioglu et al. 2020). Much of the solid wastes discharged from the leather industries are made up of skin trims, keratin wastes, fleshing wastes, chrome shaving dust, and buffing specks of dust (Kanagaraj et al. 2006). About 600,000 tons of solid waste are generated annually in worldwide from various tanning industries (Onukak et al. 2017). The tanning industry in Bangladesh generates 84,680 tons of solid waste per annum which poses a significant threat to human health as well as to surrounding environment unless these wastes undergo with proper treatment processes (Saha and Azam 2021). Thus, the leather industries and related enterprises cause severe environmental pollution by generating both organic and inorganic pollutants. The leather solid wastes are typical observed as piles at riverside and other open places in Bangladesh as they are illegally dumped from tanning industries without any prior treatments. Most of the tanning industries in Bangladesh did not adopt any solid wastes management policy. On the other hand, footwear and leather goods industries discard 20–30% of finished leathers as waste during leather products manufacturing processes (Senthil et al. 2015a, b). Finished leather scraps, cutting scraps, and used leathers (which are thrown away after the use of leather products) are also the major sources of leather solid wastes (Teklay et al. 2017a, b; Kanagaraj et al. 2006). The primary constituent of these solid wastes is protein. If the protein and other chemicals which are included in the chemically modified protein are not utilized properly, they will cause severe environmental pollution (Kanagaraj et al. 2006). The wastes produced during the production and use of leathers might include solid waste, liquid waste (effluent), and emissions of gases which constitute a serious threat to the environmental ecosystem (Sundar et al. 2011). Currently, the disposal of leather solid wastes has become a critical issue in Bangladesh which got out of control due to lack of capacity, poor public awareness, and ignorance of environmental impacts. Due to the generation of expanding volume of wastes and accompanying detrimental effects on the environment, human health, and public safety, managing leather solid wastes has become an increasingly urgent concern in many developing countries (Sumathi and Senthil 2016). About 80% of the solid waste generated from the tanning industry during leather manufacturing processes pose serious environmental impacts due to its toxic nature and improper disposal practices. These solid wastes are contaminating soil, surface waterbodies, and groundwater supplies and thus disrupt the environmental ecosystem as well as pose serious risks to human and animal health. Tannery solid wastes consist of proteinaceous untanned and tanned materials emit unpleasant gases when they are being dumped in open areas without proper treatments. Most of the time, the solid wastes are not sorted out according to their characteristics categories, and improper discharge of chromium-containing solid wastes can pollute soil and groundwater by leaching toxic chromium. Burning solid tannery waste is another typical practice in many developing countries like Bangladesh. Along with the solid wastes, leather industries emit large quantities of wastewater mixed with toxic chromium species into surrounding environment.
Chromium pollution causes different adverse impacts on the human body. It seriously endangers the health of millions of the tannery surrounding people in Bangladesh and severely pollutes local the environment as well (Jannat et al. 2023). It is well-recognized and extensively proven in the literature that chromium is toxic, carcinogenic, mutagenic, and teratogenic to humans, animals, and plants (Jobby et al. 2018; Saranraj and Sujitha 2013). Acute tubular necrosis in the kidney, as well as diarrhea, heartburn, blindness, allergic reactions, respiratory tract infections, and dermatitis, can all result from long-term exposure to some chromium compounds in humans (Adeel et al. 2012; Wang et al. 2016). A trace amount of hexavalent chromium is thought to be carcinogenic, it causes high blood pressure and kidney failure, and its presence in aquatic environments in larger quantities is highly alarming due to the numerous effects on various species (Wang 1986). Land filling by the tannery solid wastes is not an appropriate solution for the leather sector. Therefore, turning tannery solid waste into value-added useful by-products is a long-term, sustainable solution for the leather industry.
To meet the demand of rapidly growing industrialization and increasing population, numerous researches have been reported on the production of renewable biofuel from agro-waste-based biomass briquette (Velusamy et al. 2023, 2021; Velusamy et al. 2022a, b). The utilization of waste materials for the preparation of environment-friendly composites offers a promising solution to the environmental pollution challenges that people are facing today (Rahman et al. 2023). By transforming waste into valuable by-products composites not only mitigates the burden of waste disposal but also contributes to resource conservation and reduced reliance on virgin materials (Yepes et al. 2019). Synthesis of green composites from tannery wastes has gained significant attention in recent years as environmentally sustainable alternative to traditional materials (Muralidharan et al. 2022; Parisi et al. 2021; Liu et al. 2019a, b; Senthil et al. 2015a, b; Ruiz et al. 2015). Recently, Gargano et al. (2023) reported the enhancement of poor-quality leather by using collagen extracted from tannery solid waste. Other previous studies also showed the utilization of tannery solid waste to create value-added products as potential efforts to reduce environmental pollution (Sundaramurthy et al. 2021; Hashem et al. 2021). The synthesis of green composites involves the incorporation of tannery waste into a matrix material, such as natural rubber or poly(lactic acid) (PLA) (Rigueto et al. 2020). Environmentally benign green composites can be synthesized from the combination of tannery solid waste with natural rubber (Urrego et al. 2019) which can be considered as viable approach to reduce environmental pollution in the respective ecosystem. The utilization of tannery waste in composite production not only provides a sustainable solution for waste management but also offers potential benefits in terms of energy efficiency and resource conservation. Green composites produced from tannery waste with textiles offer a potential sustainable alternative to traditional materials (Sivakumar and Mohan 2020). The conversion of tannery waste into green composites materials aligns with the principles of sustainable manufacturing and the circular economy, as well as it contributes to the development of green packaging (Moktadir et al. 2018). On the other hand, a large amount of fibrous wastages are generated from agricultural activities which can be putrescible. Plant fibers (PFs) are attractive materials with a wide range of potential uses considering their affordability, cost-effectiveness, high specific strength, moderate mechanical qualities, non-abrasiveness, biodegradability, and environmentally friendly nature (Bharath et al. 2020; Islam et al. 2018). Due to the warm weather in some countries like Bangladesh, the demand for banana plantation is rapidly expanding day by day which ultimately causes the increase in the annual amount of banana plant waste (the trunk). Therefore, it is necessary to turn these wastes into wealth, and this can be accomplished by extracting the fibers from banana trunks and utilize them in plastic, textile, and paper industries (Ebisike et al. 2013). Pineapple leaf fiber (PALF) is enriched with cellulose and highly available which has the potential to be reinforced with polymers (Devi et al. 1997). Betel nut fibers are by-product obtained from the processing of betel nut fruit after drying which are mostly utilized as fuel for the household (Yusriah et al. 2014). Moringa oleifera (MO) is produced abundantly in semi-arid conditions in Bangladesh, India, Afghanistan, and Malaysia (Islam et al. 2018). These plant fibers (banana fiber, pineapple fiber, betel nut fiber, and Moringa oleifera fiber) can effectively be combined with leather fibers for preparing interesting biodegradable composite materials. The plant fibers endowed with hydroxyl group, carboxy group, aromatic C=C, etc., can form very strong bonds with the peptide linkage and imides. A good number of studies were conducted previously on composite preparation from leather solid wastes, and their results were reported in the literature. However, to the best of our knowledge, very few reports are available in the literature on the preparation of artificial leathers from tannery solid wastes with the combination of bio-degradable plant fibers. The objective of the current study was to utilize leather solid wastes as much as possible to produce eco-friendly and bio-degradable composite sheets with the combination of fibrous agricultural wastes. The study also focused on the management of leather solid wastes with the goal of synthesizing environmentally viable green materials from waste products which will reduce environmental pollution from the leather sectors in Bangladesh.
Materials and methods
Leather shaving dust was collected from Apex Tannery Limited, Hemayetpur, Savar, Dhaka 1340, and scraps of leather were collected from the leather products workshop, Institute of Leather Engineering and Technology, University of Dhaka. The latitude and longitude of Savar, Dhaka, are 23.858334 and 90.266670, respectively. Four different plant fibers such as banana (Musa acuminata), pineapple (Ananas comosus), betel nut (Areca catechu), and moringa (Moringa oleifera) were extracted from the collected agricultural waste from different regions. Banana fibers (BF) were collected from bark, pineapple fibers (PF) were collected from leaf, and betel nut fibers (BNF) and moringa fibers (MF) were collected from fruit. These polar, hydrophilic lignocellulosic fibers are made up of helically wound microfibrils in a lignin and hemicelluloses matrix. Typically, the composition is cellulose: 60–80%, lignin: 5–20%, and moisture content up to 20% (Thakre et al. 2019). Natural rubber latex, ethylene glycol (C2H6O2), aluminum oxide (Al2O3) and silicone spray (releasing agent), KOH, H2SO4, and C-2 (semi-synthetic fat) used were analytical grade, Merck, Germany. Ethylene glycol is an end-functionalized chemical that was used in the mixture as a crosslinking agent, Al2O3 was used as a reinforcement material, and semi-synthetic fat was used to increase the flexibility of the composite. The dry rubber content (DRC) of NRL was 60.23% analyzed by the standard method of PST-221.
Preparation of leather fiber (LF)
About 50 g of cleaned leather solid waste (scraps/shaving dust) was taken in a beaker. About 1000 mL of 2% KOH solution was added and stirred with a glass rod. The mixture was left for 1 h for the uniform treatment. After 1 h, the mixture was washed with distilled water several times until the pH of the solution become 7.0. Treated fibers were dried in the oven at 50–60 °C temperature for several hours. These were converted into small fibrous forms by using the crushing machine (FRITSCH, PSDFS 90L2, Germany) (a sieve diameter of 0.5 mm was used) to transform them into smaller forms. The procedure was repeated to obtain the smallest size of leather fibers, and the average size of leather fibers ranged 0.5–1.0 cm in length (Fig. 1).
Activation and preparation of plant fibers (PFs)
Waste banana bark, pineapple leaf wastage, betel nut fruit shell after use, and waste moringa fruit were collected from different sources. All the collected plant fibers were extracted in a manual retting process (Mohankumar et al. 2021). All the study materials were cleaned several times with water and dried the fibers in sunlight. All the used natural fibers are hydrophilic which indicates the consistency of hydroxyl group. To enhance the bonding of natural fibers and polymer matrix, alkali treatment of the fibers is required to modify the surface area of the fiber (Nayak et al. 2022; Madhu et al. 2019). The collected raw plant fibers were cut into small sizes and treated with 5% KOH for about 1 h to remove impurities and other greasy contents. The fibers were washed with distilled water several times to ensure a pH of 7. Then, treated fibers were dried initially at 60 °C for overnight and later at 105 °C for 1 h (Nayak et al. 2022). In this process, KOH reacts with the –OH group of the natural fibers and removes the hemicelluloses, lignin, wax, and oily materials from the external surface of the fiber, which increases the roughness of the fiber (Bharath et al. 2020). As a result, it helps to join them with the polymer tightly and become stronger. The chemical reaction of KOH with natural fibers is given below:
These were converted into small fibrous forms by using the crushing machine (FRITSCH, PSDFS 90L2, Germany) to transform them into powder form. The procedure was repeated to obtain the smaller form of different fibers, and the average length was 0.5–1.0 cm (Fig. 2).
The physical–chemical composition and mechanical properties of BF (Bharathi et al. 2021), PALF (Kengkhetkit and Amornsakchai 2012), BNHF (Yusriah et al. 2012), and MOF (Binoj 2018) after alkali treatment are shown in Table 1.
Preparation of composite sheets
The activated plant fibers—BF, PF, BNF, and MF—were mixed with the leather fibers (LF) obtained from cutting scraps at the ratio of 1:1 (w/w). Each plant fiber was mixed individually with the leather fiber. About 13 g of individual mixed fiber was added to water. The duration of soaking was kept 24 h, and after soaking, mixed fibers were minced manually. About 26 mL of natural rubber latex, 1 mL of ethylene glycol, 1 mL of C-2(semi-synthetic fat), and 4% Al2O3 were added to the minced fibers. pH was adjusted to 4–4.5 by using concentrated H2SO4. The pH value is important in leather manufacturing as leather is produced with a pH of about 4.5–5.5. The range of pH 4.5–5.5 ensures that the fat and tannins bound in the leather remain as well as resist microbial growth in the composite. Dechromination occurs if the pH of the tanning bath becomes less than 3.0 whereas chromium precipitates as chromic hydroxide at higher than 5.5 pH value. The prepared slurry was poured into the die and pressed manually to drain the water. The moist sheet was compressed for 10 s using a hydraulic press maintained by 1500-psi pressure. After drying in the sunlight for 2–3 days, it was plated using a hydraulic press at 2000 psi and 70 °C temperature for 10 s. As a releasing agent, silicone spray was used before using the die every time (Fig. 3).
The following information was considered during the preparation of composite sheets:
-
1.
CS—as a control sheet made of only LF.
-
2.
BFS—composite sheet made of LF and BF at a ratio of 1:1 (w/w).
-
3.
PFS—composite sheet made of LF and PF at a ratio of 1:1 (w/w).
-
4.
BNFS—composite sheet made of LF and BNF at a ratio of 1:1 (w/w).
-
5.
MFS—composite sheet made of LF and MF at a ratio of 1:1 (w/w).
Characterization techniques
Mechanical characterization
Three specimens with a length of 110 mm and a diameter of 25 mm were formed like dumbbells to test the mechanical qualities. The tensile tester STD 172 machine (IUP-6) was used to assess the tensile strength (TS), stitch tear strength (STS) (IUP-08), and elongation at break (EB) (IUP-06) at a rate of 5 mm/min followed by the standard method SLS 1996. All the tests were performed three times, and the average results were reported here. The composites were conditioned at a temperature of 23 ± 2 °C for 48 h and relative humidity of 65 ± 2% before testing. Water absorption (%) and desorption (%) properties of the control sheets and other composite sheets were also measured according to Sekar et al. (2007). TS, STS, and EB were determined using the following Eqs. (2), (3), and (4), respectively.
Physical characterization
The chemical composition and functional groups of the composites were analyzed by the Fourier transform infrared (FTIR) spectroscopy. A Nicolet 360 FTIR spectrometer, a resolution of 4.0 cm−1, was used to measure the spectra in the 4000–500 cm−1 frequency range. To examine the thermal consistency of the control sheet and composite sheets, thermogravimetric analysis (TGA) was performed under a nitrogen atmosphere with the help of 8000 thermal analyzer (PerkinElmer, USA) at a heating rate of 10 °C per minute and in the temperature range of 25–750 °C. To observe the structure of the composite, X-ray diffraction analysis was done. XRD pattern of the composite had been done through Rigaku MiniFlex Diffractometer (Shimadzu, XRD-7000) operating under CuKα radiation at 40 kV and 150 mA in reflection mode, with a wavelength of 1.541 Å, and the sample was incrementally scanned from 10 to 70 °C. The crystallinity index (CI) was determined using the following equation (Madhu et al. 2019):
where I002 represents the intensity of the crystalline peak, and Iam represents the intensity of the amorphous peak in the crystallographic planes. The morphology of the surface of the samples was visualized by a field emission scanning electron microscope (FESEM Model JSM-7610F). The thickness of the composite leather sheets was CS—1.78 mm, BFS—1.55 mm, PFS—1.38 mm, BNFS—1.17 mm, and MFS—1.9 mm.
Results and discussion
The tensile strength of the composite sheets depends upon the nature of the cellulose fibers. Different physical tests such as tensile strength, stitch tear strength, elongation, water adsorption, and desorption test were performed, and their results are shown in Table 2.
Tensile strength
Table 2 shows that PFS composite sheet had higher tensile strength (3.89 MPa) in comparison with control sheets (CS), leather fiber. The tensile strength of CS was observed at 3.46 MPa, whereas this value was found at 3.59, 3.25, and 2.66 MPa in the case of BFS, BNFS, and MFS, respectively. The tensile strength of pineapple fiber and leather fiber composite (3.89 MPa) was higher than that of NRL cellulose composite (3.39 ± 0.49 MPa). This value is higher than a similar neat control sheet (1.85 ± 0.34 MPa) prepared by Teklay et al. (2017a, b). The tensile strength of leather waste with jute fiber at different ratios was found 2.05–2.20 MPa which is lower than the current study (2.66–3.89 MPa) (Teklay et al. 2017a, b). Senthil et al. (2014) obtained a tensile strength value of 3.06 ± 0.12% of leather composite with different plant fibers which is consistent with the current study. The higher tensile strength value (9.84 MPa) of leather composite with natural rubber was observed by Teklay et al. (2018), but 4% Al2O3 and other initiator chemicals were used which might have enhanced the chemical reaction and finally influenced to increase the strength of the composite. Due to strong interaction and bonding between different functional groups such as –OH, –COOH, and –C=O, etc., of natural fibers with amino acid, composites prepared from amide of leather fiber have become stronger.
Stitch tear strength
Table 2 shows the stitch tear strength values of all composite sheets. The stitch tearing strength of the CS was found 35.62 N/mm, and out of four composite sheets, PFS had better tearing strength, and BFS had the lowest stitch tearing strength (23.35 N/mm). Stitch tearing strength of the composite sheets of the current study ranged between 23.35 and 33.23 N/mm which was higher than leather board composite with jute fiber (16.54–22.57 N/mm) at different ratios (Teklay et al. 2017a, b).
Elongation at break
Results in Table 2 represent the elongation at break (%) of all the leather composite sheets with the control sheet. The highest elongation at break (%) value was found (22.23%) for MFS composite sheet, and the lowest (11.11%) was found in BFS (11.11%) while this value for control sheet was 20.34%. The elongation at break (%) value of MFS and PFS composites was higher than the CS. The elongation at break (%) value of the current study ranged between 11.11 and 22.23 which was higher than those values 8.08–14.31 obtained in leather composite with jute fiber (Teklay et al. 2017a, b) and 5.09–5.62 leather composite (Senthil et al. 2014).
Water absorption and desorption
Most of all PFs are hydrophilic in nature. The water absorption and desorption ability of a composite material plays an important role in deciding whether it is suitable for footwear and leather goods manufacture. Water adsorption performance of the leather waste composite sheets such as BFS, PFS, and BNFS was 67.6%, 43.26%, and 68%, respectively, which were greater than the standard value (minimum 35%; SATRA: TM 9:1993) whereas water desorption value of those composite sheets was 67.6%, 43.26%, and 68%, respectively. The water desorption value is also higher than the SATRA standard (40%). Similar results of water absorption and desorption were found in the reported articles (Teklay et al. 2017a, b; Senthil et al. 2014).
Fourier transform infrared (FTIR) studies
The region of the aromatic skeletal vibration band near the top of 1505 cm−1 typically serves as a proxy for lignin content (Rojith and Singh 2012). In polysaccharides, vibration at 1060 cm−1 is related to C‒O, C‒C stretching, and C‒O bending. The lignin's guaiacyl unit vibration is 1250 cm−1. The phenolic and aliphatic methyl groups of lignin are the source of the weak band at 1373 cm−1. Lignin samples frequently exhibit aromatic skeletal vibrations at 1600 cm−1, 1513 cm−1, and 1443 cm−1 (Rojith and Singh 2012). The water associated with lignin may alternatively be represented as the band at 1600 cm−1. Peak near 2930 cm−1 resulted from aromatic methoxyl group C‒H stretching. Hydroxyl groups of phenolic and aliphatic structures cause a peak to appear about 3420 cm−1.
The FTIR spectra of different fibers and those of composite sheets are presented in Fig. 4. Amides bonds of collagen can be seen at wavelengths of 1638, 1543, and 1238 cm−1, which correspond to amides I, II, and III, respectively, in the FTIR spectrum of the control sheet (Ramnath et al. 2012). The absorption band at 1638 and 1543 cm−1 expresses the presence of an aromatic ring. Stretching bands for –OH and –NH2 that are overlaid cause strong absorption in the 3200–3600 cm−1 area. The C–H of rubber latex is represented by a strong and distinct peak at 2933 cm−1. Interestingly, after the formation of composites, absorption peaks at 3287, 2919, and 2855 cm−1 have become stronger, sharper, and dominated indicating that –OH groups are free on the surface of the composite rather than they are involved in bonding with other functional groups. The sample’s bound –OH groups are represented by a wide peak at 1080–1000 cm−1. The FTIR spectrum of the PFS composite sample exhibits a broad peak from 1038 to 1670 cm−1 that is made up of bonds from the glycoside linkage and C–O–C and C–O stretch (primary and secondary hydroxide groups) as well as perhaps lignin (Sekar et al. 2009). In this spectrum, the H–CH and O–CH in-plane bending vibrations are represented by the peak at 1442 cm−1. The –C–H bending at C-6 in the cellulose molecular structure is represented by the peak at 1228 cm−1. Interestingly, a strong absorption peak at 828 cm−1 appeared in the composite sheets indicating the p-substituted benzene ring which was absent in the fiber samples. However, the in-plane –CH bending is seen at 1374 cm−1 (Sekar et al. 2009). In Fig. 4, the IR spectra of BFS, PFS, BNFS, and MFS somehow resemble the same pattern. This is because all of the samples used to represent collagen and cellulose that are identical.
Thermal studies
For the thermal stability analysis of different fiber composite sheets and leather fiber sheets, approximately 5–6 mg of composite samples were placed in a platinum pan and heated up to 750 °C at a constant rate of 10 °C per min under a nitrogen atmosphere at 25 mL min−1. The thermal stability of the studied samples was analyzed in terms of weight loss with the increase in temperature. Thermograms of all the composite sheets are shown in Fig. 5. It is observed that there was a two-step weight loss in the control group. Up to 260 °C, the sample had its first weight loss (⁓20%) due to the loss of bound and free water as well as other volatiles. After that cellulose starts to decompose at about 300 °C.
The thermal decomposition pattern of all the composite sheets is very similar, and 10–18% final residue remained at 500 °C temperature. Among the observed composites, the leather sheet with banana fiber is thermally more stable than others. The decomposition of the sample’s protein and collagen caused the second significant weight loss (~61%), which happened between 300 and 400 °C. In this stage, decomposition of leather occurred. The shoulder peak provides a significant smooth loss of weight for the thermal decomposition of the leather fiber. A similar shoulder thermal decomposition peak of chrome-tanned leather at about 400 °C was observed in the previous literature (Liu et al. 2019a, b; Gil et al. 2012) and also observed a distinct peak at a temperature range of 430–480 °C. The TGA curves show that the thermal stability of all the composites is very similar, although the banana fiber composite shows a little bit higher thermal stability than others. At 700 °C, ⁓3.12% of the residue was visible in the case of BF composite with leather waste.
Structural analysis
The XRD patterns of PFS and BNFS are shown in Fig. 6. Comparing the XRD pattern of PFS composite with BNFS composite (Fig. 6), the broad XRD peak at 18° and a sharp peak at 22.5oindicate crystalline nature of the PFS composite, whereas BNFS composite shows only a broad peak at 21° indicating amorphous nature (Jayaramudu et al. 2011; Tserki et al. 2005). The broadening of the peak at 2θ = 21° occurs mainly due to the existence of amorphous hemicelluloses and lignin content of BNFS whereas the sharp peak at 2θ = 22.5o is because of the presence of α-cellulose of pineapple fibers.
The percentage of crystallinity index of PFS with leather dust was found 51% whereas BNFS was amorphous in nature.
Scanning electron microscope (SEM)
Figure 7 shows the SEM images of the PFS composite. The leather fibers and polymer binder along with plant fiber can both be seen in these images. It is easy to observe that the network of fibers is adhering to the NRL in PFS composite sheet. This demonstrates that the leather is a composite of protein fiber. The binding of NRL with pineapple fibers and leather fibers binder is clearly in the SEM image of PFS. It is noticed that the network of fibers has adhered to the NRL in the PFS sheet. All the individual strands have almost the same diameter. The length of PF is observed⁓10 µm. This demonstrates that the PFS is a composite of PF and LF.
Conclusions
Tannery solid waste as well as leather scraps were utilized effectively as suitable raw materials for making new bio-composite sheets. Physical and thermal properties of the composite sheets were examined, and the results showed that these new composites materials could serve many purposes. Compared to the control sample, the LF–plant fiber composite sheets demonstrated better mechanical characteristics. Due to the fibrous structures of composite sheets, thermal stabilities have not degraded suddenly, and they showed slower decomposition as expected for composite materials. The tensile strengths of BFS, PFS, BNFS, and MFS composites were 3.59, 3.89, 3.25, and 2.66 MPa, respectively, whereas the stitch tear strengths were found as 23.35, 33.23, 29.35, and 31.58 (N/mm), respectively. Estimation of water absorption in composite sheets showed the range from 33.33 to 68 which were considerably higher than the standard value (minimum 35%; SATRA: TM 9:1993) except for MFS. The physio-mechanical, thermal, and morphological studies showed that the new composite sheets could be employed as a raw material to produce different light consumer items such as chappal, insole, belts, purses, small leather goods, mouse pads, and other interior decorative goods. New composite sheets could also be used as replacement of leather or other synthetic sheets, and they could work as reinforcing layer or direct raw materials for making various light products. Transformation of solid leather wastes into valuable by-products not only mitigates the burden of waste disposal but also contributes to solid waste management, resource conservation, and reduces reliance on virgin materials. This new approach of solid leather waste utilization is cost-effective, environment friendly, and would be suitable for the sustainable development of leather industries worldwide and thereby will secure environmental sustainability.
Availability of data and materials
All data generated or analyzed during this study are included in this article.
References
Adeel SS, Wajid A, Hussain S, Malik F, Sami Z, Haq IU, Hameed A, Channa RA (2012) Recovery of chromium from the tannery wastewater by use of Bacillus subtilis in Gujranwala, Pakistan. IOSR J Pharm Biol Sci 2(2):36–45
Bharath KN, Madhu P, Gowda TG, Sanjay MR, Kushvaha V, Siengchin S (2020) Alkaline effect on the characterization of discarded waste of Moringa oleifera fiber as a potential eco-friendly reinforcement for biocomposites. J Polym Environ 28(11):2823–2836. https://doi.org/10.1007/s10924-020-01818-4
Bharathi SV, Vinodhkumar S, Saravanan MM (2021) Strength characteristics of banana and sisal fiber reinforced composites. IOP Conf Ser Mater Sci Eng 1055(1):012024. https://doi.org/10.1088/1757-899X/1055/1/012024
Binoj JS (2018) Characterization and optimization of mechanical properties of sustainable moringa oleifera fruit husk fiber for polymer composite applications. SAE Tech Pap 2018:28. https://doi.org/10.4271/2018-28-0045
Cerqueira EF (2011) Mechanical behaviour of polypropylene reinforced sugarcane bagasse fibers composite. Procedia Eng 10:2046–2051. https://doi.org/10.1016/j.proeng.2011.04.339
Devi LU, Bhagawan SS, Thomas S (1997) Mechanical properties of pineapple leaf fiber-reinforced polyester composite. J Appl Polym Sci 64(9):1739–1748. https://doi.org/10.1002/(SICI)1097-4628(19970531)64:9%3C1739::AID-APP10%3E3.0.CO;2-T
Ebisike K, AttahDaniel BE, Babatope B, Olusunle SO (2013) Studies on the extraction of naturally-occurring banana fibers. Int J Eng Sci 2(9):9
Gargano M, Florio C, Sannia G, Lettera V (2023) From leather wastes to leather: enhancement of low quality leather using collagen recovered from leather tanned wastes. Clean Technol Environ Policy. https://doi.org/10.1007/s10098-023-02552-w
Gil RR, Girón RP, Lozano MS, Ruiz B, Fuente E (2012) Pyrolysis of biocollagenic wastes of vegetable tanning. Optimization and kinetic study. J Anal Appl Pyrol 98:129–136. https://doi.org/10.1016/J.JAAP.2012.08.010
Gopanna A, Mandapati RN, Thomas SP (2019) Fourier transform infrared spectroscopy (FTIR), Raman spectroscopy and wide-angle X-ray scattering (WAXS) of polypropylene (PP)/cyclic olefin copolymer (COC) blends for qualitative and quantitative analysis. Polymer 76:4259–4274. https://doi.org/10.1007/s00289-018-2599-0
Hashem M, Sheikh M, Biswas M, Hasan M, Payel S (2021) Composite fabrication from fat extracted limed fleshing: solid waste management in tannery. Bangladesh J Sci Ind 3(56):215–222. https://doi.org/10.3329/bjsir.v56i3.55969
International Union of Leather Technologists and Chemist Societies (2001) IUP-08. Measurement of tearing strength
International Union of Leather Technologists and Chemist Societies (2001) IUP-06. Measurement of tensile strength and percentage of elongation
Islam MR, Isa N, Yahaya AN, Beg MD, Yunus RM (2018) Mechanical, interfacial, and fracture characteristics of poly (lactic acid) and Moringa oleifera fiber composites. Adv Polym Technol 37(6):1665–1673. https://doi.org/10.1002/adv.21823
Jannat N, Nahar H, Khan NS (2023) Potential removal of chromium from tannery wastewater by water hyacinth roots. Water Conserv Sci Eng 8:21. https://doi.org/10.1007/s41101-023-00196-x
Jayaramudu J, Maity A, Sadiku ER, Guduri BR, Rajulu AV, Ramana CV, Li R (2011) Structure and properties of new natural cellulose fabrics from Cordia dichotoma. Carbohyd Polym 86(4):1623–1629. https://doi.org/10.1016/j.carbpol.2011.06.071
Jobby R, Jha P, Yadav AK, Desai N (2018) Biosorption and biotransformation of hexavalent chromium [Cr(VI)]: a comprehensive review. Chemosphere 207:255–266. https://doi.org/10.1016/j.chemosphere.2018.05.050
Kale RD, Jadhav NC (2019) Utilization of waste leather for the fabrication of composites and to study its mechanical and thermal properties. SN Appl Sci 1(10):1–9. https://doi.org/10.1007/s42452-019-1230-9
Kanagaraj J, Velappan KC, Babu NK, Sadulla S (2006) Solid wastes generation in the leather industry and its utilization for cleaner environment—a review. J Sci Ind Res 65:541–548
Kanagaraj J, Senthilvelan T, Panda RC, Kavitha S (2015) Eco-friendly waste management strategies for greener environment towards sustainable development in leather industry: a comprehensive review. J Clean Prod 89:1–7. https://doi.org/10.1016/j.jclepro.2014.11.013
Kengkhetkit N, Amornsakchai T (2012) Utilization of pineapple leaf waste for plastic reinforcement: a novel extraction method for short pineapple leaf fiber. Ind Crops Prod 40:55–61. https://doi.org/10.1016/j.indcrop.2012.02.037
Langhorst AE, Burkholder J, Long J, Thomas R, Kiziltas A, Mielewski D (2018) Blue-agave fiber-reinforced polypropylene composites for automotive application. Bio Resour 13(1):820–835
Liu J, Luo L, Hu Y, Wang F, Zheng X, Tang K (2019a) Kinetics and mechanism of thermal degradation of vegetable-tanned leather fiber. J Leather Sci Eng 1(1):1–13. https://doi.org/10.1186/s42825-019-0010-z
Liu B, Li Y, Wang Q, Bai S (2019b) Green fabrication of leather solid waste/thermoplastic polyurethanes composite: physically de-bundling effect of solid-state shear milling on collagen bundles. Compos Sci Technol. https://doi.org/10.1016/j.compscitech.2019.06.001
Madhu P, Sanjay MR, Pradeep S, Bhat KS, Yogesha B, Siengchin S (2019) Characterization of cellulosic fibre from Phoenix pusilla leaves as potential reinforcement for polymeric composites. J Market Res 8(3):2597–2604. https://doi.org/10.1016/j.jmrt.2019.03.006
Mahesh D, Kowshigha KR, Raju NV, Aggarwal PK (2020) Characterization of banana fiber-reinforced polypropylene composites. J Indian Acad Wood Sci 17(1):1–8. https://doi.org/10.1007/s13196-019-00244-x
Mohankumar D, Amarnath V, Bhuvaneswari V, Saran SP, Saravanaraj K, Gogul MS, Sridhar S, Kathiresan G, Rajeshkumar L (2021) Extraction of plant based natural fibers–a mini review. IOP Conf Ser Mater Sci Eng 1145(1):012023. https://doi.org/10.1088/1757-899X/1145/1/012023
Moktadir A, Rahman T, Rahman H, Ali S, Paul S (2018) Drivers to sustainable manufacturing practices and circular economy: a perspective of leather industries in Bangladesh. J Clean Prod 174:1366–1380. https://doi.org/10.1016/j.jclepro.2017.11.063
Muralidharan V, Palanivel S, Balaraman M (2022) Turning problem into possibility: a comprehensive review on leather solid waste intra-valorization attempts for leather processing. J Clean Prod 367:133021. https://doi.org/10.1016/j.jclepro.2022.133021
Nayak S, Khuntia SK, Mohanty SD, Mohapatra J, Mall TK (2022) An experimental study of physical, mechanical and morphological properties of alkali treated moringa/areca based natural fiber hybrid composites. J Nat Fibers 19(2):630–641. https://doi.org/10.1080/15440478.2020.1758282
Onukak IE, Mohammed-Dabo IA, Ameh AO, Okoduwa SI, Fasanya OO (2017) Production and characterization of biomass briquettes from tannery solid waste. Recycling 2(4):17. https://doi.org/10.3390/recycling2040017
Palanisamy S, Kalimuthu M, Santulli C, Nagarajan R, Karuppiah G (2022) Effect of extraction methods on the properties of bast fibres. In: Rajeshkumar G, Devnani GL, Sinha S, Sanjay MR, Siengchin S (eds) Bast fibers and their composites: processing, properties and applications. Springer, Berlin, pp 17–37. https://doi.org/10.1007/978-981-19-4866-4_2
Parisi M, Nanni A, Colonna M (2021) Recycling of chrome-tanned leather and its utilization as polymeric materials and in polymer-based composites: a review. Polymers 3(13):429. https://doi.org/10.3390/polym13030429
Rahman MA, Haque S, Athikesavan MM, Kamaludeen MB (2023) A review of environmental friendly green composites: productionmethods, current progresses, and challenges. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-022-24879-5
Ramnath V, Sekar S, Sankar S, Sankaranarayanan C, Sastry TP (2012) Preparation and evaluation of bio-composites as wounddressing material. J Mater Sci Mater Med 23(12):3083–3095. https://doi.org/10.1007/s10856-012-4765-5
Rigueto C, Rosseto M, Krein D, Ostwald B et al (2020) Alternative uses for tannery wastes: a review of environmental, sustainability, and science. J Leather Sci Eng. https://doi.org/10.1186/s42825-020-00034-z
Rojith G, Singh IB (2012) Delignification, cellulose crystallinity change and surface modification of coir pith induced by oxidative delignification treatment. Int J Environ Bioenergy 3(1):46–55
Ruiz MR, Budemberg ER, da Cunha GP, Bellucci FS, da Cunha HN, Job AE (2015) An innovative material based on natural rubber and leather tannery waste to be applied as antistatic flooring. J Appl Polym Sci 132(3):41297
Saha B, Azam FAB (2021) Probable ways of tannery’s solid and liquid waste management in Bangladesh—an overview. Text Leather Rev 4(2):1–20. https://doi.org/10.31881/TLR.2020.25
Saikia P, Goswami T, Dutta D, Dutta NK, Sengupta P, Neog D (2017) Development of a flexible composite from leather industry waste and evaluation of their physico-chemical properties. Clean Technol Environ Policy 19(8):2171–2178. https://doi.org/10.1007/s10098-017-1396-z
Saranraj P, Sujitha D (2013) Microbial bioremediation of chromium in tannery effluent: a review. Int J Microbiol Res 4(3):305–320. https://doi.org/10.5829/idosi.ijmr.2013.4.3.81228
Sekar S, Mohan R, Ramasastry M, Das BN, Sastry TP (2007) Preparation and particle characterization of composite boards using chrome shavings and various binders. Leather Age 19:86–92
Sekar S, Mohan R, Ramasastry M, Das BN, Sastry TP (2009) Preparation and characterization of composite boards using chrome shavings and plant fibers. J Indian Leather Technol Assoc 10:765–770
Senthil R, Hemalatha T, Kumar BS, Uma TS, Das BN, Sastry TP (2014) Recycling of finished leather wastes: a novel approach. Clean Technol Environ Policy 17(1):187–197. https://doi.org/10.1007/s10098-014-0776-x
Senthil R, Hemalatha T, Manikandan R, Das BN, Sastry TP (2015a) Leather boards from buffing dust: a novel perspective. Clean Technol Environ Policy 17(2):571–576. https://doi.org/10.1007/s10098-014-0831-7
Senthil R, Inbasekaran S, Gobi N, Das BN, Sastry TP (2015b) Utilisation of finished leather wastes for the production of blended fabrics. Clean Technol Environ Policy 17(6):1535–1546. https://doi.org/10.1007/s10098-014-0881-x
Singh SK (2015) Fabrication and characterization of bio composite materials based on sunnhemp fibre. Int J Mod Eng Res 5(4):24–29
Singh AA, Biswas P, Biswas K (2014) Structure, mechanical and thermal properties of coconut fiber reinforced polypropylene composites with 2% MAPP as a compatibilizer. Appl Polym Compos 2(2):109–119
Sivakumar V, Mohan R (2020) Sustainable solid waste management in leather and textile industry leather and textile waste fibre-polymer composite and nanocomposite overview and review. Text Leather Rev. https://doi.org/10.31881/TLR.2020.04
SLC-Society of Leather Technologists and Chemists. Official Methods of Analysis (1996)
Sumathi VI, Senthil RE (2016) Physico-chemical properties of reconstituted fibers composite prepared from leather waste. Int J Pharm Bio Sci 7(4):105–110
Sundar VJ, Raghavarao J, Muralidharan C, Mandal AB (2011) Recovery and utilization of chromium-tanned proteinous wastes of leather making: a review. Crit Rev Environ Sci Technol 41(22):2048–2075. https://doi.org/10.1080/10643389.2010.497434
Sundaramurthy I, Thiyagarajan G, Panda R, Sankar S (2021) Collagen and carbon-ferrous nanoparticles used as a green energy composite material for energy storage devices. CMS 1(14):80–92. https://doi.org/10.2174/2666145413666201207202502
Teklay A, Gebeyehu G, Getachew T, Yaynshet T, Sastry TP (2017a) Conversion of finished leather waste incorporated with plant fibers into value added consumer products–an effort to minimize solid waste in Ethiopia. Waste Manag 68:45–55. https://doi.org/10.1016/j.wasman.2017.07.024
Teklay A, Gebeyehu G, Getachew T, Yaynshet T, Sastry TP (2017b) Preparation of value added composite boards using finished leather waste and plant fibers - a waste utilization effort in Ethiopia. Clean Technol Environ Policy 19(5):1285–1296. https://doi.org/10.1007/s10098-016-1327-4
Teklay A, Gebeyehu G, Getachew T, Yaynshet T, Inbasekaran S, Sastry TP (2018) Preparation of value added composite sheet from solid waste leather-a prototype design. Sci Res Essays 13(2):11–13. https://doi.org/10.5897/SRE2017.6551
Thakre AR, Baxi RN, Shelke DR, Bhuyar DS (2019) Composites of polypropylene and natural fibers: a review. Int J Res Eng IT Soc Sci 8(5):56–59
Thanikaivelan P, Rao JR, Nair BU, Ramasami T (2005) Recent trends in leather making: processes, problems, and pathways. Critical Reviews Env Sci Tech 35(1):37–79. https://doi.org/10.1080/10643380590521436
Tserki V, Zafeiropoulos NE, Simon F, Panayiotou C (2005) A study of the effect of acetylation and propionylation surface treatments on natural fibres. Compos A Appl Sci Manuf 36(8):1110–1118. https://doi.org/10.1016/j.compositesa.2005.01.004
Urrego W, Vasquez NC, Velasquez SM, Posada J (2019) Mechanical and rheometric properties of natural rubber composites filled with untreated and chemically treated leather wastes. J Compos Mater. https://doi.org/10.1177/002199831880519
Velusamy S, Subbaiyan A, Thangam RS (2021) Combustion characteristics of briquette fuels from sorghum panicle–pearl millets using cassava starch binder. Environ Sci Pollut Res Int 28:21471–21485. https://doi.org/10.1007/s11356-02-11790-0
Velusamy S, Subbaiyan A, Murugesan SR, Shanmugamoorthy M, Sivakumar V, Velusamy P, Veerasamy S, Mani K, Sundararaj P, Periyasamy S (2022a) Comparative analysis of agro waste material solid biomass briquette for environmental sustainability. Adv Mater Sci Eng 2022:1–7. https://doi.org/10.1155/2022/3906256
Velusamy S, Subbaiyan A, Kandasamy S, Shanmugamoorthy M, Thirumoorthy P (2022b) Combustion characteristics of biomass fuel briquettes from onion peels and tamarind shells. Arch Environ Occup Health 77(3):251–262. https://doi.org/10.1080/19338244.2021.1936437
Velusamy S, Subbaiyan A, Shanmugamoorthy M, Thirumoorthy P (2023) Characterization of solid biomass briquette biofuel from the wastes of Senna auriculata and Ricinus communis using Tapioca starch for sustainable environment. Environ Sci Pollut Res 30:10110–10127. https://doi.org/10.1007/s11356-022-22823-1
Wang W (1986) The effect of river water on phytotoxicity of Ba, Cd, and Cr. Environ Pollut B 11(3):193–204. https://doi.org/10.1016/0143-148X(86)90023-6
Wang D, He S, Shan C, Zhang W, Pan B, Ma H, Zhang X (2016) Chromium speciation in tannery effluent afteralkaline precipitation: isolation and characterization. J Hazard Mater 316:169–177. https://doi.org/10.1016/j.jhazmat.2016.05.021
Yepes WU, Cardona N, Velasquez SM, Giraldo Vásquez DH, Posada JC (2019) Mechanical and rheometric properties of natural rubber composites filled with untreated and chemically treated leather wastes. J Compos Mater 53(11):1475–1487
Yorgancioglu A, Başaran B, Sancakli A (2020) Value addition to leather industry wastes and by-products: hydrolyzed collagen and collagen peptides. Text. Ind Waste 7:131–141. https://doi.org/10.5772/intechopen.92699
Yusriah L, Sapuan SM, Zainudin ES, Mariatti M (2012) Exploring the potential of betel nut husk fiber as reinforcement in polymer composites: effect of fiber maturity. Procedia Chem 4:87. https://doi.org/10.1016/j.proche.2012.06.013
Yusriah L, Sapuan SM, Zainudin ES, Mariatti M (2014) Characterization of physical, mechanical, thermal and morphological properties of agro-waste betel nut (Areca catechu) husk fibre. J Clean Prod 72:174–180
Acknowledgements
The authors would like to express their sincere thanks to Dr. Abdul Gafur, Principal Scientific Officer, Bangladesh Council of Scientific and Industrial Research (BCSIR) for instrumental support.
Funding
The authors declare that no fund was received for the current research.
Author information
Authors and Affiliations
Contributions
MT helped in experimental work, methodology, data collection, and original draft manuscript preparation. MAM helped in design, conceptualization, methodology, sample analysis, and review and editing. MAK helped in design, conceptualization, methodology, and review and editing. MJR helped in conceptualization, methodology, instrumental support, and review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tauhiduzzaman, M., Mottalib, M.A., Rahman, M.J. et al. Preparation and characterization of composite sheets from solid leather waste with plant fibers: a waste utilization effort. Clean Techn Environ Policy 26, 1025–1038 (2024). https://doi.org/10.1007/s10098-023-02642-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10098-023-02642-9